The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Sperm Collection and Preparation
2.3. Mitochondrial Staining
2.4. Statistical Analysis
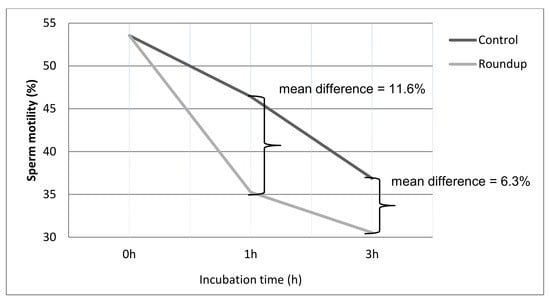
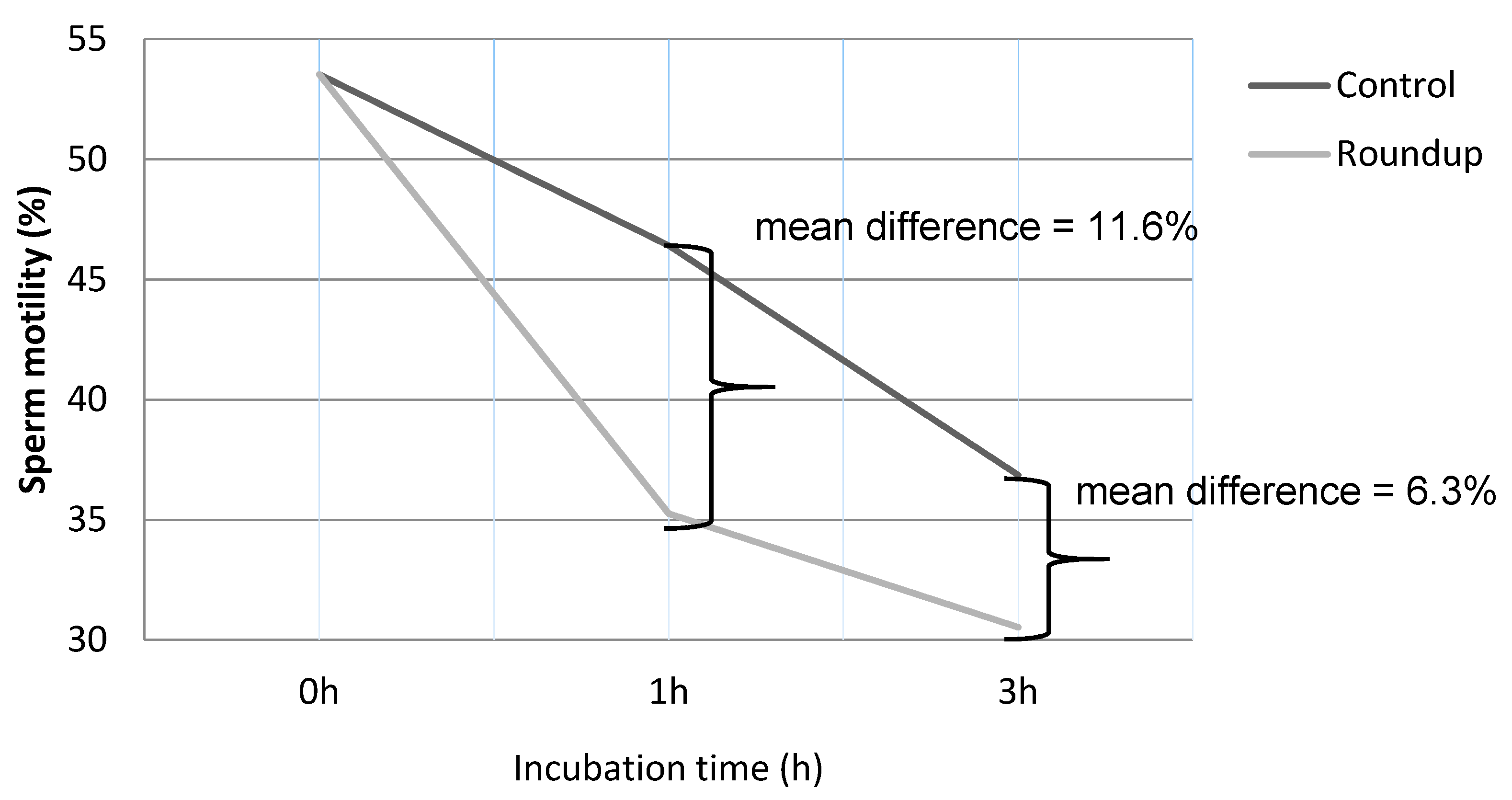
3. Results
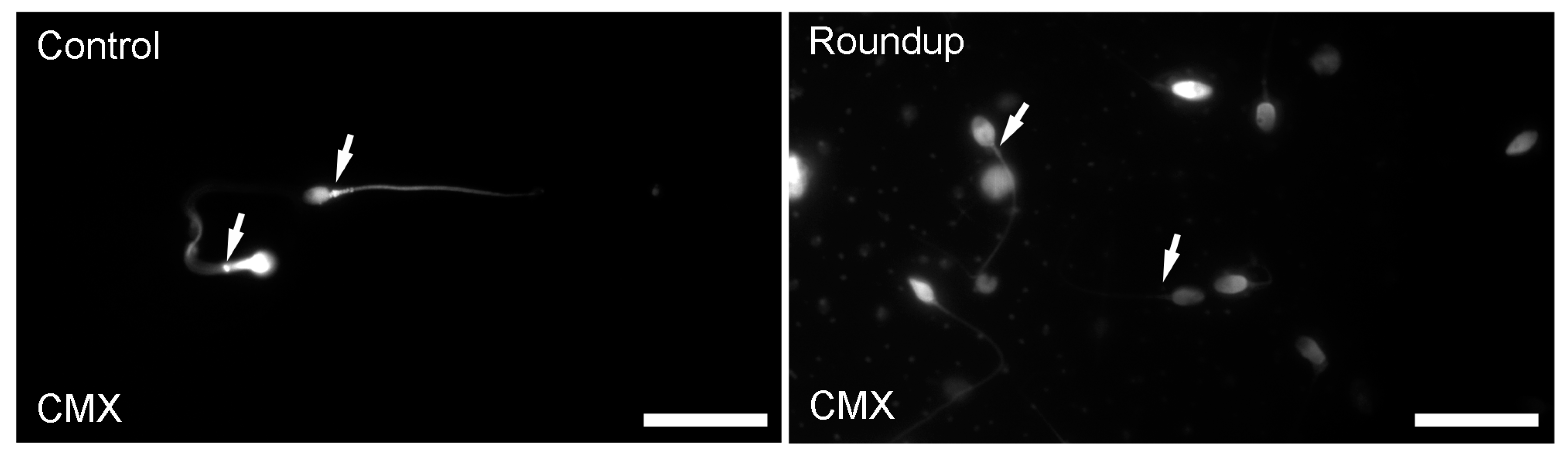
Mitochondrial Functionality
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giudice, L.C. Environmental toxicants: Hidden players on the reproductive stage. Fertil. Steril. 2016, 106, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Banerjee, R. Environmental toxins: Alarming impacts of pesticides on male fertility. Hum. Exp. Toxicol. 2014, 33, 1017–1039. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Smedbol, E.; Chalifour, A.; Hénault-Ethier, L.; Labrecque, M.; Lepage, L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef] [PubMed]
- Williams G, M.; Kroes, R.; Munro, I.C. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000, 31 Pt 1, 117–165. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, A.; Gnazzo, V.; Acosta, H.; López, S.L.; Carrasco, A.E. Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. Chem. Res. Toxicol. 2010, 23, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Ji, Y.; Song, X.; Guo, H.; Han, L.; Zhang, F.; Liu, X.; