1. Introduction
Lymphoma is one of the most common hematopoietic neoplasm seen in dogs, accounting for 7–24% of all cancers diagnosed in canines [
1]. Multicentric lymphoma is the most common anatomic form accounting for over 80% of all diagnosed cases [
2]. A common manifestation of multicentric lymphoma is the development of non-painful generalized lymphadenopathy with peripheral lymph nodes often bilaterally and symmetrically enlarged [
3]. The treatment approach for canine lymphoma is determined by the stage of the disease. The current standard of care for dogs with lymphoma involves the use of multiagent chemotherapy, which achieves response rates of 65% to 96% and first-remission durations of 6 to 9 months [
4]. There are various multiagent chemotherapy protocols such as different variations of ‘CHOP’ (cyclophosphamide, doxorubicin, vincristine, prednisolone). Despite favorable treatment outcomes initially, the majority of dogs will experience relapse, necessitating the reinstitution of additional chemotherapies for the management of refractory disease. Second remission is harder to achieve and may be of shorter duration [
5]. Some relapsed dogs become refractory to the initial treatment and hence may require a different chemotherapeutic agent/rescue therapy to achieve second remission. For rescue therapy, again a multiagent protocol is followed; however, a single agent therapy may be preferred for clients with financial or logistical restrictions. Also cross-resistance between chemotherapeutic agents can decrease the efficacy of rescue protocols. Alkylating agents seldom show cross-resistance and are quite effective in achieving clinical remission [
6]. Alkylating agents include chemotherapeutic drug classes such as nitrosoureas, nitrogen mustards, and alkyl sulfonates, and they function by attaching an alkyl group to the DNA.
The nitrosourea drug, lomustine (CCNU, CeeNU, C
9H
16ClN
30
3, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea), is used clinically for treating a wide variety of human malignancies, most commonly brain tumors and lymphoma [
7,
8]. In veterinary medicine, lomustine has been successfully used primarily for the treatment of resistant lymphoma [
5,
9,
10], but also for the treatment of mast cell tumors [
11], intracranial meningioma [
12], epitheliotropic lymphoma [
13], and histiocytic sarcoma [
14] in dogs either alone or in combination with other chemotherapeutic agents. There are several studies in the literature indicating good efficacy of lomustine in combination with other chemotherapeutic agents such as L-asparaginase, doxorubicin,
etc. for the treatment of resistant canine lymphoma [
5,
9,
10]. However, its potential as a single agent for first line therapy or to treat relapsed canine lymphoma is less promising. Lomustine as a single agent may be preferred when treatment costs prohibit exploring other options or when a multidrug treatment is not tolerated by the patient due to extreme side effects.
In a clinical setting, lomustine is usually administered to dogs orally at 50–90 mg/m
2 every three weeks [
5,
10,
15]. Lomustine is a highly lipophilic nitrosourea compound, which undergoes hydrolysis
in vivo to form isomeric metabolites, primarily trans-4-hydroxylomustine (trans-4) and cis-4-hydroxylomustine (cis-4) (
Figure 1) in various animal species including humans [
16,
17,
18]. The comparative biological effects of lomustine and its metabolites were studied in mice. The single dose lethal dose (LD)
10 (dose causing death of 10% of the mice when administered intraperitoneally) and single dose effective dose (ED)
50 (dose producing 45 day survival in 50% of the mice after intraperitoneal implantation of 10
5 L1210 cells) were compared to determine the therapeutic index for each compound [
19]. The metabolites showed an approximately two-fold greater therapeutic index compared to the parent drug. It was therefore suggested that hydroxylation at the 4'-position of the cyclohexyl ring of lomustine, confers both isomers (cis-4 and trans-4) of the metabolite with enhanced alkylating activity and reduced toxic effects relative to lomustine, resulting in better therapeutic index of each of the metabolites relative to the parent compound [
19].
