2. Case Descriptions
From 2010 through 2012, a total of 12 cases of suspected leptospirosis were prospectively enrolled at the Ross University Veterinary Clinics, in accordance with an approved Institutional Animal Care and Use Committee protocol. From this group, four confirmed acute cases and one chronic case of leptospirosis were diagnosed. Another four animals in the group were diagnosed with
Ehrlichia canis infection, and the remaining cases were undiagnosed [
11]. The chronic subclinical case of leptospirosis was described previously [
10] and the four acute, fatal cases of leptospirosis are described below.
Ehrlichia- and
Babesia-specific PCR tests were performed to rule out other etiologies [
12]. None of the cases discussed here were found positive for either of these pathogens.
Results of complete blood count (CBC), serum biochemistry testing, and urinalysis are detailed in
Table 1. Full necropsies were performed in fatal cases. A complete set of tissue samples were collected from each case, fixed in 10% neutral-buffered formalin, routinely processed and stained with hematoxylin and eosin (HE). Select sections of the kidney and the liver were evaluated using Warthin-Starry stain.
Table 1.
Results of complete blood count (CBC), serum biochemistry testing, and urinalysis.
Table 1.
Results of complete blood count (CBC), serum biochemistry testing, and urinalysis.
| | Case 1 | Case 2 | Case 3 | Case 4 |
|---|
| CBC Results | | | | |
| TNCC (6–17 × 109/L) | 12.42 | 32.28 | 41.8 | 18.19 |
| Neutrophils, seg (3–11.4 × 109/L) | 9.3 | 29.1 | ND | 16.6 |
| Neutrophils, band (0–0.3 × 109/L) | 0.2 | 0 | ND | 0.2 |
| Lymphocytes (1–4.8 × 109/L) | 0.1 | 0.3 | ND | 0.9 |
| Monocytes (0.15–1.35 × 109/L) | 7.2 | 2.9 | ND | 0.5 |
| Eosinophils (0.1–0.75 × 109/L) | 0 | 0 | ND | 0 |
| Basophils (0 × 109/L) | 0 | 0 | ND | 0 |
| PCV (37%–55%) | 30 | 49 | 34 | 30 |
| RBC (5.5–8.5 × 1012/L) | 5.04 | 7.9 | 5.68 | 4.73 |
| Hemoglobin (12–18 g/dL) | 10.5 | 16.7 | 10.5 | 9.3 |
| Reticulocytes (0–60 × 106/L) | 0 | 0 | ND | 47.3 |
| MCV (60–77 fL) | 56 | 64 | 60 | 62 |
| MCH (19.5–24.5 pg) | 20.8 | 21.1 | 18.5 | 19.6 |
| MCHC (31.0–34.0 g/dL) | 37.6 | 33 | 31.1 | 31.5 |
| Platelets (200–500 × 109/L) | 19 | 156 | 61 | 3 |
| MPV (3.9–11.1 fL) | 7.9 | 7.8 | 9.9 | 8.2 |
| Serum Biochemistry | | | | |
| Refractometer protein (6–7.5 g/dL) | 6.5 | 8.8 | ND | 5.4 |
| Total Protein (5.4–8.2 g/dL) | 4.4 | ICT | ICT | 5.3 |
| Albumin (2.5–4.4 g/dL) | 2.8 | ICT | ICT | 2.1 |
| Globulin (2.3–5.2 g/dL) | 1.6 | ICT | ICT | 3.2 |
| Alkaline phosphatase (20–150 U/L) | 1697 | 545 | 448 | 596 |
| ALT (10–118 U/L) | 147 | ICT | 62 | 217 |
| Amylase (200–1200 U/L) | 2536 | ND | 2387 | 322 |
| Glucose (60–110 mg/dL) | 87 | 84 | 112 | 100 |
| Total Bilirubin (0.1–0.6 mg/dL) | 11.2 | 21.8 | 18 | 4.7 |
