Flaxseed and Carbohydrase Enzyme Supplementation Alters Hepatic n-3 Polyunsaturated Fatty Acid Molecular Species and Expression of Genes Associated with Lipid Metabolism in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Diets
2.2. Lipid Profiling
2.3. Analysis of Phospholipid Molecular Species
2.4. RT-qPCR of Lipid Metabolism-Related Genes
2.5. Statistics
3. Results
3.1. Diet Lipid Profile
3.2. Chicken Production Performance
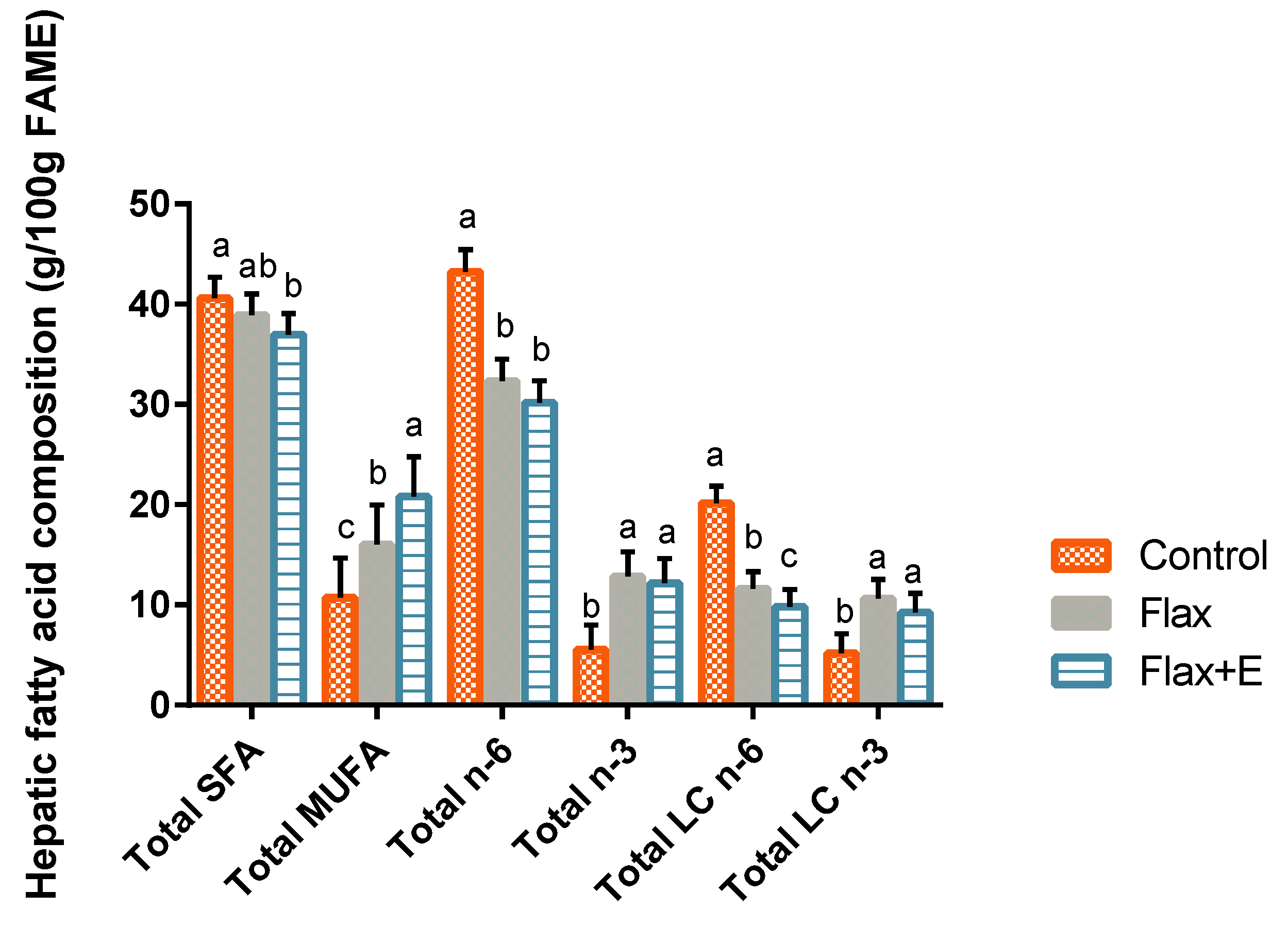
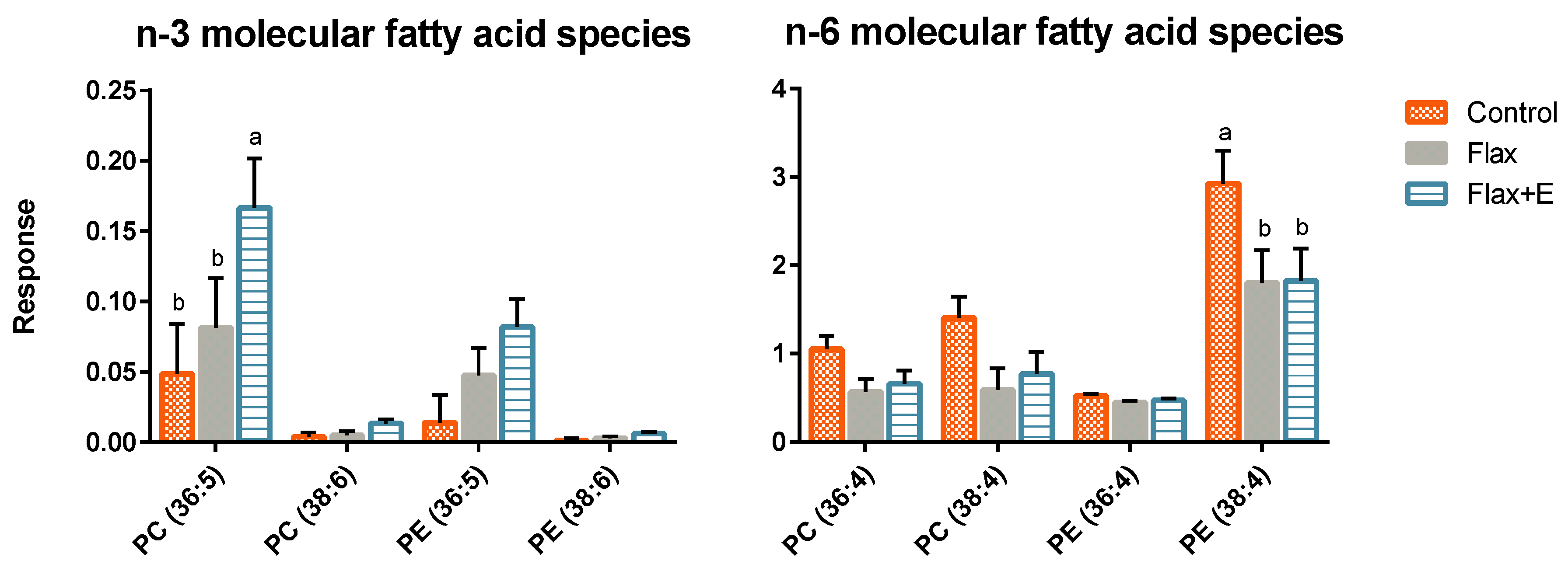
3.3. Liver Lipid Profile
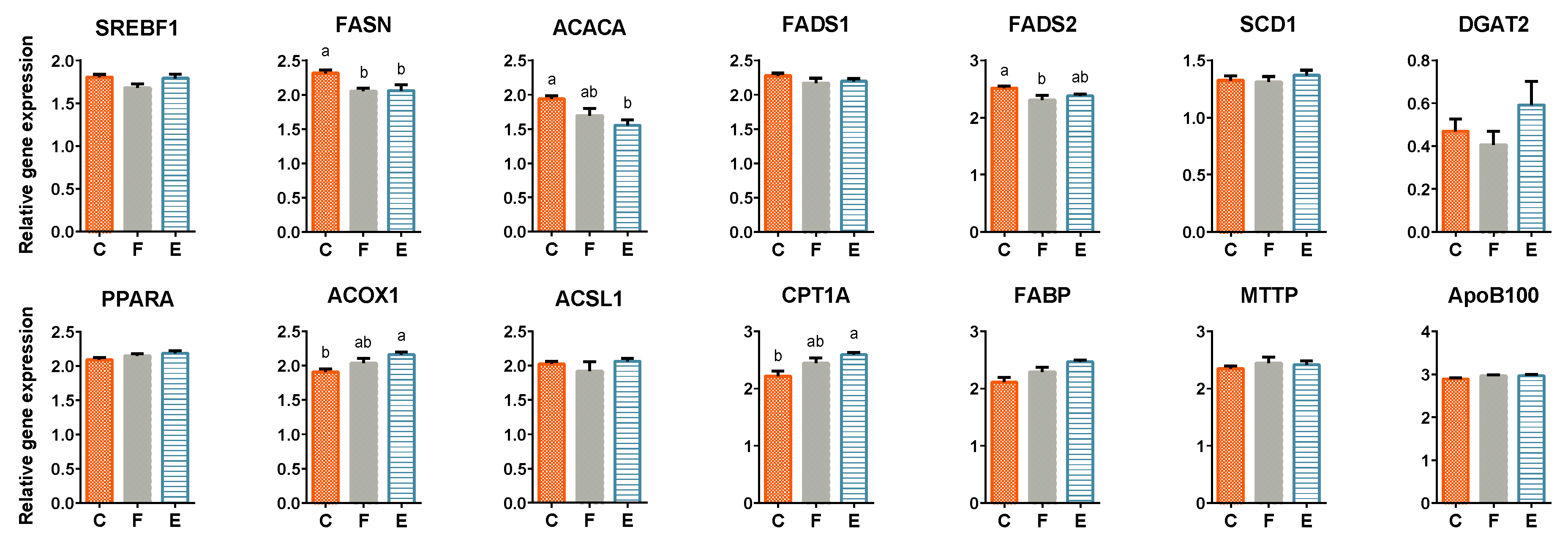
3.4. Liver Lipid Metabolism-Related Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tocher, D.R.; Bendiksen, E.Å.; Campbell, P.J.; Bell, J.G. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Shomonov-Wagner, L.; Raz, A.; Leikin-Frenkel, A. Alpha linolenic acid in maternal diet halts the lipid disarray due to saturated fatty acids in the liver of mice offspring at weaning. Lipids Health Dis. 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhuang, J.; Rao, K.; Li, X.; Zhao, R. Effect of early feed restriction on hepatic lipid metabolism and expression of lipogenic genes in broiler chickens. Res. Vet. Sci. 2010, 89, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Ntambi, J.M.; Friedman, J.M. Stearoyl-CoA desaturase-1 and the metabolic syndrome. Curr. Drug Targets Immune Endocr. Metab. Disord. 2003, 3, 271–280. [Google Scholar] [CrossRef]
- Jump, D.B.; Botolin, D.; Wang, Y.; Xu, J.; Christian, B.; Demeure, O. Fatty Acid Regulation of Hepatic Gene Transcription. J. Nutr. 2005, 135, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.; Osorio, J.S.; Trevisi, E.; Yanqui-Rivera, F.; Estill, C.T.; Bionaz, M. 2,4-Thiazolidinedione Treatment Improves the Innate Immune Response in Dairy Goats with Induced Subclinical Mastitis. PPAR Res. 2017, 2017, 7097450. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr. Opin. Lipidol. 2008, 19, 242–247. [Google Scholar] [CrossRef]
- Ajuyah, A.O.; Ahn, D.U.; Hardin, R.T.; Sim, J.S. Dietary Antioxidants and Storage Affect Chemical Characteristics of ω-3 Fatty Acid Enriched Broiler Chicken Meats. J. Food Sci. 1993, 58, 43–46. [Google Scholar] [CrossRef]
- Azcona, J.O.; Schang, M.J.; Garcia, P.T.; Gallinger, C.; Ayerza, R., Jr.; Coates, W. Omega-3 enriched broiler meat: the influence of dietary α-linolenic-ω-3 fatty acid sources on growth, performance and meat fatty acid composition. Can. J. Anim. Sci. 2008, 88, 257–269. [Google Scholar] [CrossRef]
- Bhatty, R.S.; Cherdkiatgumchai, P. Compositional analysis of laboratory-prepared and commercial samples of linseed meal and of hull isolated from flax. J. Am. Oil Chem. Soc. 1990, 67, 79–84. [Google Scholar] [CrossRef]
- Jia, W.; Slominski, B.A. Means to improve the nutritive value of flaxseed for broiler chickens: the effect of particle size, enzyme addition, and feed pelleting. Poult. Sci. 2010, 89, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Apperson, K.D.; Cherian, G. Effect of whole flax seed and carbohydrase enzymes on gastrointestinal morphology, muscle fatty acids, and production performance in broiler chickens. Poult. Sci. 2017, 96, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Kansas Lipidomics Research Center. Available online: http://www.k-state.edu/lipid/lipidomics/ (accessed on 8 March 2019).