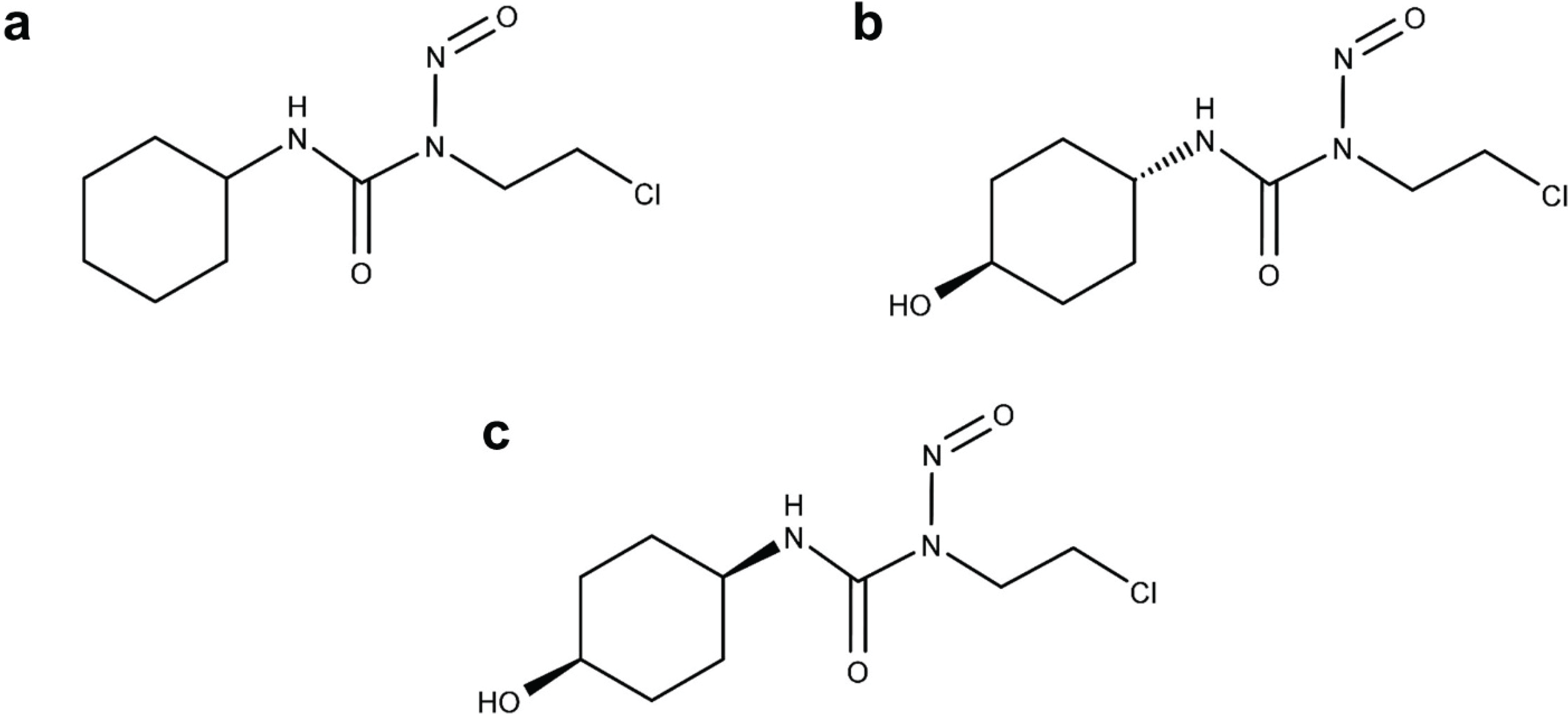
Figure 1.
Chemical structure of lomustine (a), trans-4-hydroxylomustine (b) and cis-4-hydroxylomustine (c).
Figure 1.
Chemical structure of lomustine (a), trans-4-hydroxylomustine (b) and cis-4-hydroxylomustine (c).
Hence it is plausible that the metabolites of lomustine could be better options for chemotherapy than the parent drug, with greater efficacy and a more favorable side effect profile. In addition to the above metabolites, minor metabolites are also formed due to hydroxylation at the second and third position of the cyclohexyl ring [
16,
19,
20]. The primary objectives of this study were: (1) to determine the preliminary metabolism characteristics of lomustine in dogs; (2) to evaluate the comparative cytotoxicity of lomustine and its trans-4 and cis-4 metabolites in canine lymphoma cell lines 17–71 and GL-1 and also in peripheral blood mononuclear cells (PBMC) from lymphoma dogs.
2. Materials and Methods
2.1. Animals and Sample Collections
The in vivo metabolism characteristics of lomustine were studied in two healthy research hounds. The dogs received 1.7 mg/kg (53.6 mg/m2) oral administration of commercially available lomustine capsules, and blood samples were collected at 15, 30, 45 min, 1, 2, 4, 6, 8 and 12 h after drug administration. Samples were placed in EDTA microcentrifuge tubes and kept on ice until centrifugation (10 min 5,000 g). Additionally, lomustine was also administered to a dog with lymphoma. The dog was presented at the veterinary teaching hospital and was newly diagnosed for lymphoma. No signs of any concurrent illness were noted in the dog. Lomustine and its metabolites were analyzed in the plasma samples immediately using the high performance liquid chromatography (HPLC) method described below. All study procedures were approved by the university’s Institutional Animal Care and Use Committee.
