Effect of Concentration on Amine-Modified Kenaf as a Sorbent for Carbon Dioxide Adsorption in a Pressure Swing Adsorption System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Preparation of the Amine-Modified Kenaf Sample
2.3. Structural Characterization Analysis
2.4. Elemental Characterization Analysis
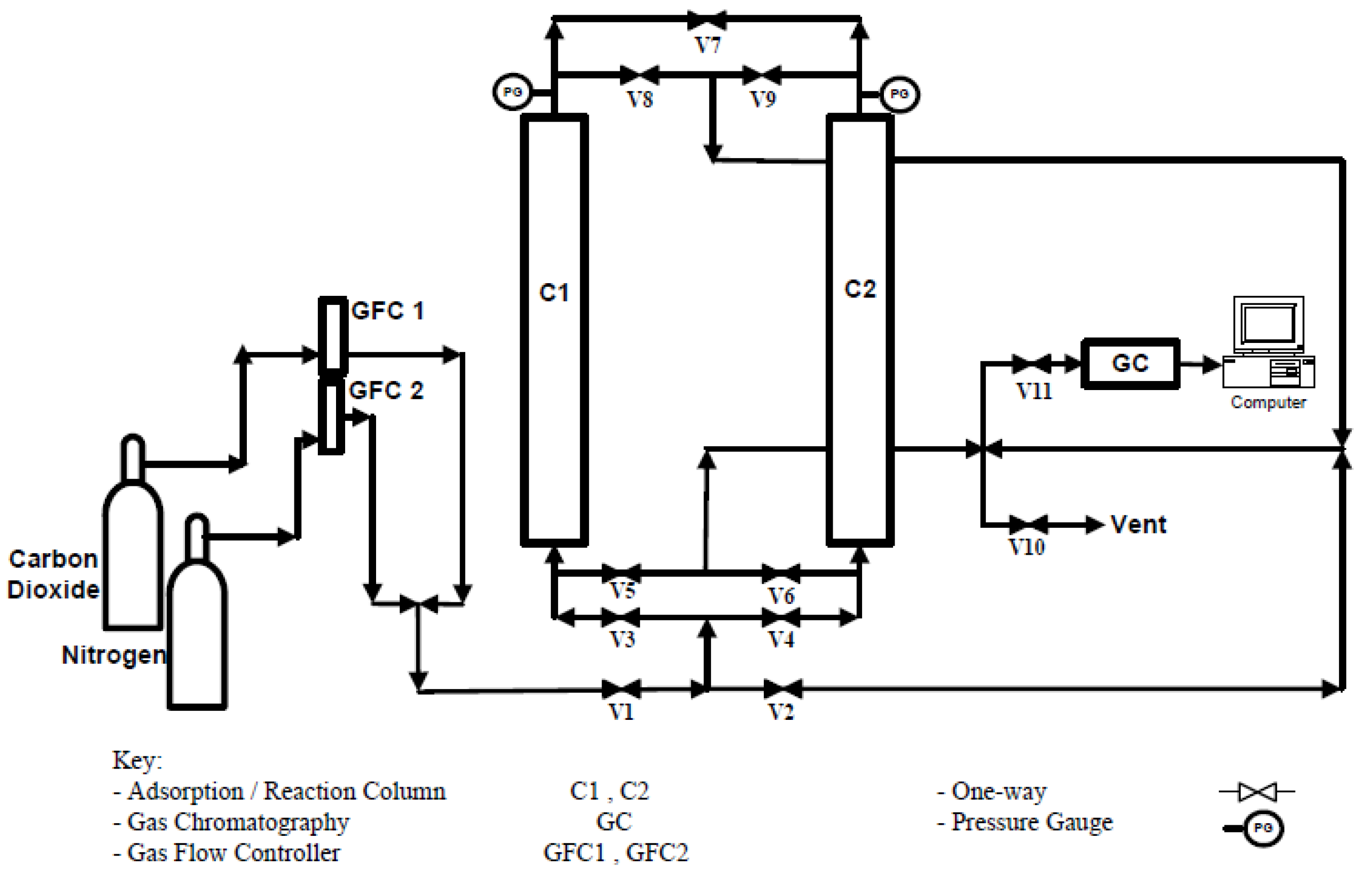
2.5. CO2 Adsorption and Regeneration Studies
3. Results and Discussion
3.1. Structural Characterization Analysis
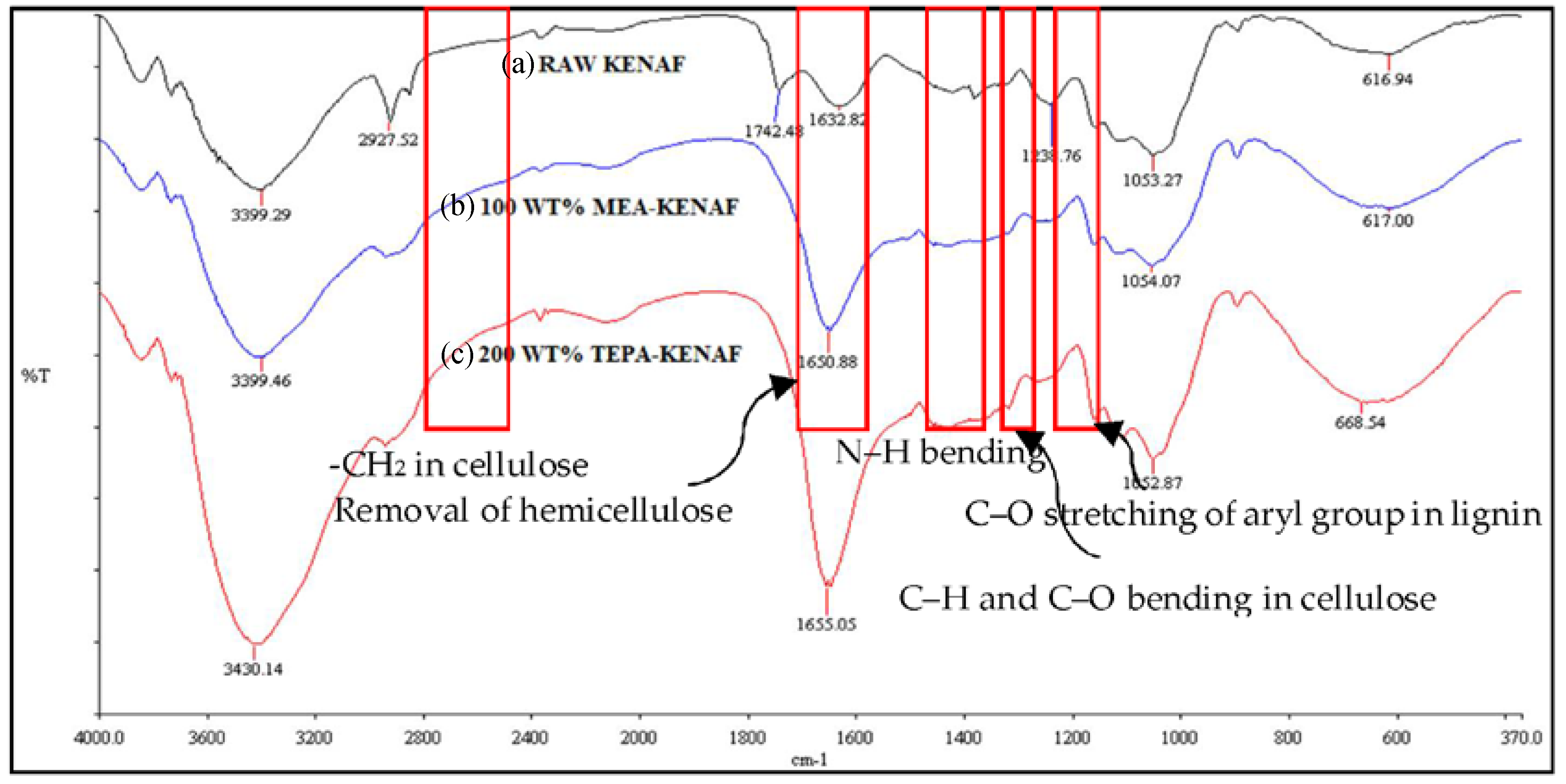
3.1.1. FTIR Characterization Study
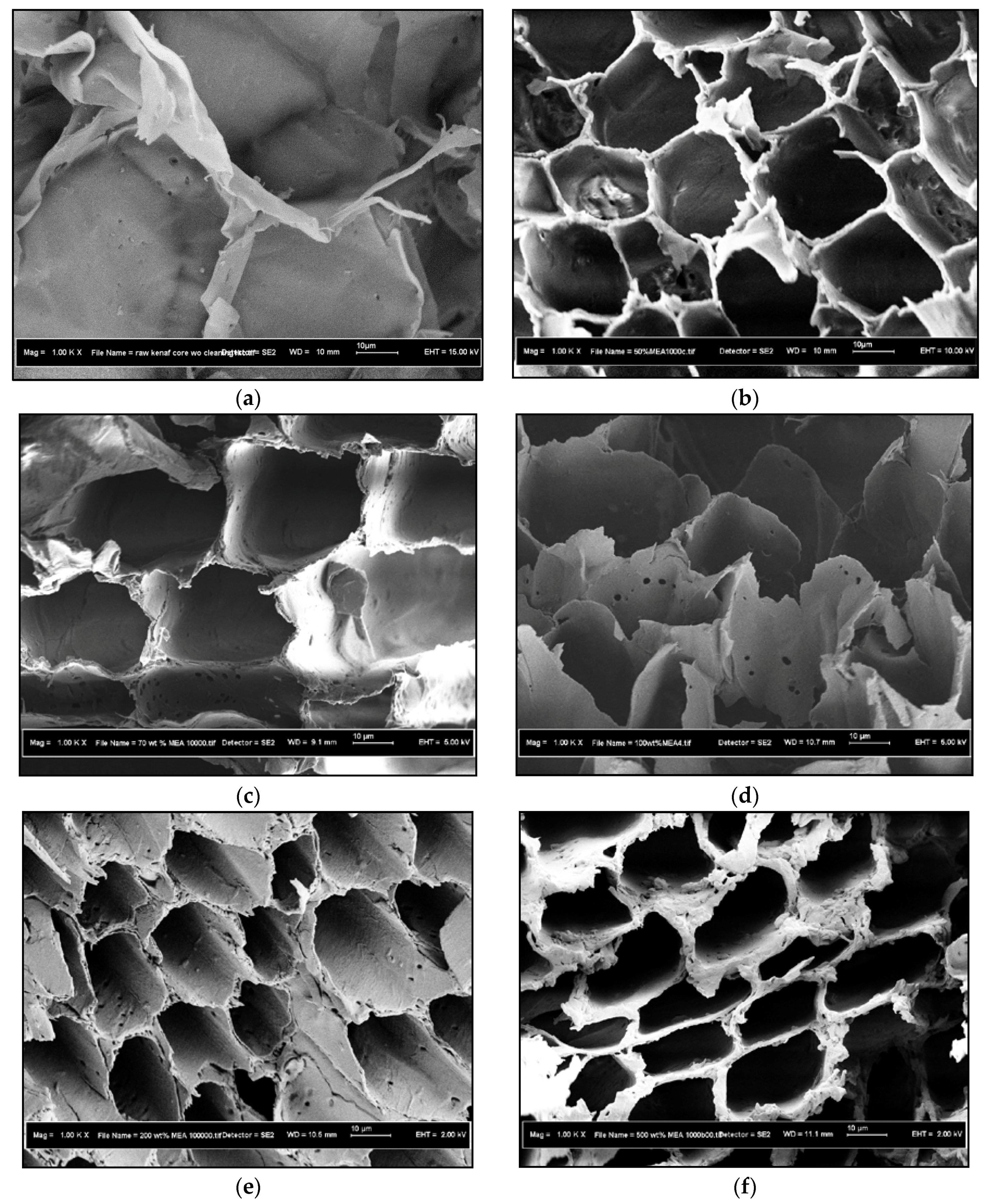
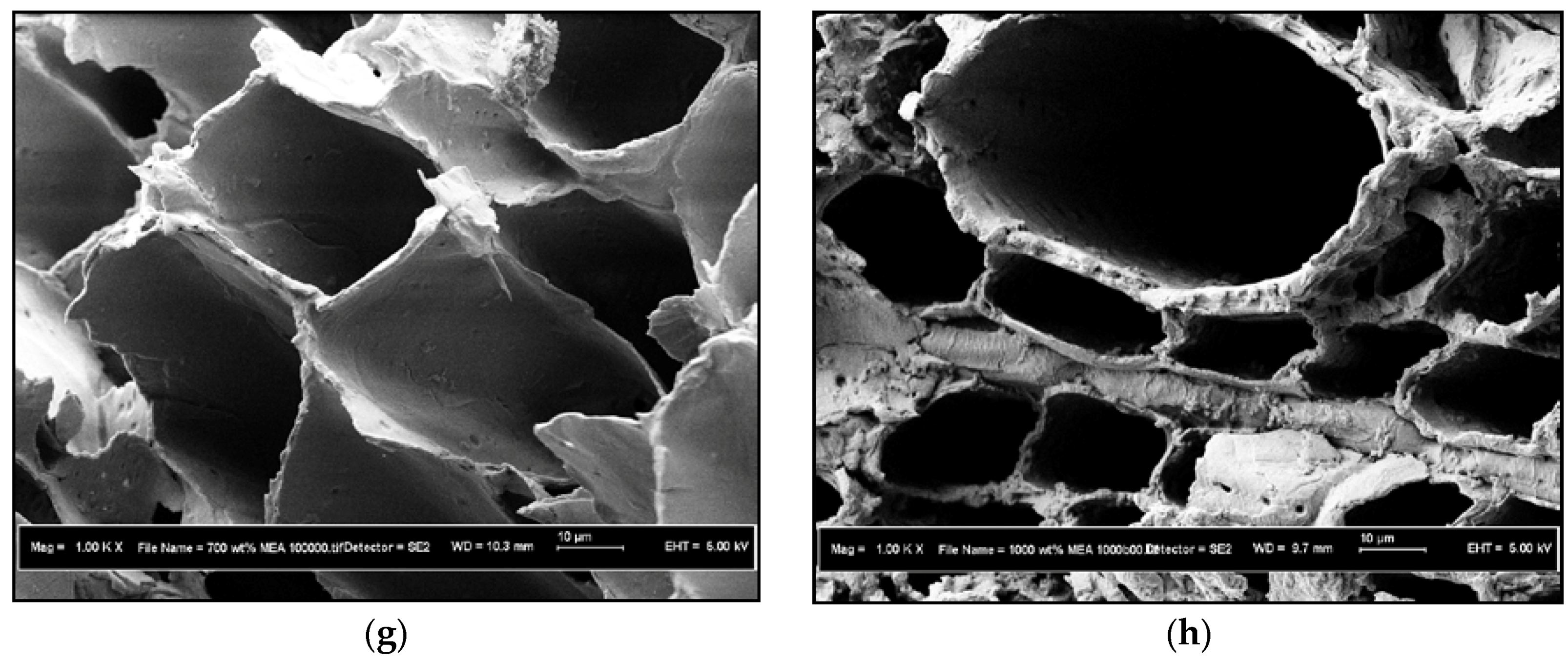
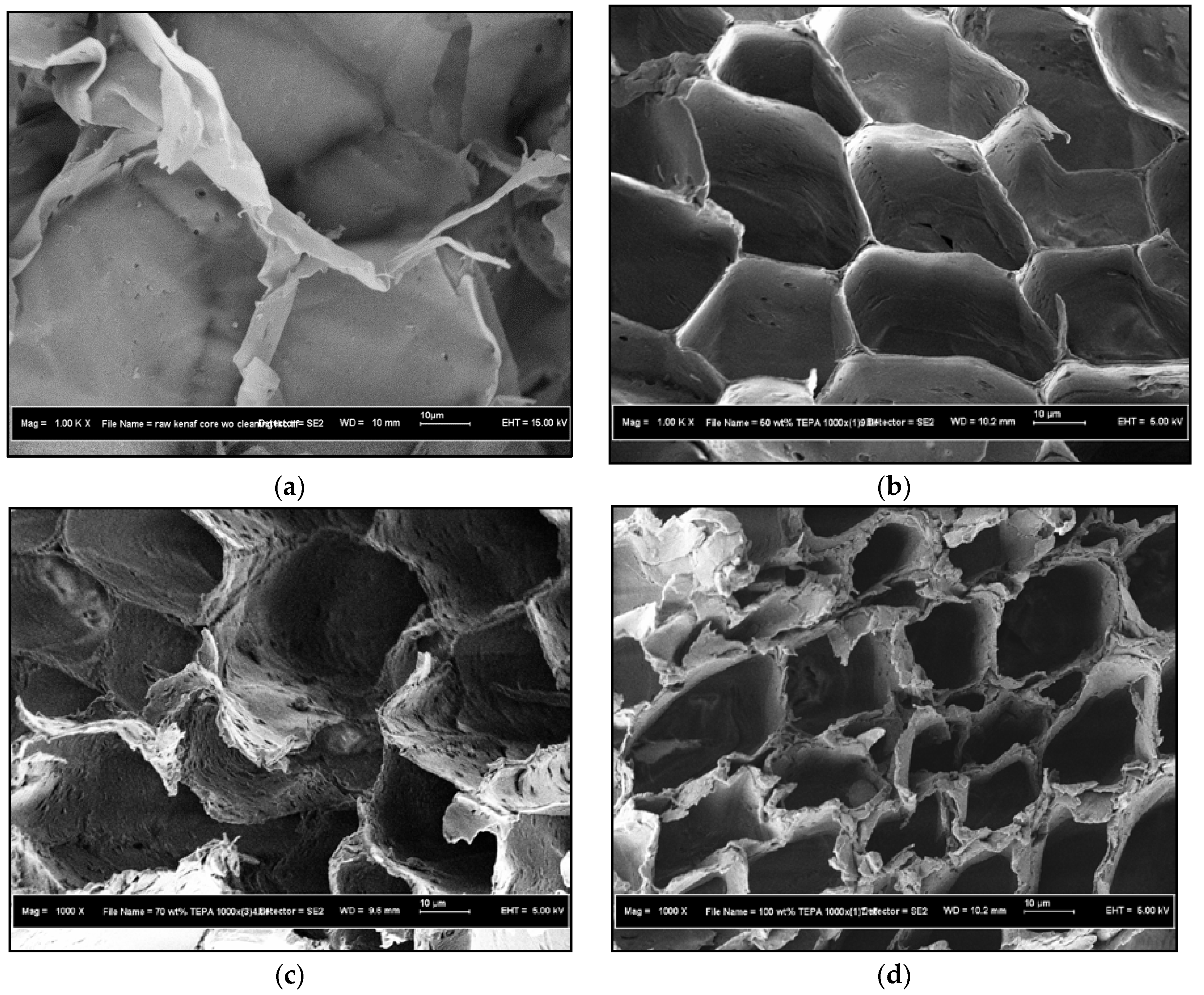
3.1.2. FESEM Characterization Study
3.1.3. EDX Characterization Study
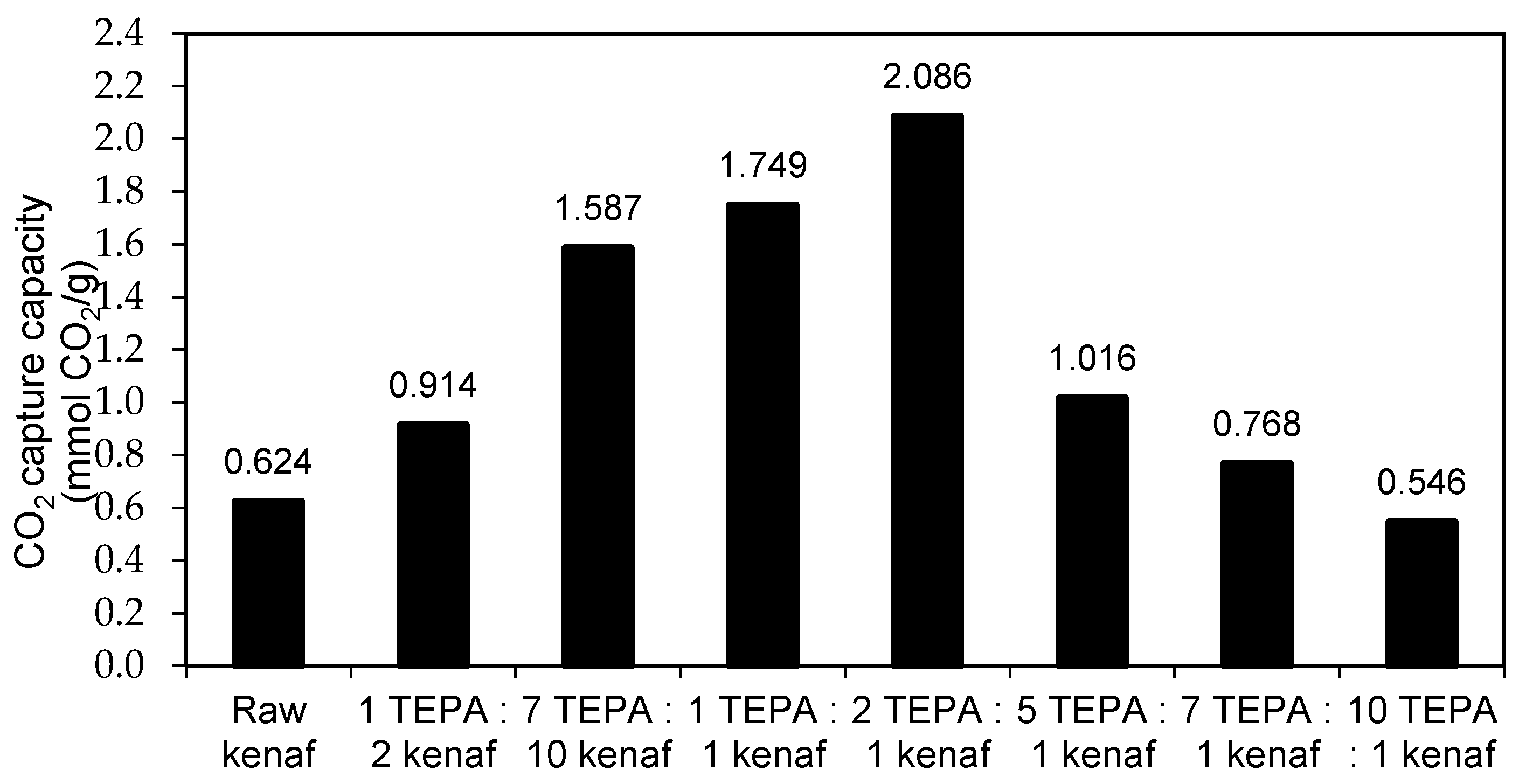
3.2. Effect of Amine Concentration on CO2 Adsorption Study
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Wang, B.; Sun, Q.; Liu, H. Waste is a misplayed resource: Synthesis of zeolites from fly ash for CO2 capture. Energy Procedia 2017, 114, 2537–2544. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Sun, Q.; Zheng, L. A novel method for the preparation of CO2 sorption sorbents with high performance. Appl. Energy 2014, 123, 179–184. [Google Scholar] [CrossRef]
