Preconcentration of Pb with Aminosilanized Fe3O4 Nanopowders in Environmental Water Followed by Electrothermal Atomic Absorption Spectrometric Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Aminosilanized Fe3O4 Magnetite
2.3. Characterization
2.4. Magnetic Solid-Phase Extraction Procedure
3. Results and Discussion
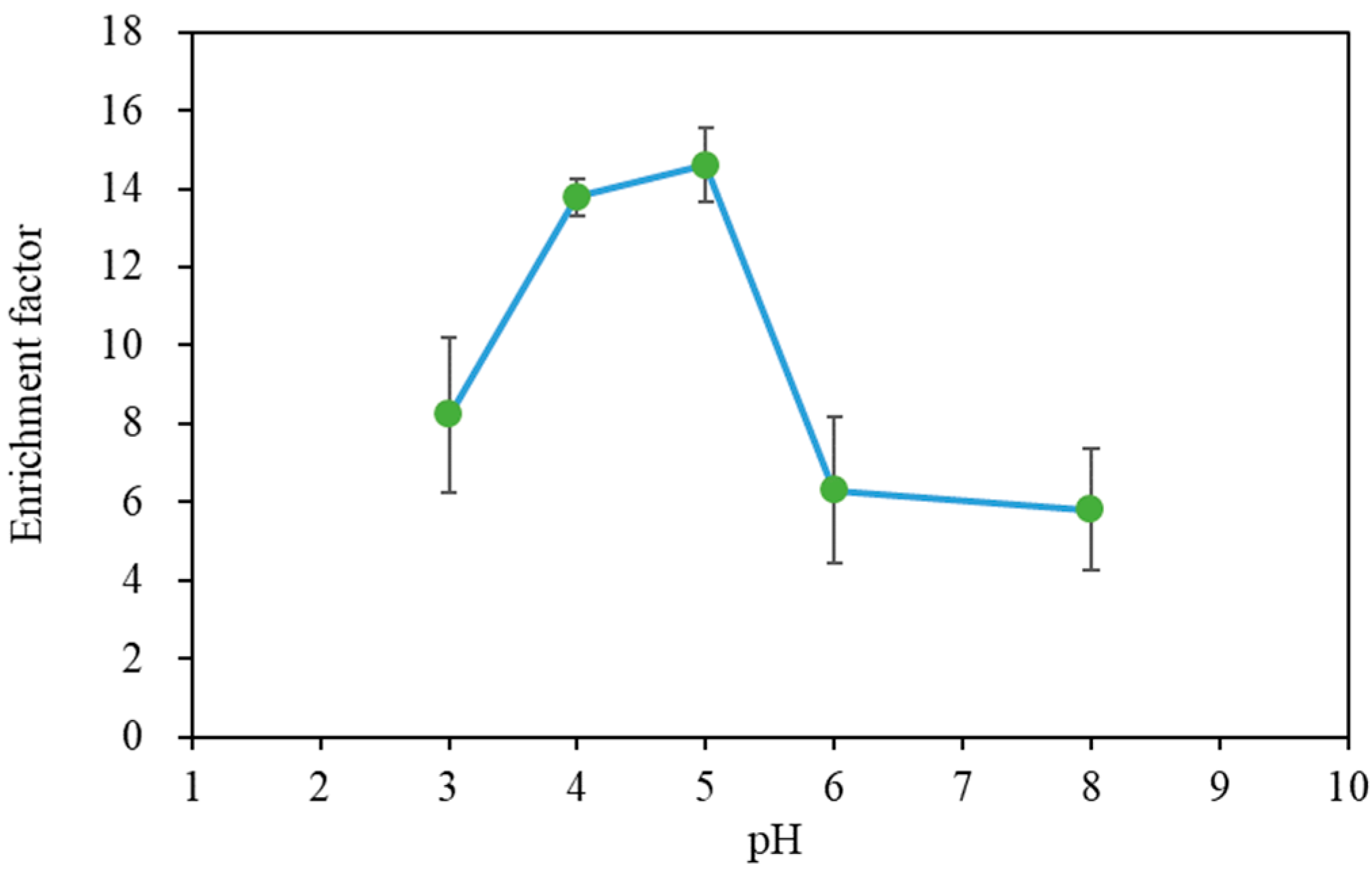
3.1. Acquisition of Base Data
3.2. Characterization of Aminosilanized Magnetite Fe3O4
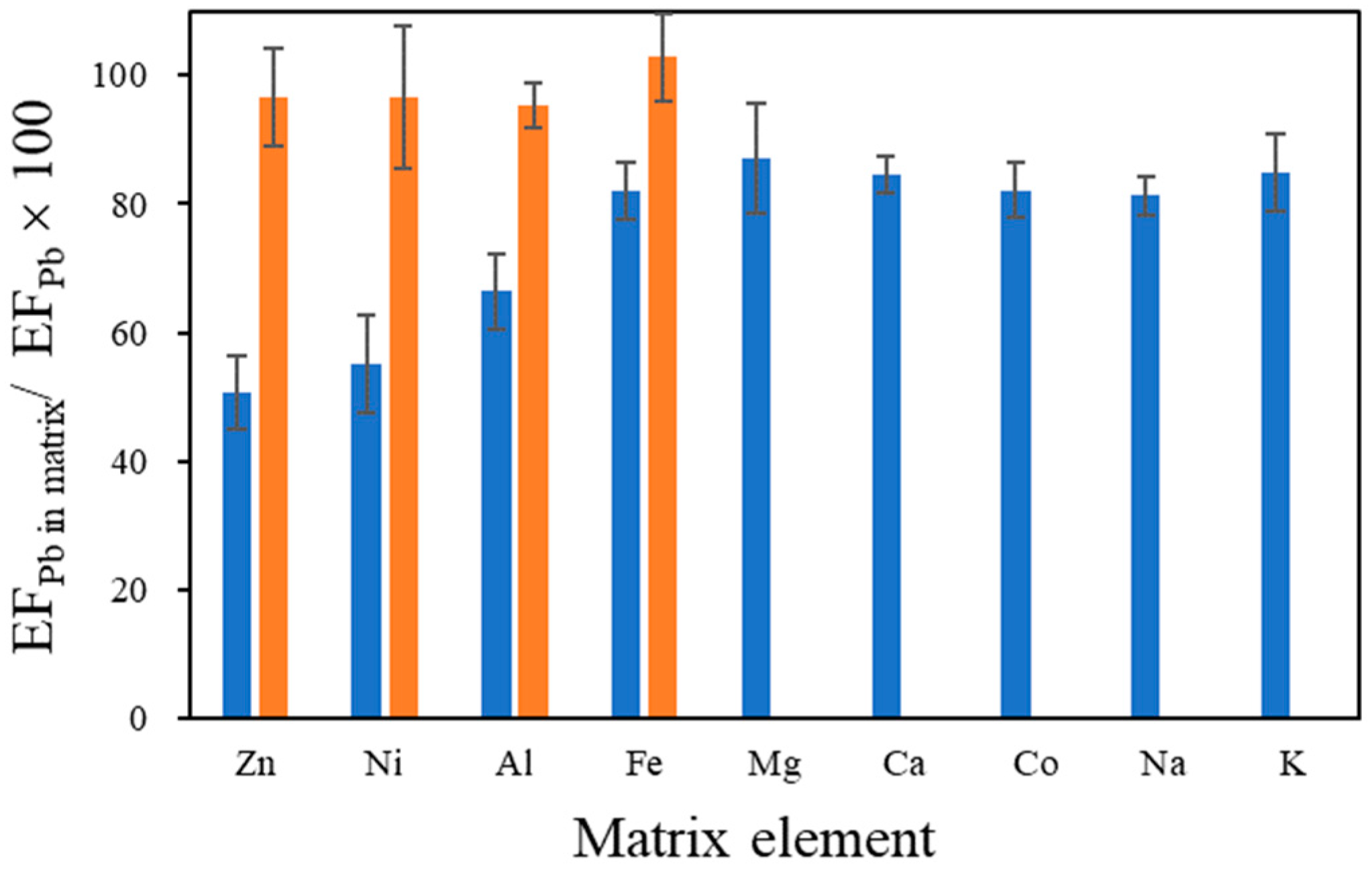
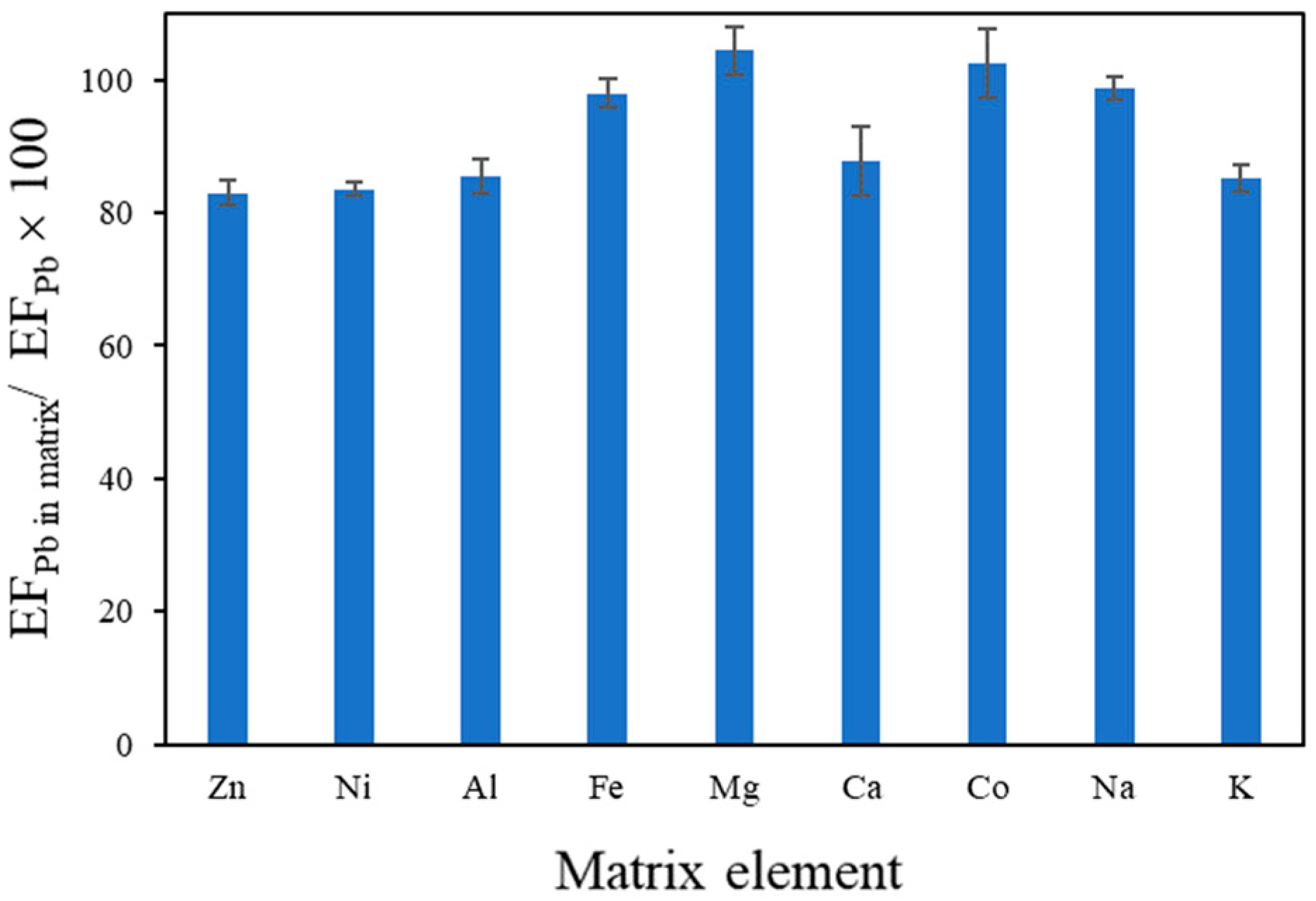
3.3. Effect of Coexisting Elements
3.4. Analytical Performance
3.5. Comparison with Other Techniques
3.6. Determination of Pb in Environmental Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Notes
References
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef]
- Hung, C.; Hu, B. Silica-coated magnetic nanoparticles modified with γ-mercaptopropyltrimethoxysilane for fast and selective solid phase extraction of trace amounts of Cd, Cu, Hg, and Pb in environmental and biological samples prior to their determination by inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B 2008, 67, 437–444. [Google Scholar]
- Wei, Y.; Yang, R.; Yu, X.Y.; Wang, L.; Liu, J.H.; Huang, X. Stripping voltammetry study of ultra-trace toxic metal ions on highly selectivity adsorptive porous magnesium oxide nanoflowers. Analyst 2012, 137, 2183–2191. [Google Scholar] [CrossRef]
- Liu, B.; Lv, X.; Meng, X.; Yu, G.; Wanga, D. Removal of Pb (II) from aqueous solution using dithiocarbamate modified chitosan beads with Pb (II) as imprinted ions. Chem. Eng. J. 2013, 220, 412–419. [Google Scholar] [CrossRef]