2.6. Statistical Analysis
Statistical analysis was done using SPSS software. Descriptive statistics were produced for all continuous variables. Mean and standard deviation were calculated. Normality of data was assessed using the Shapiro-Wilks test. Paired t-tests were performed to evaluate differences between technical repeats of each drug and dose combination. Multiple one way ANOVA and Tukey’s post hoc test were performed to evaluate the following within and between group differences: Between drugs within each dose in each cell type at each time point, between doses (concentrations) within each drug in each cell type at each time, between time points within dose in cell types for the each drug. The threshold for statistical significance was set at a p value of 0.05. For the canine lymphoma cell lines, two out of seventy two groups did not show normal distribution using Shapiro-Wilks test. However when these groups were further evaluated for normality using kurtosis, skewness, and Q-Q plots, the data showed normal distribution. Hence a parametric test was used to reduce the probability of type II error. Power analysis: Cytotoxicity data from two canine lymphoma cell lines were used to estimate sample size requirements for PBMC. Using the data from the 17–71 and GL-1 cell lines, based on a priori information of alpha = 0.05, power of 0.8 and an expected difference in mean of 3.77 (corresponding to the maximum standard deviation seen at any concentration), we would require 3 three samples. The lowest concentration of 300 ng/mL was excluded from the power analysis as no statistically significant cell killing was seen in either of the cell lines with any of the drugs at that concentration.
3. Results
Lomustine was not detected in plasma samples of the three dogs following oral administration of the drug.
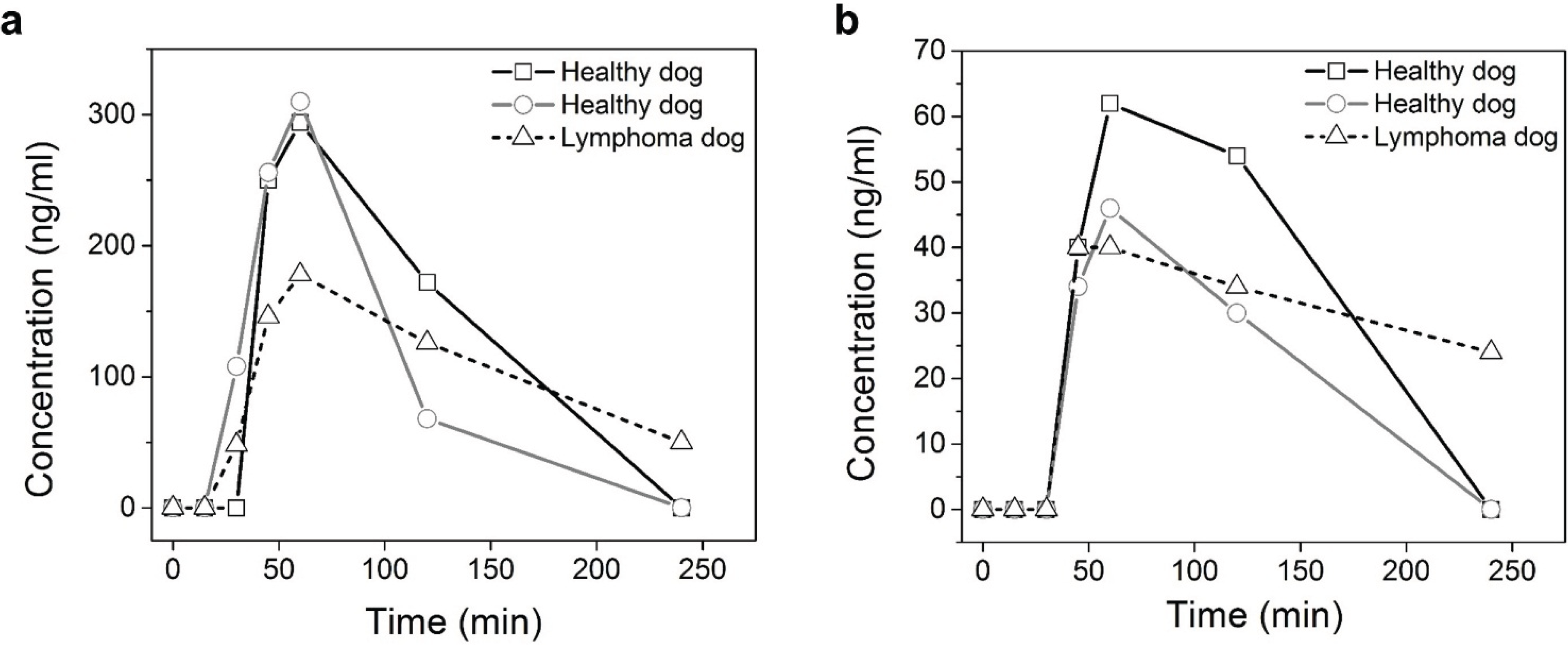
Figure 2a shows that trans-4 is the major metabolite of lomustine and the maximum concentration was about 325 ng/mL detected 1 h after drug administration for both the research hounds. The maximum concentration of cis-4 in the three dogs was around 40–65 ng/mL (
Figure 2b).