- Luca, R.; Olav, B. Overview on pressure swing adsorption (PSA) as CO2 capture technology: State-of-the-art, limits and potentials. Energy Procedia 2017, 114, 2390–2400. [Google Scholar]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.; Baltrus, J.P.; Stevens, R.W.; Khatri, R.; Chuang, S.S.C.; Filburn, T. Improved immobilized carbon dioxide capture sorbents. Fuel Process. Technol. 2005, 86, 1449–1455. [Google Scholar] [CrossRef]
- Zanganeh, K.E.; Shafeen, A.; Salvador, C. CO2 capture and development of an advanced pilot–scale cryogenic separation and compression unit. Energy Procedia 2009, 1, 247–252. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Resnik, K.P. Aqua ammonia process for simultaneous removal of CO2, SO2 and NOx. Int. J. Environ. Technol. Manag. 2004, 4, 89–104. [Google Scholar] [CrossRef]
- Haszeldine, R.S. Carbon capture and storage: How green can black be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Bara, J.E.; Camper, D.E.; Gin, D.L.; Noble, R.D. Room-temperature ionic liquids and composite materials: Platform technologies for CO2 capture. Acc. Chem. Res. 2010, 43, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.; Walker, G.M.; Allen, S.J. Investigations on the Adsorption of Acidic Gases using Activated Dolomite. Chem. Eng. J. 2006, 117, 239–244. [Google Scholar] [CrossRef]