- Freschi, G.P.G.; Fortunato, F.M.; Freschi, C.D.; Neto, J.A.G. Simultaneous and direct determination of as, Bi, Pb, Sb, and Se and Co, Cr, Fe, and Mn in milk by electrothermal atomic absorption spectrometry. Food Anal. Method 2012, 5, 861–866. [Google Scholar] [CrossRef]
- Zeng, C.; Tan, H.; Gu, Y.; Liang, C. Enhancement effect of room temperature ionic liquids on the chemical vapor generation of lead, cadmium and bismuth for thermospray flame furnace atomic absorption spectrometric determination. Anal. Methods 2014, 6, 4710–4715. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Li, Q.; Ma, J.; Zhu, M. Preparation characterization and application of mesoporous silica-grafted graphene oxide for highly selective lead adsorption. Chem. Eng. J. 2015, 273, 630–637. [Google Scholar] [CrossRef]
- Jia, X.; Han, Y.; Liu, X.; Duan, T.; Chen, H. Dispersive liquid–liquid microextraction combined with flow injection inductively coupled plasma mass spectrometry for simultaneous determination of cadmium, lead and bismuth in water samples. Microchim. Acta 2010, 171, 49–56. [Google Scholar] [CrossRef]
- Bahadit, Z.; Bulut, V.N.; Ozdes, D.; Duran, C.; Bektas, H.; Sovlak, M. Selective separation, preconcentration and determination of Pb (II) ions in environmental samples by coprecipitation with a 1,2,4-triazole derivative. J. Ind. Eng. Chem. 2014, 20, 1030–1034. [Google Scholar]
- Mortada, W.I.; Kenavy, I.M.; Hassanien, M.M. A cloud point extraction procedure for gallium, indium and thallium determination in liquid crystal display and sediment samples. Anal. Methods 2015, 7, 2114–2120. [Google Scholar] [CrossRef]