| BUN (25–180 mg/dL) | >180 | >180 | 125 | 121 |
| Creatinine (0.3–1.4 mg/dL) | ICT | ICT | ICT | 2.6 |
| Calcium (8.6–11.8 mg/dL) | 9.7 | 14.7 | >16 | 13.4 |
| Phosphorus (2.9–6.6 mg/dL) | 17.5 | >20.0 | 16.1 | 16.8 |
| Sodium (138–160 mmol/L) | 132 | 149 | 146 | 142 |
| Potassium (3.7–5.8 mmol/L) | 8.1 | ICT | 4.7 | 4.1 |
| Na: K Ratio | 16.3 | ICT | 31.1 | 34.6 |
| Urinalysis | | | | |
| Spec. Gravity | 1.011 | 1.027 | ND | 1.011 |
| pH | 6 | 5 | ND | 7 |
| Protein (mg/dL) | 15–30 | 100–300 | ND | 15–30 |
| Glucose (mg/dL) | Negative | 100–250 | ND | 100–250 |
| Ketones (mg/dL) | ≥160 | Negative | ND | Negative |
| Bilirubin (mg/dL) | Negative | ≥6.0 | ND | Negative |
| Hemoglobin/Blood (Ery/uL) | 50–250 | 80–250, hm | ND | ≥250 |
DNA was isolated from whole blood (200 μL), and tissues (10–20 mg) using a DNeasy Blood and Tissue Mini-Kit (Qiagen, Valencia, CA, USA), following the manufacturer’s directions. Urine samples (1–1.5 mL) were centrifuged for 5 min at 10,000 g
, and pellets were processed using the DNeasy Blood and Tissue Mini-Kit (Qiagen). A
Leptospira- specific fluorescence resonance energy transfer-polymerase chain reaction (FRET-PCR) was performed on a LightCycler 2.0 real-time PCR platform using thermal protocol and PCR conditions as described elsewhere [
10].
Leptospira serovars Canicola, Copenhageni (Icterohaemorrhagiae), Grippotyphosa, Hebdomadis, Autmnalis, Australis, Ballum, and Pomona (NVSL, Ames, IA, USA) were maintained at 30 °C in Polysorbate −80 bovine serum albumin medium (NVSL), and used to perform the microscopic agglutination test (MAT), as described previously [
13].
2.1. Case 1
A four-month old, intact male mixed-breed puppy (1.6 kg) presented for acute onset of vomiting, diarrhea, anorexia, and lethargy. The owner reported two prior vaccinations with a DHPPC combination, but not Leptospira. The patient was hypothermic (T = 96.6 °F), tachycardic (200/min), and hypotensive (systolic blood pressure of 85 mm Hg). CBC with differential revealed lymphopenia with monocytosis, mild microcytic anemia, and severe thrombocytopenia with no visible platelet clumping or giant platelets. Serum biochemistry revealed several abnormalities, most notably elevated liver enzymes, bilirubin, BUN, and phosphorus, and low sodium with high potassium. Urine was dilute with mild hematuria and trace amounts of protein; waxy casts and clumps of transitional epithelial cells were seen in the sediment. The patient was euthanized and submitted for necropsy.
Gross necropsy findings included poor body condition (1.5/5), generalized icterus, and petechial to ecchymotic hemorrhages in the lungs and kidneys. The liver appeared pale and had an enhanced lobular pattern. The main microscopic lesions were confined to the kidneys and the liver (
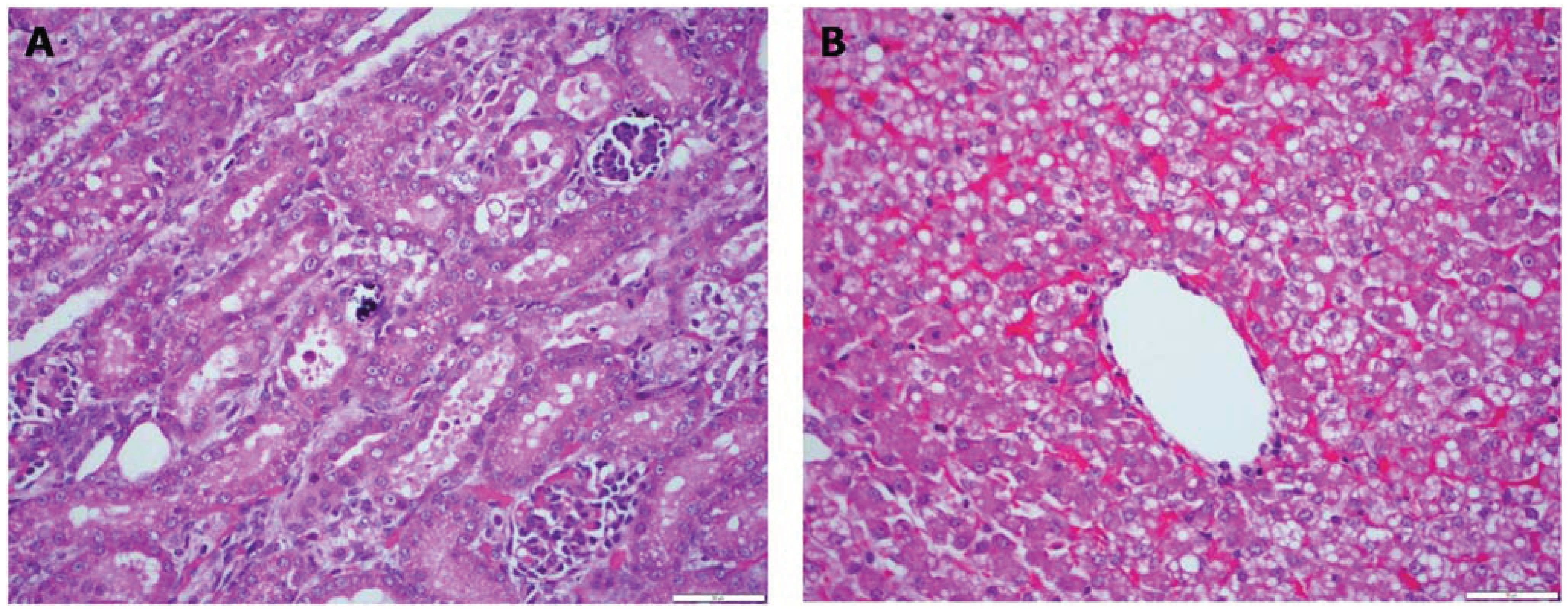
Figure 1). In the kidneys, there was acute tubular degeneration and necrosis, with multifocal mineralization of the tubular basement membrane. In the liver, there was marked dissociation of the hepatocellular cords, multifocal hepatocellular necrosis, central congestion and prominent vacuolation of hepatocytes. Bile stasis was prominent in major biliary ducts.
Figure 1.
Case 1. Multifocal areas of acute renal tubular degeneration and necrosis, with multifocal mineralization of the tubular basement membrane (A). Marked dissociation of the hepatocellular cords, multifocal hepatocellular necrosis, central congestion and prominent vacuolation of hepatocytes (B). H&E stain 40×.
Figure 1.
Case 1. Multifocal areas of acute renal tubular degeneration and necrosis, with multifocal mineralization of the tubular basement membrane (A). Marked dissociation of the hepatocellular cords, multifocal hepatocellular necrosis, central congestion and prominent vacuolation of hepatocytes (B). H&E stain 40×.
Testing for Leptospira using FRET-PCR was performed on blood, liver, and kidney samples. Liver and kidney samples were positive for Leptospira DNA, and blood was weakly positive, with detectable DNA on only one of three replicates. No samples were available for serologic testing.
2.2. Case 2
A seven-month-old, intact female mixed-breed dog (9.0 kg) presented for weakness and vomiting. The patient was afebrile (T = 99.7 °F), tachycardic (180/min), hypotensive (blood pressure 90 mm Hg), and icteric on presentation. Neutrophilia, lymphopenia, and monocytosis were noted, along with mild thrombocytopenia and occasional giant platelets. Serum biochemistry was complicated by severe icterus, but liver enzymes, bilirubin, BUN, calcium, and phosphorus were elevated. Urinalysis revealed bilirubinuria and hematuria with granular casts, leukocytes, and bacteria (cocci) seen in sediment. There was no improvement following treatment with intravenous fluids, ampicillin, and famotidine, and the patient was euthanized eight hours after admission.
Gross necropsy findings included only adequate body condition (2.5/5), generalized icterus, petechial to ecchymotic hemorrhages in the subcutaneous tissue, lungs, urinary bladder and kidneys. The liver appeared diffusely congested and there were multifocal, segmental areas of hemorrhage in the gastric and intestinal mucosae. The main microscopic lesions were confined to the kidneys and the liver. In the kidneys, there was acute tubular degeneration and necrosis, with multifocal mineralization of the tubular basement membrane. Multifocal, discrete aggregates of lymphocytes and plasma cells in the renal interstitium were also observed. In the liver, there was marked dissociation of the hepatocellular cords, and multifocal hepatocellular necrosis.