- Mandal, B.P.; Bandyopadhyay, S.S. Simultaneous absorption of CO2 and H2S into aqueous blends of n–methyldiethanolamine and diethanolamine. Environ. Sci. Technol. 2006, 40, 6076–6084. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arenillas, A.; Rubiera, F.; Pis, J.J. CO2 capture by adsorption with nitrogen enriched carbons. Fuel 2007, 86, 2204–2212. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhkout, Y.; Sayari, A. Influence of regeneration conditions on the cyclic performance of amine-grafted mesoporous silica for CO2 capture: An experimental and statistical study. Chem. Eng. Sci. 2010, 65, 4166–4172. [Google Scholar] [CrossRef]
- Samanta, A.; Zhou, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post–combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2011, 51, 1438–1463. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. Chemsuschem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.G.; Gonzalez, A.S.; Pevida, C.; Pis, J.J.; Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for post combustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef]
- González, A.S.; Plaza, M.G.; Rubiera, F.; Pevida, C. Sustainable biomass–based carbon adsorbents for post–combustion CO2 capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef]
- Nasri, N.S.; Hamza, U.D.; Ismail, S.N.; Ahmed, M.M.; Mohsin, R. Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J. Clean. Prod. 2013, 71, 148–157. [Google Scholar] [CrossRef]
- Lee, C.S.; Ong, Y.L.; Aroua, M.K.; Daud, W.M.A.W. Impregnation of palm shell based activated carbon with sterically hindered amines for CO2 adsorption. Chem. Eng. J. 2013, 219, 558–564. [Google Scholar] [CrossRef]
- Ello, A.S.; Luiz, K.C.; de Souza, A.T.; Mietek, J. Development of microporous carbons for CO2 capture by KOH activation of african palm shells. J. CO2 Util. 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Silvestre-Albero, J.; Moreno-Pirajan, J.C. CO2 adsorption on binderless activated carbon monoliths. Adsorption 2011, 17, 497–504. [Google Scholar] [CrossRef]
- Shen, W.; He, Y.; Zhang, S.; Li, J.; Fan, W. Yeast–based microporous carbon material for carbon dioxide capture. ChemSusChem 2012, 5, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Shen, W.; Wang, J.; Fan, W. Superior carbon–based CO2 adsorbents prepared from poplar anthers. Carbon 2014, 69, 255–263. [Google Scholar] [CrossRef]