- Crecente, R.M.P.; Lovera, C.G.; Barciela, G.J.; Mendez, A.J.; Martin, S.G.; Latorre, C.H. Multiwalled carbon nanotubes as a sorbent material for the solid phase extraction of lead from urine and subsequent determination by electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B 2014, 101, 15–20. [Google Scholar] [CrossRef]
- Shih, T.T.; Hsu, I.H.; Chen, S.N.; Chen, P.H.; Deng, M.J.; Chen, Y.; Lin, Y.W.; Sun, Y.C. A dipole-assisted solid-phase extraction microchip combined with inductively coupled plasma-mass spectrometry for online determination of trace heavy metals in natural water. Analyst 2015, 141, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Rohanifar, A.; Rodriguez, L.B.; Devasurendra, A.M.; Alipourasiabi, N.; Anderson, J.L.; Kirchhoff, J.R. Solid-phase microextraction of heavy metals in natural water with a polypyrrole/carbon nanotube/1,10–phenanthroline composite sorbent material. Talanta 2018, 188, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Z.; Liu, Y.; Yang, X.; Luoa, L.; Chena, J.; Yaob, S. Preparation of core–shell magnetic ion-imprinted polymer for selective extraction of Pb (II) from environmental samples. Chem. Eng. J. 2011, 178, 443–450. [Google Scholar] [CrossRef]
- Babazadeh, M.; Hosseinzadeh, K.R.; Abolhasani, J.; Ghorbani-Kalhor, E.; Hassanpour, A. Solid phase extraction of heavy metal ions from agricultural samples with the aid of a novel functionalized magnetic metal–organic framework. RSC Adv. 2015, 5, 19884–19892. [Google Scholar] [CrossRef]
- Salla, V.; Hardaway, C.J.; Sneddon, J. Preliminary investigation of Spartina alterniflora for phytoextraction of selected heavy metals in soils from Southwest Louisiana. Microchem. J. 2011, 98, 207–212. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Rezaee, M. Extraction of trace amounts of mercury with sodium dodecyl sulphate -coated magnetite nanoparticles and its determination by flow injection inductively coupled plasma-optical emission spectrometry. Talanta 2010, 81, 831–836. [Google Scholar] [CrossRef]
