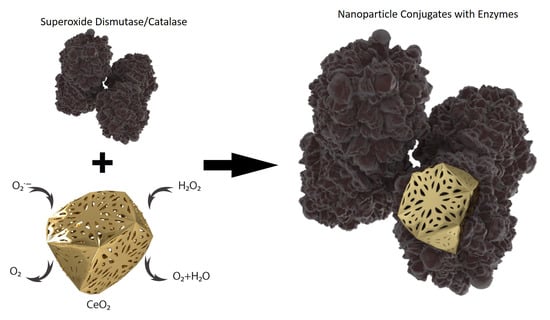
Antioxidant Activity of SOD and Catalase Conjugated with Nanocrystalline Ceria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanocrystalline Ceria
2.3. Physicochemical Characterization of Ceria Nanoparticles
2.4. Preparation of Ceria-Enzyme Conjugates
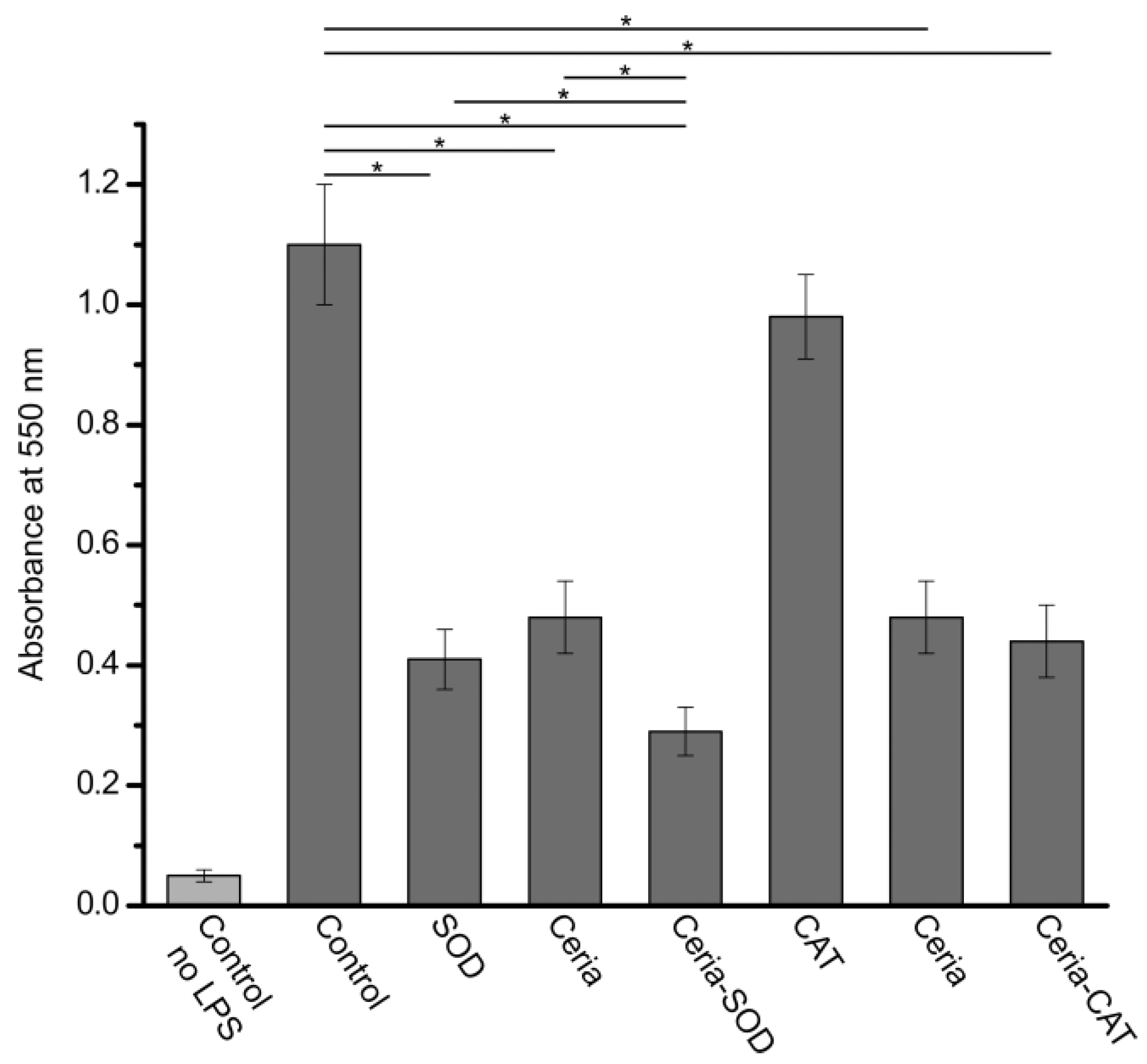
2.5. Assessment of Antioxidant Activity
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Howard, M.D.; Greineder, C.F.; Hood, E.D.; Muzykantov, V.R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control. Release 2014, 177, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Nanni, S.; Figueira, I.; Ivanov, I.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Pinto, P.; Silva, R.F.M.; Brites, D.; et al. Bioaccessible (poly)phenol metabolites from raspberry protect neural cells from oxidative stress and attenuate microglia activation. Food Chem. 2017, 215, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, V.V.; Muro, S.; Arguiri, E.; Khoshnejad, M.; Tliba, S.; Christofidou-Solomidou, M.; Muzykantov, V.R. Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates. J. Control. Release 2016, 234, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, reactive oxygen species and human disease: A critical evaluation with special reference to atherosclerosis. Br. J. Exp. Pathol. 1989, 70, 737–757. [Google Scholar] [PubMed]
- Naik, E.; Dixit, V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Umpierrez, G.E.; Cuervo, R.; Kitabchi, A.E. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004, 53, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Maximov, V.D.; Yu, J.; Zhu, H.; Vertegel, A.A.; Kindy, M.S. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J. Cereb. Blood Flow Metab. 2013, 33, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Vertegel, A.; Reukov, V.; Maximov, V. Enzyme–Nanoparticle Conjugates for Biomedical Applications. In Enzyme Stabilization and Immobilization; Minteer, S.D., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 679, pp. 165–182. [Google Scholar]
- Jolly, S.R.; Kane, W.J.; Bailie, M.B.; Abrams, G.D.; Lucchesi, B.R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ. Res. 1984, 54, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Temsah, R.M.; Netticadan, T.; Chapman, D.; Takeda, S.; Mochizuki, S.; Dhalla, N.S. Alterations in sarcoplasmic reticulum function and gene expression in ischemic-reperfused rat heart. Am. J. Physiol. 1999, 277, H584–H594. [Google Scholar] [PubMed]
- Wheler, K.; Smutney, F.; Dikalova, M.; Samulski, T. Antioxidant Gene Therapy and Hepatic Ischemia-Reperfusion Injury. Hepatology 2010, 5, 705–711. [Google Scholar]
- Fan, C.G.; Zwacka, R.M.; Engelhardt, J.F. Therapeutic approaches for ischemia/reperfusion injury in the liver. J. Mol. Med. 1999, 77, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.K.; Miao, L.; St. Clair, D.K.; St. Clair, W.H. Redox-modulated phenomena and radiation therapy: The central role of superoxide dismutases. Antioxid. Redox Signal. 2014, 20, 1567–1589. [Google Scholar] [CrossRef] [PubMed]