- Boonpoke, A.; Chirakorn, S.; Laosiripojana, N.; Towprayon, S.; Chidthaisong, A. Synthesis of activated carbon and MCM–41 from bagasse and rice husk and their carbon dioxide adsorption capacity. J. Sustain. Energy Environ. 2011, 2, 77–81. [Google Scholar]
- Alhwaige, A.A.; Ishida, H.; Agag, T.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Primo, A.; Forneli, A.; Corma, A.; Garci’a, H. From biomass wastes to highly efficient CO2 adsorption: Graphitisation of chitosan and alignates biopolymers. ChemSusChem 2012, 5, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.R.; Akil, H.M. The CO2 adsorptive and regenerative behaviors of rhizopus oligosporus and carbonaceous hibiscus cannabinus exposed to thermal swings. Microporous Mesoporous Mater. 2008, 110, 363–369. [Google Scholar] [CrossRef]
- Garcia, A.M.; Cuerda-Correa, E.M.; Marin, M.O.; Paralejo, A.D.; Diez, M.A.D. Development and characterization of carbon–honeycomb monoliths from kenaf natural fibers: A preliminary study. Ind. Crops Prod. 2011, 35, 105–110. [Google Scholar] [CrossRef]
- Murphy, P.T.; Moore, K.J.; Richard, T.L.; Bern, C.J. Enzyme enhanced solid-state fermentation of kenaf core fiber for storage and pretreatment. Bioresour. Technol. 2007, 98, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Ireana Yusra, A.F.; Bhat, A.H.; Jawaid, M. Cell wall ultrastructure, anatomy, lignin distribution and chemical composition of malaysian cultivated kenaf fiber. Ind. Crops Prod. 2010, 31, 113–121. [Google Scholar] [CrossRef]
- Siqueira, R.M.; Freitas, G.R.; Peixoto, H.R.; do Nascimento, J.F.; Musse, A.P.S.; Torres, A.E.B.; Azevedo, D.C.S.; Bastos-Neto, M. Carbon dioxide capture by pressure swing adsorption. Energy Procedia 2017, 114, 2182–2192. [Google Scholar] [CrossRef]
- Zaini, N. Amine-Functionalized Kenaf as Carbon Dioxide Adsorbent in Pressure Swing Adsorption System. Ph.D. Thesis, Universiti Teknologi Malaysia, Johor Bahru, Malaysia, 2006. [Google Scholar]
- Khalil, H.P.A.; Ismail, H.; Roazman, H.D.; Ahmad, M.N. The effect of acetylation on interfacial shear strength between plant fiber and various matrices. Eur. Polym. J. 2001, 37, 1037–1045. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Compos. Part A 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Tahir, P.M.; Zaini, L.H.; Azry, S.S.; Makinejad, M.D. Characteristics of nanofibers extracted from kenaf core. Bioresources 2010, 5, 2556–2566. [Google Scholar]