Testing for Leptospira DNA using FRET-PCR was performed on blood and urine, and leptospiral DNA was detected in the urine. Serology, using the MAT revealed titers of 1:400 and 1:800 for serovars Icterohaemorrhagiae and Grippotyphosa, respectively.
2.3. Case 3
A four-month old female Bull Mastiff-Rottweiler cross puppy (9.1 kg) was presented for lethargy and anorexia of a few days duration. There was no history of prior vaccination for Leptospira; a combination DAPPC vaccine had been given two months prior. The dog was sternally recumbent, afebrile (T = 101.3 °F), severely dehydrated, and tachycardic (250/min). Icterus of the sclera, pinnae, and mucous membranes was noted. The liver was palpably enlarged. CBC was performed but no differential count was included; leukocytosis and thrombocytopenia were detected. Icterus interfered with serum biochemistry testing, but alkaline phosphatase, amylase, bilirubin, BUN, calcium, and phosphorus were all elevated. The patient was euthanized, and necropsy was performed.
Gross necropsy findings included only adequate body condition (2.5/5), generalized icterus, petechial to ecchymotic hemorrhages in the subcutaneous tissue, lungs, and kidneys. The kidneys were slightly enlarged and edematous. The liver was diffusely pale and had an enhanced lobular pattern. There were multifocal areas of hemorrhage in the intestinal mucosa. The main microscopic lesions were confined to the kidneys and the liver. Due to postmortem autolysis, optimal examination of the tissues was prevented. In the kidneys, there was acute tubular degeneration and necrosis, with multifocal mineralization of the tubular basement membrane. There were also multifocal, discrete aggregates of round cells (presumed to be lymphocytes and plasma cells) in the renal interstitium. In the liver, there was marked dissociation of the hepatocellular cords, and multifocal hepatocellular necrosis.
Blood, urine, liver, and kidney samples were tested for Leptospira using FRET-PCR, and the urine and liver tested positive for Leptospira DNA. No samples were available for serologic testing.
2.4. Case 4