Figure 2.
Plasma concentration of trans-4-hydroxylomustine (a) and cis-4-hydroxylomustine (b) following single 1.7 mg/kg oral administration of lomustine in three dogs.
Figure 2.
Plasma concentration of trans-4-hydroxylomustine (a) and cis-4-hydroxylomustine (b) following single 1.7 mg/kg oral administration of lomustine in three dogs.
The results show that there is a minor partitioning of these drugs into/onto RBC (
Table 1). About 9–15% of the drug was found to be in the RBC compartment. For our metabolism studies, drug concentrations were measured in plasma and not whole blood. This small difference in the drug levels in plasma and whole blood could have led to a slight underestimation of the actual drug concentrations in blood.
Table 1.
In vitro concentrations of lomustine, trans-4-hydroxylomustine and cis-4-hydroxylomustine (ng/mL) in whole blood, plasma and red blood cells.
Table 1.
In vitro concentrations of lomustine, trans-4-hydroxylomustine and cis-4-hydroxylomustine (ng/mL) in whole blood, plasma and red blood cells.
| Drug | Plasma mean ± SD | Whole blood mean ± SD | RBC± SD | Ke/p |
|---|
| Lomustine | 287.18 ± 27.42 | 309.87 ± 14.03 | 37.49 ± 4.16 | 0.12 |
| Trans-4-hydroxylomustine | 256.42 ± 41.13 | 273.38 ± 16.11 | 27.71 ± 5.01 | 0.10 |
| Cis-4-hydroxylomustine | 228.98 ± 12.40 | 253.81 ± 15.90 | 40.75 ± 5.66 | 0.17 |
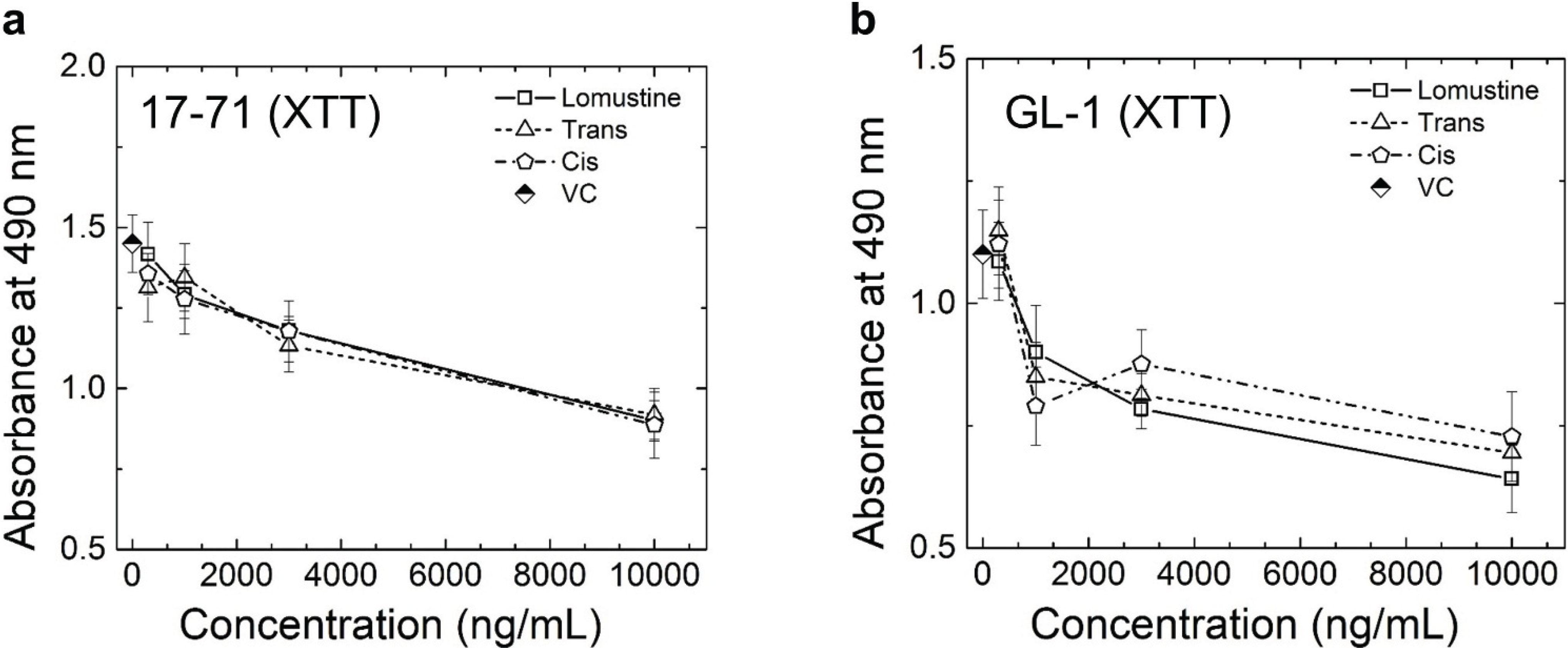
The results obtained from the XTT assay (
Figure 3) and annexin/PI assay (
Figure 4) in the 48 h cytotoxicity study show that there is a statistically significant cell killing at 3,000 and 10,000 ng/mL concentrations, when comparing to control for lomustine, trans-4 and cis-4 metabolites in canine lymphoma cell lines 17–71. In the canine lymphoma cell line GL-1, in addition to the above concentrations, 1,000 ng/mL also showed significant cytotoxicity compared to the VC. After treating the cells with the highest drug concentration, 10,000 ng/mL, about 50% and 40% of the cells were still viable at 48 h in 17–71 and GL-1 cells, respectively (
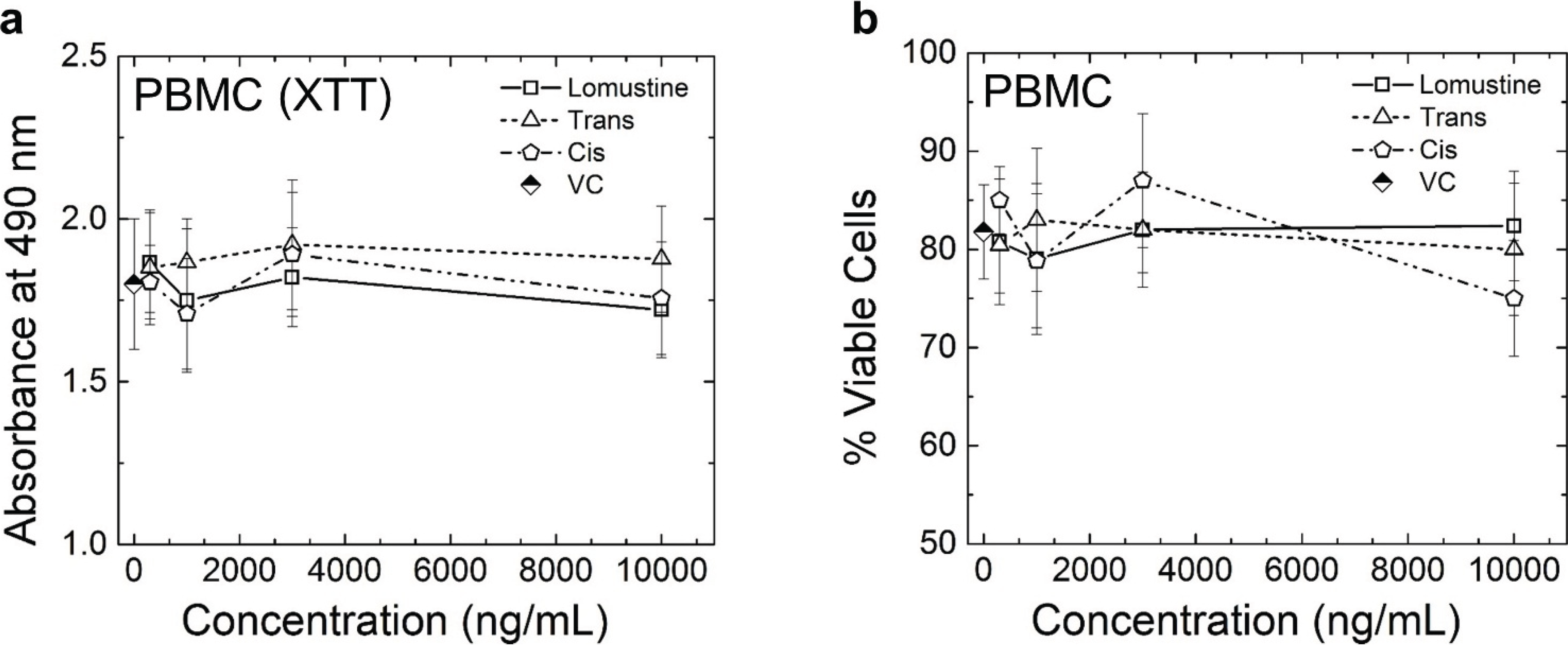
Figure 4). The results obtained from both XTT and Annexin/PI assay do not show any statistically significant difference in the cytotoxicity caused by lomustine, trans-4, and cis-4 in 17–71 and GL-1 cells at 48 h. Lomustine and its metabolites did not show any cell killing in the PBMC obtained from three lymphoma dogs at 48 h after exposure to lomustine, trans-4, and cis-4 (
Figure 5). Our results show that terminally differentiated lymphocytes in circulation may not be as sensitive to alkylating agents as lomustine at 48 h. It is possible that PBMC may require a longer incubation time for the cytotoxicity of lomustine to become apparent.