- Nacos, M.; Katapodis, P.; Pappas, C.; Daferera, D.; Tarantilis, P.A.; Christakopoulos, P.; Polissiou, M. Kenaf xylan–A source of biologically active acidic oligosaccharides. Carbohydr. Polym. 2006, 66, 126–134. [Google Scholar] [CrossRef]
- Troedec, M.; Sedan, D.; Peyratout, C.; Bonnet, J.; Smith, A.; Guinebretiere, R.; Gloaguen, V.; Krausz, P. Influence of various chemical treatments on the composition and structure of hemp fibers. Compos. Part A 2008, 39, 514–522. [Google Scholar] [CrossRef]
- Anita, R.; Sohail, A.; Suzana, Y. Effect of monoethanolamine loading on the physicochemical properties of amine–functionalized Si-MCM-41. Sains Malays. 2014, 43, 253–259. [Google Scholar]


| Ratio MEA:Kenaf | Amount of MEA Needed (g) | Elemental Compositions (wt %) | pH Value | ||
|---|---|---|---|---|---|
| Carbon (C) | Oxygen (O) | Nitrogen (N) | |||
| 1:2 | 0.75 | 55.51 | 42.16 | 2.34 | 10.60 |
| 7:10 | 1.05 | 57.59 | 39.44 | 2.98 | 10.62 |
| 1:1 | 1.50 | 60.40 | 32.40 | 7.20 | 10.81 |
| 2:1 | 3.00 | 54.22 | 37.51 | 8.27 | 11.15 |
| 5:1 | 7.50 | 60.27 | 30.56 | 9.17 | 11.27 |
| 7:1 | 10.50 | 51.88 | 38.11 | 10.02 | 11.38 |
| 10:1 | 15.00 | 50.96 | 38.02 | 11.02 | 11.67 |
| Ratio TEPA:Kenaf | Amount of TEPA Needed (g) | Elemental Compositions (wt %) | pH Value | ||