- Liu, X.W.; Hu, Q.Y.; Fang, Z.; Zhang, X.J.; Zhang, B.B. Magnetic chitosan nanocomposites: A useful recyclable took for heavy metal ion removal. Langmuir 2009, 25, 3–8. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Tahera, M.A.; Behzadi, M.; Moghaddam, F.H. Simultaneous preconcentration of bismuth and lead ions on modified magnetic core–shell nanoparticles and their determination by ETAAS. Chem. Eng. J. 2015, 281, 444–452. [Google Scholar] [CrossRef]
- Sitko, R.; Janik, P.; Feist, B.; Talik, E.; Gagor, A. Suspended aminosilanized graphene oxide nanosheets for selective preconcentration of lead ions and ultrasensitive determination by electrothermal atomic absorption spectrometry. ACS Appl. Mater. Interfaces 2014, 6, 20144–20153. [Google Scholar] [CrossRef]
- Li, R.; Zhang, M.; Yang, Y.; Zhang, Z.; Qin, R.; Wang, L. Adsorption of Pb (II) ions in aqueous solutions by common reed ash-derived SBA-15 modified by amino-silanes. Desalin. Water Treat. 2015, 55, 1554–1566. [Google Scholar] [CrossRef]
- Kheshtzar, I.; Ghorbani, M.; Gatabi, M.P.; Lashkenari, M.S. Facile synthesis of smart aminosilane modified-SnO2/porous silica nanocomposite for high efficiency removal of lead ions and bacterial inactivation. J. Hazard. Mater. 2018, 359, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E.; Majzik, A.; Horivát, Z.S.; Illés, E. Magnetite in aqueous medium: Coating its surface and surface coated with it. Rom. Rep. Phys. 2006, 58, 281–286. [Google Scholar]