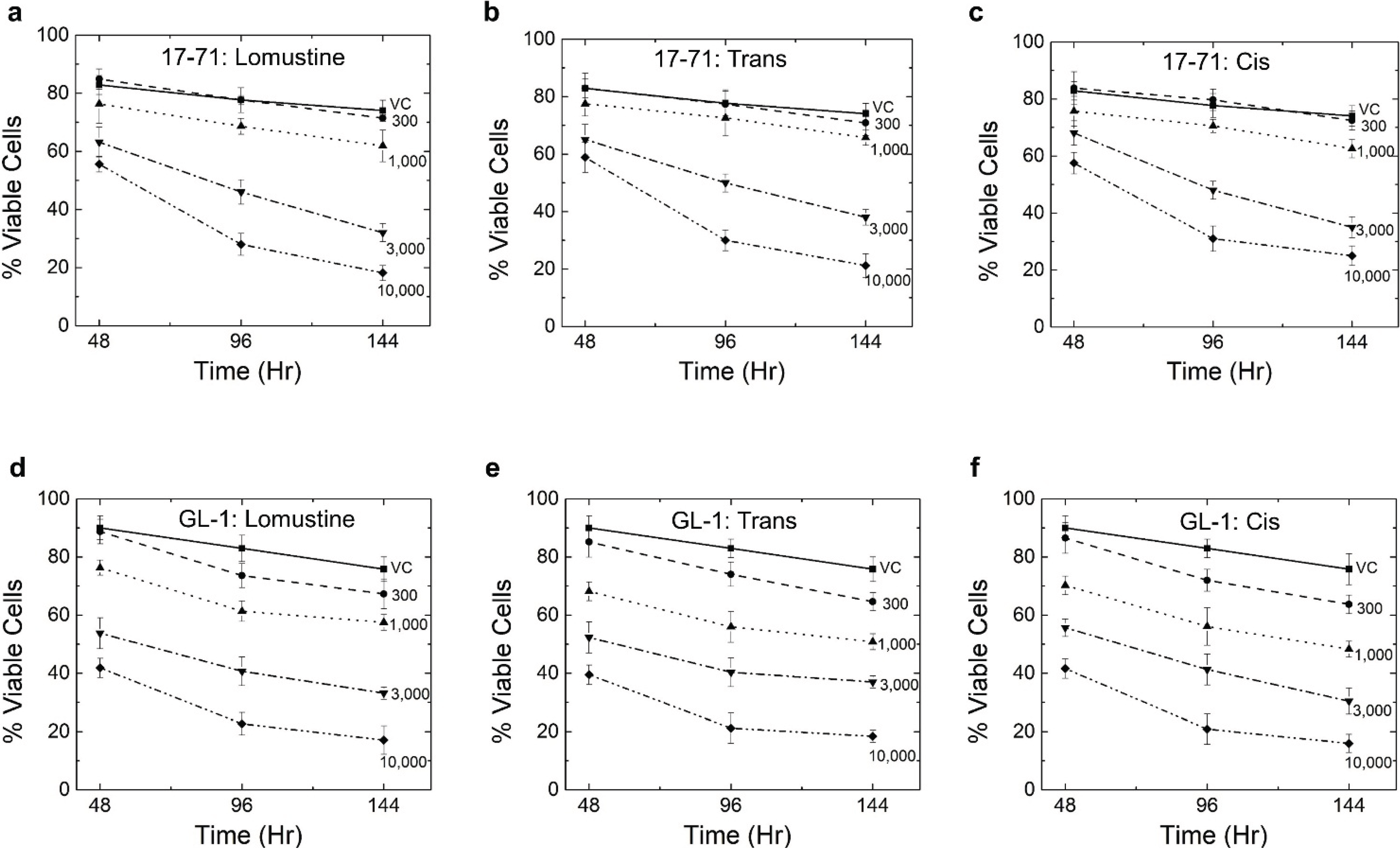
In the time-dependent cytotoxicity study, all three compounds showed a greater degree of cell killing at 96 and 144 h as compared to 48 h, thus confirming the premise that cytotoxicity with these drugs is delayed in nature (
Figure 6). However, no statistically significant difference was seen in the cytotoxicity among the three compounds. In the 17–71 cell, the 300 ng/mL samples did not show any cell killing or reduction in viability even at 144 h after drug treatment. The 3,000 and 10,000 ng/mL concentrations resulted in a comparable level of cell killing at 144 h. Also, using 10,000 ng/mL at 96 h data, more than 60% of the cells were non-viable. Due to this ‘saturation’ in cell killing, very little difference was seen in cytotoxicity at 144 h as compared to 96 h at 10,000 ng/mL. These results show that there is a delay in cell killing by lomustine and its metabolites, and hence measuring cell viability/apoptosis after 48 h of incubation may not be the best indicator of the cytotoxic potential of the compounds.