- Dziubla, T.D.; Shuvaev, V.V.; Hong, N.K.; Hawkins, B.J.; Madesh, M.; Takano, H.; Simone, E.; Nakada, M.T.; Fisher, A.; Albelda, S.M.; et al. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials 2008, 29, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.; Simone, E.; Wattamwar, P.; Dziubla, T.; Muzykantov, V. Nanocarriers for vascular delivery of antioxidants. Nanomedicine 2011, 6, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.D.; Hood, E.D.; Greineder, C.F.; Alferiev, I.S.; Chorny, M.; Muzykantov, V. Targeting to Endothelial Cells Augments the Protective Effect of Novel Dual Bioactive Antioxidant/Anti-Inflammatory Nanoparticles. Mol. Pharm. 2015, 11, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Hood, E.D.; Chorny, M.; Greineder, C.F.; Alferiev, S.I.; Levy, R.J.; Muzykantov, V.R. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials 2014, 35, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; Bhathena, P.R.; Dweik, R.A.; Kavuru, M.; Erzurum, S.C. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 2000, 355, 624. [Google Scholar] [CrossRef]
- Comhair, S.A.A.; Ricci, K.S.; Arroliga, M.; Lara, A.R.; Dweik, R.A.; Song, W.; Hazen, S.L.; Bleecker, E.R.; Busse, W.W.; Chung, K.F.; et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Casano, L.M.; Gómez, L.D.; Lascano, H.R.; González, C.A.; Trippi, V.S. Inactivation and degradation of CuZn-SOD by active oxygen species in wheat chloroplasts exposed to photooxidative stress. Plant Cell Physiol. 1997, 38, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Fridovich, I. Superoxide Radical Inhibits Catalase. J. Biol. Chem. 1982, 257, 5751–5754. [Google Scholar] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Shimizu, N.; Kobayashis, K.; Hayashi, K. The Reaction of Superoxide Radical with Catalase. J. Biol. Chem. 1984, 259, 4414–4418. [Google Scholar] [PubMed]
- Mao, G.D.; Thomas, P.D.; Lopaschuks, G.D.; Poznanskyq, M.J.; Tg, C. Superoxide Dismutase (SOD)-Catalase Conjugates. J. Biol. Chem. 1993, 268, 416–420. [Google Scholar] [PubMed]
- Bunn, H.F. The use of hemoglobin as a blood substitute. Am. J. Hematol. 1993, 42, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Baranchikov, A.E.; Polezhaeva, O.S.; Ivanov, V.K.; Tretyakov, Y.D. Lattice expansion and oxygen non-stoichiometry of nanocrystalline ceria. CrystEngComm 2010, 12, 3531–3533. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Mullins, D.R. The surface chemistry of cerium oxide. Surf. Sci. Rep. 2015, 70, 42–85. [Google Scholar] [CrossRef]
- Grulke, E.; Reed, K.; Beck, M.; Huang, X.; Cormack, A.; Seal, S. Nanoceria: factors affecting its pro- and anti-oxidant properties. Environ. Sci. Nano 2014, 1, 429–444. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E. S.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- Reukov, V.; Maximov, V.; Vertegel, A. Proteins conjugated to poly(butyl cyanoacrylate) nanoparticles as potential neuroprotective agents. Biotechnol. Bioeng. 2011, 108, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, O.S.; Shekunova, T.O.; Ivanov, V.K.; Shcherbakov, A.B.; Popov, A.L.; Davydova, G.A.; Selezneva, I.I.; Kopitsa, G.P.; Tret’yakov, Y.D. One-stage synthesis of ceria colloid solutions for biomedical use. Dokl. Chem. 2011, 437, 103–106. [Google Scholar] [CrossRef]