|---|---|---|---|---|---|
| Carbon (C) | Oxygen (O) | Nitrogen (N) | |||
| 1:2 | 0.75 | 63.52 | 29.09 | 7.39 | 10.82 |
| 7:10 | 1.05 | 57.27 | 35.09 | 7.65 | 11.02 |
| 1:1 | 1.50 | 56.89 | 34.72 | 8.39 | 11.14 |
| 2:1 | 3.00 | 54.51 | 33.08 | 12.41 | 11.39 |
| 5:1 | 7.50 | 51.55 | 32.99 | 15.46 | 11.83 |
| 7:1 | 10.50 | 51.90 | 29.84 | 18.27 | 12.01 |
| 10:1 | 15.00 | 48.15 | 31.94 | 19.91 | 12.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaini, N.; Kamarudin, K.S.N. Effect of Concentration on Amine-Modified Kenaf as a Sorbent for Carbon Dioxide Adsorption in a Pressure Swing Adsorption System. ChemEngineering 2018, 2, 23. https://doi.org/10.3390/chemengineering2020023
Zaini N, Kamarudin KSN. Effect of Concentration on Amine-Modified Kenaf as a Sorbent for Carbon Dioxide Adsorption in a Pressure Swing Adsorption System. ChemEngineering. 2018; 2(2):23. https://doi.org/10.3390/chemengineering2020023
Chicago/Turabian StyleZaini, Nabilah, and Khairul Sozana Nor Kamarudin. 2018. "Effect of Concentration on Amine-Modified Kenaf as a Sorbent for Carbon Dioxide Adsorption in a Pressure Swing Adsorption System" ChemEngineering 2, no. 2: 23. https://doi.org/10.3390/chemengineering2020023