A 7-week-old old male, mixed breed puppy (2.0 kg) presented for acute onset of vomiting, diarrhea, and anorexia. The puppy was afebrile (T = 99.9 °F) and tachycardic (160/min), with generalized lymphadenopathy. Mild neutrophilia and lymphocytopenia, mild anemia, and severe thrombocytopenia were noted on CBC with differential. Liver enzymes, bilirubin, BUN, creatinine, calcium, and phosophorus were all elevated. Abdominal ultrasound revealed hyperechoic kidneys and a hypoechoic, enlarged liver. Upon treatment with intravenous fluids, the patient was noted to be anuric; attempted diuresis with furosemide resulted in only 1 mL of urine; urine was dilute, with significant hematuria, and clumps of transitional epithelial cells were noted in urine sediment. The patient became progressively more icteric, remained anuric, and was euthanized.
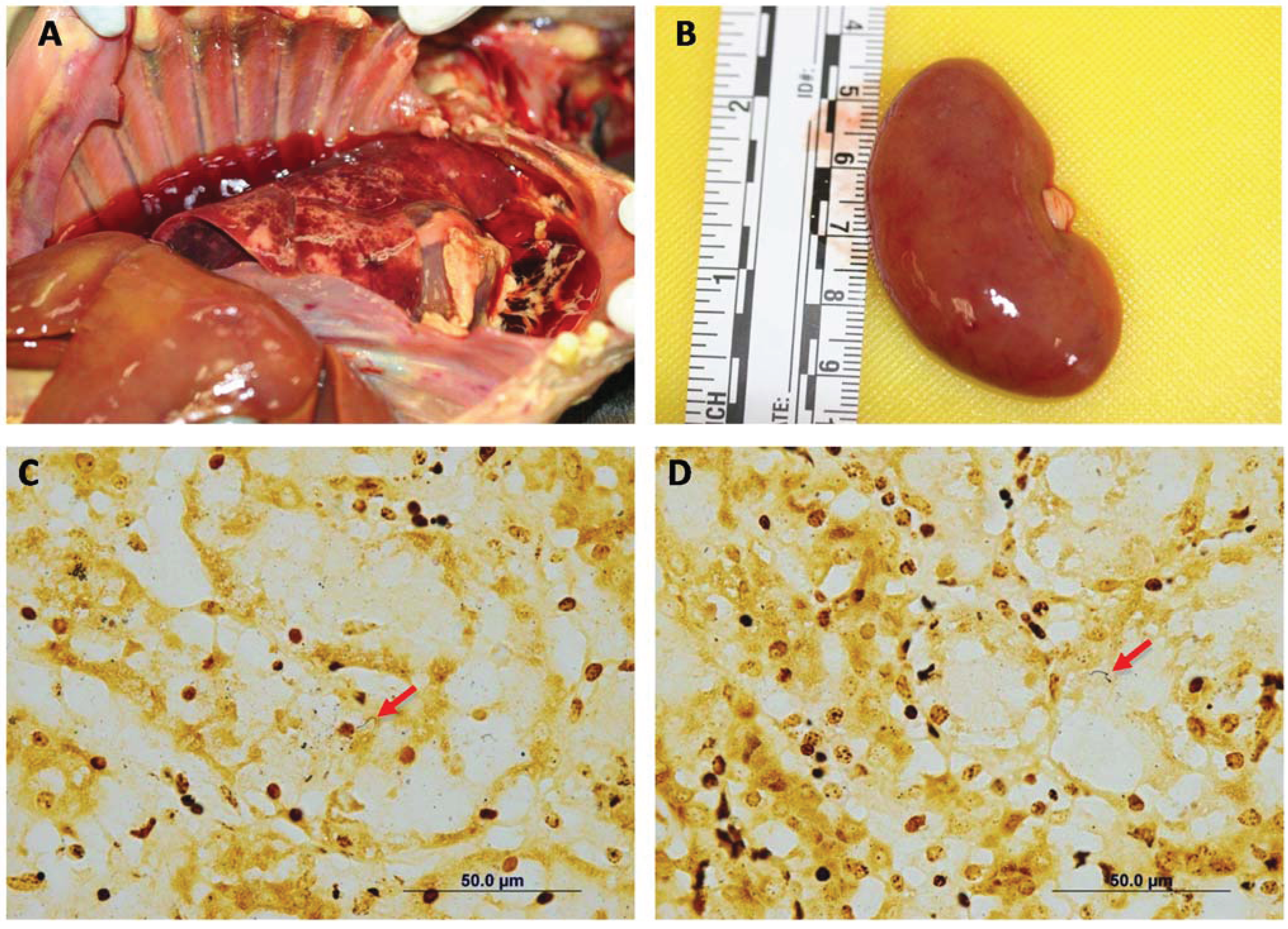
Gross necropsy findings included only adequate body condition (2.5/5), generalized icterus and edema (anasarca). There were multifocal petechial to ecchymotic hemorrhages in the lungs, kidneys (
Figure 2A,B), urinary bladder, and gastric and intestinal mucosae. The liver appeared pale and had an enhanced lobular pattern. Multifocal small (1–4 mm) areas of pallor were present in the myocardium. The main microscopic lesions were confined to the kidneys, the liver and the heart. In the kidneys, there was acute tubular degeneration and necrosis, with multifocal mineralization of the tubular basement membrane. In the liver, there was marked dissociation of the hepatocellular cords, multifocal hepatocellular degeneration and necrosis. The heart had multifocal to coalescing areas of myocardial degeneration and necrosis, myocarditis and myocardial hemorrhages (
Figure 3). Warthin-Starry stain demonstrated spiral organisms in the kidneys and the liver (
Figure 2C,D).
Blood, liver, and kidney samples were tested using FRET-PCR, and all three samples were positive for leptospiral DNA. No samples were available for serological testing.
Figure 2.
Case 4. Common gross necropsy findings included generalized icterus, petechial to ecchymotic hemorrhages in the lungs (A) and kidneys (B). In most cases, the liver appeared pale and had an enhanced lobular pattern (A). Silver stain showing spiral organisms in the renal interstitium (C) and the liver parenchyma (D). Warthin Starry Stain 100×.
Figure 2.
Case 4. Common gross necropsy findings included generalized icterus, petechial to ecchymotic hemorrhages in the lungs (A) and kidneys (B). In most cases, the liver appeared pale and had an enhanced lobular pattern (A). Silver stain showing spiral organisms in the renal interstitium (C) and the liver parenchyma (D). Warthin Starry Stain 100×.
Figure 3.