- Cherian, G. Egg yolk conjugated linoleic acid alters phospholipid molecular species in chick tissues. Eur. J. Lipid Sci. Technol. 2009, 111, 546–552. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide. Statistics. Release 9.4; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Smink, W.; Gerrits, W.J.; Hovenier, R.; Geelen, M.J.; Verstegen, M.W.; Beynen, A.C. Effect of dietary fat sources on fatty acid deposition and lipid metabolism in broiler chickens. Poult. Sci. 2010, 89, 2432–2440. [Google Scholar] [CrossRef]
- Head, B.A.; Vercese, F.; Cherian, G. Total tract lipid digestibility, muscle fatty acids and oxidative stability during storage in broilers fed flax with carbohydrase enzyme. Poult. Sci. 2017, 96 (Suppl. 1), P428. [Google Scholar]
- Hermier, D. Lipoprotein metabolism and fattening in poultry. J. Nutr. 1997, 127 (Suppl. 5), 805S–808S. [Google Scholar] [CrossRef]
- Kloareg, M.; Noblet, J.; van Milgen, J. Deposition of dietary fatty acids, de novo synthesis and anatomical partitioning of fatty acids in finishing pigs. Br. J. Nutr. 2007, 97, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, L.R.; Hughes, R.; Geier, M.; Gibson, R. The effect of diet containing high alpha-linolenic acid on omega-3 fatty acids and health status of the heart in broilers. Bul. Peternak. 2017, 41, 48–53. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kim, Y.C.; Ntambi, J.M. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J. Lipid Res. 2001, 42, 1018–1024. [Google Scholar] [PubMed]
- Betti, M.; Perez, T.I.; Zuidhof, M.J.; Renema, R.A. Omega-3-enriched broiler meat: 3. Fatty acid distribution between triacylglycerol and phospholipid classes. Poult. Sci. 2009, 88, 1740–1754. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, L.R.; Hughes, R.J.; Geier, M.S.; Makrides, M.; Gibson, R.A. Dietary alpha-linolenic acid enhances omega-3 long chain polyunsaturated fatty acid levels in chicken tissues. Prostaglandins Leukot Essent Fat. Acids 2012, 87, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Konishi, Y.; Zaima, N.; Kajihara, S.; Nakanishi, H.; Taguchi, R.; Setou, M. Visualization of the cell-selective distribution of PUFA-containing phosphatidylcholines in mouse brain by imaging mass spectrometry. J. Lipid Res. 2009, 50, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Else, P.L. Membranes as possible pacemakers of metabolism. J. Biol. 1999, 199, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.D.; Jump, D.B. Polyunsaturated fatty acid regulation of hepatic gene transcription. Lipids 1996, 31, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Bezard, J.; Blond, J.P.; Bernard, A.; Clouet, P. The metabolism and availability of essential fatty acids in animal and human tissues. Reprod. Nutr. Dev. 1994, 34, 539–568. [Google Scholar] [CrossRef] [PubMed]
- Assaf, S.; Lagarrigue, S.; Daval, S.; Sansom, M.; Leclercq, B.; Michel, J.; Pitel, F.; Alizadeh, M.; Vignal, A.; Douaire, M. Genetic linkage and expression analysis of SREBP and lipogenic genes in fat and lean chicken. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 137, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.; Wang, F.; Zhou, S.; Shangguan, M.; Xue, L.; Zhang, B.; Ding, F.; Hui, D.; Liang, A.; He, D. Treatment with PPARalpha agonist clofibrate inhibits the transcription and activation of SREBPs and reduces triglyceride and cholesterol levels in liver of broiler chickens. PPAR Res. 2015, 2015, 347245. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Pan, Z.; Kou, J.; Li, L.; Xia, L.; Hu, S.; Liu, H.; Wang, J. De novo lipogenesis in the liver and adipose tissues of ducks during early growth stages after hatching. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 163, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Ko, Y.H.; Chin, H.J.; Hsu, C.; Ding, S.T.; Chen, C.Y. The effect of feed restriction on expression of hepatic lipogenic genes in broiler chickens and the function of SREBP1. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 153, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Lopez-Bote, C.J.; Menoyo, D.; Bautista, J.M. Abdominal fat deposition and fatty acid synthesis are lower and beta-oxidation is higher in broiler chickens fed diets containing unsaturated rather than saturated fat. J. Nutr. 2000, 130, 3034–3037. [Google Scholar] [CrossRef]