Figure 3.
Graphs showing the results of XTT assay in 17–71 (a) and GL-1 (b) cells at 48 h. Vehicle control (VC) was 0.1% methanol. The results show the mean and standard deviation of three independent experiments (n = 3).
Figure 3.
Graphs showing the results of XTT assay in 17–71 (a) and GL-1 (b) cells at 48 h. Vehicle control (VC) was 0.1% methanol. The results show the mean and standard deviation of three independent experiments (n = 3).
Figure 4.
Graphs showing the percent viable cells obtained from Annexin/PI assay in 17–71 (a) and GL-1 (b) cells at 48 h. Vehicle control (VC) was 0.1% methanol. The results show the mean and standard deviation of three independent experiments (n = 3).
Figure 4.
Graphs showing the percent viable cells obtained from Annexin/PI assay in 17–71 (a) and GL-1 (b) cells at 48 h. Vehicle control (VC) was 0.1% methanol. The results show the mean and standard deviation of three independent experiments (n = 3).
Figure 5.
Graphs showing the results of XTT assay (a) and Annexin/PI assay (b) in peripheral blood mononuclear cells (PBMC) isolated from three lymphoma dogs. Vehicle control (VC) was 0.1% methanol. The graph shows the mean and standard deviation of results obtained from three dogs. No statistically significant difference was seen in any of the drug treated samples compared to VC.
Figure 5.
Graphs showing the results of XTT assay (a) and Annexin/PI assay (b) in peripheral blood mononuclear cells (PBMC) isolated from three lymphoma dogs. Vehicle control (VC) was 0.1% methanol. The graph shows the mean and standard deviation of results obtained from three dogs. No statistically significant difference was seen in any of the drug treated samples compared to VC.
Figure 6.
Graphs showing the results of Annexin/PI assay in 17–71 cells (a, b and c) and GL-1 cells (d, e, and f) at 48, 96 and 144 h after treatment with lomustine, trans- and cis-4-hydroxylomustine. Vehicle control (VC) was 0.1% methanol. The results show the mean and standard deviation of three independent experiments (n = 3).
Figure 6.
Graphs showing the results of Annexin/PI assay in 17–71 cells (a, b and c) and GL-1 cells (d, e, and f) at 48, 96 and 144 h after treatment with lomustine, trans- and cis-4-hydroxylomustine. Vehicle control (VC) was 0.1% methanol. The results show the mean and standard deviation of three independent experiments (n = 3).
4. Discussion
Lomustine has been identified as a potentially important therapeutic agent for use in the treatment of various cancers in dogs; as such, we needed a sensitive and reliable analytical method to perform pharmacokinetic experiments with this agent in dogs. In particular, the method selected needed to be sufficiently sensitive to detect low concentrations of lomustine and its possible monohydroxylated metabolites in canine plasma from test animals.
The resultant HPLC method is a sensitive analytical method with the LOD of lomustine, cis-4 and trans-4 in plasma of about 10 ng/120 µL, 5 ng/120 µL, and 5 ng/120 µL, respectively, based on signal to noise ratio. Additionally, satisfactory recovery was obtained for lomustine and its monohydroxylated metabolites extracted from plasma samples. The developed extraction procedure is very simple (one step liquid/liquid extraction procedure) and suitable to analyze multiple samples in a short-time period. The CV for within-run and between-run samples were less than 15% for lomustine, trans-4, and cis-4, indicating good method precision [
21].