- Ahsan, S.; Kaneco, S.; Ohta, K.; Mizuno, T.; Suzuki, T.; Miyada, M.; Taniguchi, Y. Electrothermal atomic absorption spectrometric determination of lead in calcium drug samples by direct atomization technique. Anal. Chim. Acta 1998, 362, 279–284. [Google Scholar] [CrossRef]
- Sadeghi, O.; Tavassoil, N.; Amini, M.M.; Ebrahimzadeh, H.; Daei, N. Pyridine-functionalized mesoporous silica as an adsorbent material for the determination of nickel and lead in vegetables grown in close proximity by electrothermal atomic adsorption spectroscopy. Food Chem. 2011, 127, 364–368. [Google Scholar] [CrossRef]
- Carletto, I.S.; Carasek, E.; Welz, B. Hollow-fiber liquid-liquid-solid micro-extraction of lead in soft drinks and determination by graphite furnace atomic absorption spectrometry. Talanta 2011, 84, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.S.; Hu, B. Determination of Cd, Co, Ni and Pb in biological samples by microcolumn packed with black stone (Pierre noire) online coupled with ICP-OES. J. Hazard. Mater. 2008, 157, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.Z.; Shamspur, T.; Karimi, M.A.; Naroui, E. Preconcentration of trace amounts of Pb (II) ions without any chelating agent by using magnetic iron oxide nanoparticles prior to ETAAS determination. Sci. World J. 2012, 2012, 640437. [Google Scholar] [CrossRef]
- Ghorbani-Kalhor, E. A metal-organic framework nanocomposite made from functionalized magnetite nanoparticles and HKUST-1 (MOF-199) for preconcentration of Cd (II), Pb (II), and Ni (II). Microchim. Acta 2016, 183, 2639–2647. [Google Scholar] [CrossRef]




| Method | Sample Volume (mL) | Detection Limit (pg·mL−1) | Reference |