- Duran-Montgé, P.; Theil, P.K.; Lauridsen, C.; Esteve-Garcia, E. Dietary fat source affects metabolism of fatty acids in pigs as evaluated by altered expression of lipogenic genes in liver and adipose tissues. Animal 2009, 3, 535–542. [Google Scholar] [CrossRef]
- Jing, M.; Gakhar, N.; Gibson, R.A.; House, J.D. Dietary and ontogenic regulation of fatty acid desaturase and elongase expression in broiler chickens. Prostaglandins Leukot Essent Fat. Acids 2013, 89, 107–113. [Google Scholar] [CrossRef]
- Campioli, E.; Rustichelli, C.; Avallone, R. n-3 Dietary supplementation and lipid metabolism: Differences between vegetable- and fish-derived oils. J. Funct. Foods 2012, 4, 207–212. [Google Scholar] [CrossRef]
- Ortiz, L.T.; Rebole, A.; Alzueta, C.; Rodriguez, M.L.; Trevino, J. Metabolisable energy value and digestibility of fat and fatty acids in linseed determined with growing broiler chickens. Br. Poult. Sci. 2001, 42, 57–63. [Google Scholar] [CrossRef]
- Newman, R.E.; Bryden, W.L.; Fleck, E.; Ashes, J.R.; Buttemer, W.A.; Storlien, L.H.; Downing, J.A. Dietary n-3 and n-6 fatty acids alter avian metabolism: metabolism and abdominal fat deposition. Br. J. Nutr. 2002, 88, 11–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Vallim, T.; Salter, A.M. Regulation of hepatic gene expression by saturated fatty acids. Prostaglandins Leukot Essent Fat. Acids 2010, 82, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n−3 and n−6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
| Ingredient (g/100 g) | Control | Flax |
|---|---|---|
| Corn grain | 49.5 | 43.77 |
| Soybean meal | 34.23 | 31.53 |
| Wheat middlings | 8.82 | 7.72 |
| Corn oil | 3.36 | - |
| Canola oil | - | 2.92 |
| Limestone | 1.98 | 1.98 |
| Lysine | 0.27 | 0.27 |
| DL-methionine | 0.33 | 0.33 |
| Salt | 0.38 | 0.38 |
| Dicalcium Phosphate | 0.61 | 0.6 |
| Broiler premix 1 | 0.5 | 0.61 |
| Wheat middlings | 8.3 | 9.1 |
| Flaxseed | - | 10 |
| Calculated analysis Metabolizable energy | 3186 | 3189 |
| Crude protein (%) | 21.7 | 22 |
| Fatty acids 2 (%) | ||
| 14:00 | 0 | 0.52 |
| 16:00 | 14.64 | 7.37 |
| 18:00 | 2.19 | 2.95 |
| 18:01 | 25.1 | 34.82 |
| 18:2n-6 | 54.21 | 27.62 |
| 18:3n-3 | 3.86 | 26 |
| 20:01 | 0 | 0.64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Head, B.; Bionaz, M.; Cherian, G. Flaxseed and Carbohydrase Enzyme Supplementation Alters Hepatic n-3 Polyunsaturated Fatty Acid Molecular Species and Expression of Genes Associated with Lipid Metabolism in Broiler Chickens. Vet. Sci. 2019, 6, 25. https://doi.org/10.3390/vetsci6010025
Head B, Bionaz M, Cherian G. Flaxseed and Carbohydrase Enzyme Supplementation Alters Hepatic n-3 Polyunsaturated Fatty Acid Molecular Species and Expression of Genes Associated with Lipid Metabolism in Broiler Chickens. Veterinary Sciences. 2019; 6(1):25. https://doi.org/10.3390/vetsci6010025
Chicago/Turabian StyleHead, Brian, Massimo Bionaz, and Gita Cherian. 2019. "Flaxseed and Carbohydrase Enzyme Supplementation Alters Hepatic n-3 Polyunsaturated Fatty Acid Molecular Species and Expression of Genes Associated with Lipid Metabolism in Broiler Chickens" Veterinary Sciences 6, no. 1: 25. https://doi.org/10.3390/vetsci6010025
APA StyleHead, B., Bionaz, M., & Cherian, G. (2019). Flaxseed and Carbohydrase Enzyme Supplementation Alters Hepatic n-3 Polyunsaturated Fatty Acid Molecular Species and Expression of Genes Associated with Lipid Metabolism in Broiler Chickens. Veterinary Sciences, 6(1), 25. https://doi.org/10.3390/vetsci6010025