The metabolic profile of oral lomustine in dogs established in our study is similar to that reported in humans and rats with trans-4 being the major metabolite followed by cis-4 and the complete absence of parent lomustine. However, since the study was only done in three dogs, these results have to be considered as preliminary. In our study, the dogs received lomustine at the dose of 1.7 mg/kg, and the two research hounds showed similar peak levels of trans-4 (about 325 ng/mL), but the level for the same in the lymphoma dog was lower. The peak cis-4 lomustine level was between 40–65 ng/mL. In a published study of four human patients that received lomustine at the dose of 4.1 mg/kg, parent lomustine was not detected in the blood. The average peak concentration of trans-4 was 530 ng/mL and that for cis-4 was 320 ng/mL [
18]. In another study in human patients that used high dose lomustine (15 mg/kg), the peak plasma concentration detected ranged between 410–2,310 ng/mL for trans-4 and 180–1,820 ng/mL for cis-4 [
17], a five- and 10-fold difference between the maximum concentrations observed among patients, respectively. This supports the premise that there is a large inter-individual difference in the metabolic profile of lomustine.
As parent lomustine was not detected in the blood at any time after administration of the drug, the RBC partitioning behavior of lomustine and its metabolites was investigated. The results showed that there is a minor partitioning of these drugs into RBC. However, RBC partitioning values were not high enough to explain why lomustine was not detected in our canine plasma samples. It is likely that, like in other animal species, the absence of lomustine in dogs’ plasma is due to rapid “first pass” metabolism and not due to significant RBC partitioning.
As mentioned previously it has been reported that the major hydroxylated metabolites of lomustine may have enhanced alkylating activity and reduced toxic effects relative to parent lomustine [
19]. In this study, we wanted to investigate whether the major hydroxylated metabolites showed potential as a more effective chemotherapeutic option than lomustine using
in vitro studies. The metabolism studies discussed show that trans-4 and cis-4 are the major metabolites in dogs after oral treatment with lomustine, with trans-4 being in greater proportion.
The drug concentrations for the cytotoxicity study were derived from the metabolism studies in dogs where we found that the maximum plasma concentration of trans-4 was about 300 ng/mL and that for cis-4 was 50 ng/mL. Hence we used concentrations starting at 300 ng/mL up till 10,000 ng/mL as we did not know at what concentration the
in vitro biological effects of the drugs would be seen. We found no significant difference in the cytotoxicity caused by lomustine and its trans-4 and cis-4 metabolites in both the canine lymphoma cell lines 17–71 and GL-1. At 48 h after treatment with 10,000 ng/mL lomustine or its metabolites, 40–50% of the cells were still viable. However, after 96 h of incubation this number had dropped to about 20% indicating a time-dependent effect of lomustine cytotoxicity. In the PBMC from three lymphoma dogs, none of the drugs showed any significant cell killing compared to the control at 48 h after lomustine treatment. In two of the dogs, there were no circulating lymphoma cells detected. Hence the cytotoxicity results in PBMC of those two patients should represent the sensitivity of “normal” lymphocytes to the three agents. However, the third lymphoma patient had a lot of circulating lymphoma cells (37% of all of the nucleated cells). Hence, for this patient, it is highly probable that when we screened the sensitivity of “normal lymphocytes”, we were indeed also screening a good proportion of malignant lymphoma cells too. This would make interpretation of results from this patient more difficult and problematic. Interestingly, the
in vitro inhibitory concentration (IC)
50 for lomustine mentioned in literature varies from 50–250 μM (11,650–58,400 ng/mL) [
28,
29,
30]. These studies have utilized different cell lines and also used different techniques of detecting the cell killing. However, a time-dependent study of lomustine cytotoxicity has not been done before. Our findings suggest that apoptosis of cells after lomustine administration is not an immediate event but rather a delayed one. Also, the therapeutic concentration of lomustine measured in the three dogs (about 300 ng/mL) did not show any cytotoxic effect in the
in vitro study with the canine cell lines even at 144 h (6 days) of incubation. One reason for this disparity could be that since lomustine is metabolized rapidly to form pharmacologically active metabolites and also spontaneously breaks down into reactive intermediates which ultimately cause the alkylation of DNA, measuring the parent compound alone may not indicate the extent of the cytotoxic effect of the drug. The anticancer effect of lomustine may be brought about by the combined action of lomustine and its active metabolites whose absolute quantification in blood is precluded by their rapid breakdown in physiological body conditions.