- Vertegel, A.A.; Siegel, R.W.; Dordick, J.S. Silica nanoparticle size influences the structure and enzymatic activity of adsorbed lysozyme. Langmuir 2004, 20, 6800–6807. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Pathania, V.; Pathak, C.M. Inhibitory effect of vitamin E on proinflammatory cytokines-and endotoxin-induced nitric oxide release in alveolar macrophages. Life Sci. 2005, 76, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Johnson, M.; Walker, M.; Riley, K.; Sims, C. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, A.B.; Zholobak, N.M.; Ivanov, V.K.; Ivanova, O.S.; Marchevsky, A.V.; Baranchikov, A.E.; Spivak, N.Y.; Tretyakov, Y.D. Synthesis and antioxidant activity of biocompatible maltodextrin-stabilized aqueous sols of nanocrystalline ceria. Russ. J. Inorg. Chem. 2012, 57, 1411–1418. [Google Scholar] [CrossRef]
- Zholobak, N.M.; Ivanov, V.K.; Shcherbakov, A.B.; Shaporev, A.S.; Polezhaeva, O.S.; Baranchikov, A.Y.; Spivak, N.Y.; Tretyakov, Y.D. UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J. Photochem. Photobiol. B Biol. 2011, 102, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yotnda, P. Production and Detection of Reactive Oxygen Species (ROS) in Cancers. J. Vis. Exp. 2011, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Mertas, A.; Czuba, Z.P.; Król, W. Inhibition of Inflammatory Response by Artepillin C in Activated RAW264. 7 Macrophages. Evid.-Based Complement. Altern. Med. 2013, 2013, 735176. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Jung, M.; Teoh, W.Y.; Gunawan, C.; Vassie, J.A.; Amal, R.; Whitelock, J.M. Cellular uptake and reactive oxygen species modulation of cerium oxide nanoparticles in human monocyte cell line U937. Biomaterials 2012, 33, 7915–7924. [Google Scholar] [CrossRef] [PubMed]
- Heckman, K.L.; Decoteau, W.; Estevez, A.; Reed, K.J.; Costanzo, W.; Sanford, D.; Leiter, J.C.; Clauss, J.; Knapp, K.; Gomez, C.; et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 2013, 7, 10582–10596. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, K.; Babu, N.; Singh, A.K.; Das, S.; Kumar, A.; Seal, S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 439–448. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, D.; Rodriguez, J.; Ward, B.; Vertegel, A.; Ivanov, V.; Reukov, V. Antioxidant Activity of SOD and Catalase Conjugated with Nanocrystalline Ceria. Bioengineering 2017, 4, 18. https://doi.org/10.3390/bioengineering4010018
Gil D, Rodriguez J, Ward B, Vertegel A, Ivanov V, Reukov V. Antioxidant Activity of SOD and Catalase Conjugated with Nanocrystalline Ceria. Bioengineering. 2017; 4(1):18. https://doi.org/10.3390/bioengineering4010018
Chicago/Turabian StyleGil, Dmitry, Jeannette Rodriguez, Brendan Ward, Alexey Vertegel, Vladimir Ivanov, and Vladimir Reukov. 2017. "Antioxidant Activity of SOD and Catalase Conjugated with Nanocrystalline Ceria" Bioengineering 4, no. 1: 18. https://doi.org/10.3390/bioengineering4010018
APA StyleGil, D., Rodriguez, J., Ward, B., Vertegel, A., Ivanov, V., & Reukov, V. (2017). Antioxidant Activity of SOD and Catalase Conjugated with Nanocrystalline Ceria. Bioengineering, 4(1), 18. https://doi.org/10.3390/bioengineering4010018