|---|---|---|---|
| SPE | 100 | 110 | [25] |
| HF-LLSMET | 20 | 7 | [26] |
| MC-ICPOES | 3 | 1130 | [27] |
| MSPE-ETAAS | 25 | 3.7 | [19] |
| GO-ETAAS | 50 | 12 | [20] |
| Fe3O4-ETAAS | 50 | 0.8 | [28] |
| Fe3O4/HKUST-FAAS | 200 | 800 | [29] |
| This work | 100 | 14 |
| Sorbent | Sample | Concentration of Pb (ng·mL−1) | ||
|---|---|---|---|---|
| Add | Found | Recovery (%) | ||
| Fe3O4 | Tap water | - | n.d. | |
| 0.3 | 0.33 ± 0.01 | 110 | ||
| Mineral water | - | n.d. | ||
| 0.3 | 0.32 ± 0.01 | 106 | ||
| Ground water | - | n.d. | ||
| 0.3 | 0.33 ± 0.01 | 110 | ||
| NH2-Fe3O4 | Mineral water | - | n.d. | |
| 0.1 | 0.075 ± 0.010 | 75 | ||
| 0.5 | 0.50 ± 0.03 | 101 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusutaki, T.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Preconcentration of Pb with Aminosilanized Fe3O4 Nanopowders in Environmental Water Followed by Electrothermal Atomic Absorption Spectrometric Determination. ChemEngineering 2019, 3, 74. https://doi.org/10.3390/chemengineering3030074
Kusutaki T, Furukawa M, Tateishi I, Katsumata H, Kaneco S. Preconcentration of Pb with Aminosilanized Fe3O4 Nanopowders in Environmental Water Followed by Electrothermal Atomic Absorption Spectrometric Determination. ChemEngineering. 2019; 3(3):74. https://doi.org/10.3390/chemengineering3030074
Chicago/Turabian StyleKusutaki, Tomoharu, Mai Furukawa, Ikki Tateishi, Hideyuki Katsumata, and Satoshi Kaneco. 2019. "Preconcentration of Pb with Aminosilanized Fe3O4 Nanopowders in Environmental Water Followed by Electrothermal Atomic Absorption Spectrometric Determination" ChemEngineering 3, no. 3: 74. https://doi.org/10.3390/chemengineering3030074