Case 4. Multifocal to coalescing areas of coagulative necrosis within myocardium characterized by the presence of hypereosinophilic and shrunken cardiomyocytes. In addition, there are multifocal hemorrhages that replace and separate the adjacent cardiomyocytes. Also present are aggregates of mixed inflammatory cells, predominantly mononuclear cells, less commonly neutrophils, and occasionally lymphocytes or plasma cells in the interstitium. (A). 20×; (B). 60×.
Figure 3.
Case 4. Multifocal to coalescing areas of coagulative necrosis within myocardium characterized by the presence of hypereosinophilic and shrunken cardiomyocytes. In addition, there are multifocal hemorrhages that replace and separate the adjacent cardiomyocytes. Also present are aggregates of mixed inflammatory cells, predominantly mononuclear cells, less commonly neutrophils, and occasionally lymphocytes or plasma cells in the interstitium. (A). 20×; (B). 60×.
3. Discussion
Initial signs of the leptospiral infection in dogs are protean and include some combination of anorexia, lethargy, depression, fever, and muscle tenderness. The degree of hepatic involvement can vary, as can pulmonary hemorrhage [
14,
15]. In all four cases described here, hepatic, renal and pulmonary lesions were noted. Generalized icterus, thrombocytopenia, elevated alkaline phosphatase, elevated bilirubin, and hyperphosphatemia were seen in all cases. An inflammatory leukogram, elevated BUN or creatinine, and pancreatic damage were observed in all four patients. In addition to pulmonary hemorrhage, petechial and ecchymotic hemorrhages were commonly present in the kidneys, subcutaneous tissue, gastrointestinal tract and bladder. Commonly, the liver was pale and had an enhanced lobular pattern.
Microscopic lesions were similar in all dogs but varied in extent. Every case exhibited multifocal mineralization of the basement membrane and renal tubular degeneration and necrosis, characterized by swelling and vacuolation of tubular epithelial cells, cytoplasmic hypereosinophilia, and karyorrhexis. Sloughed epithelial cells and cellular debris were present in the lumen of the tubules. Two cases had multifocal, mild lymphocytic and plasmacytic aggregates in the renal interstitium. Lesions present in the liver were characterized by marked dissociation of the hepatocellular cords. Other lesions included hepatocellular necrosis, vacuolation of the cytoplasm of hepatocytes, sinusoidal dilation and congestion and parenchymal hemorrhages.
Interestingly, extensive myocardial damage and myocarditis were observed in one of the cases (case 4). Although abnormal ECG readings in canine leptospirosis and myocarditis in some rare cases of human leptospirosis have previously been documented [
14,
16], this report describes the first histopathologically confirmed case of canine leptospirosis with myocardial involvement. Other etiologies associated with myocarditis were not investigated in this study.
Taken individually, most of these findings are common in canine leptospirosis [
14,
15], but the severity and frequency of hemorrhage, thrombocytopenia, and hyperphosphatemia are unusual, possibly reflecting the acute and severe nature of the infections in this case series. The use of qPCR on blood, urine, and tissue samples allowed the accurate diagnosis of
Leptospira and revealed that tissue samples, followed by urine, are the most appropriate sample for PCR-based diagnosis of acutely fatal cases. Further work is needed to identify the circulating leptospiral strains on St. Kitts, and to determine if the severity of these cases is due to the infecting strain of
Leptospira or to host-associated factors. Young age of the animals in this case series reflects that of the patient base on the island. In a previous study we found that the average age of “healthy” control-group dogs presented to the Ross University Veterinary Clinics was 1.93 ± 2.17 years [
12].
The public health implications of canine leptospirosis depend on various factors, such as geographical location, climate, rainfall, and socioeconomic factors. In a recent study, we showed that one-fifth of tested water sources on St. Kitts were contaminated with leptospiral DNA [
17], and 9.1% of serum samples from pregnant women on St. Kitts and Nevis contained antibodies against
Leptospira [
4]. This work documents that
Leptospira also causes severe, acutely fatal, canine infections on St. Kitts. The presence of stray dogs, feral cats, and several other potential reservoir species, including rats, mongooses, and monkeys, presents a combination of factors that can support enzootic circulation and transmission of the pathogen. This case series is important, as it provides the first evidence of myocarditis in canine leptospirosis, and the presence of fatal cases of canine leptospirosis on this small Caribbean island.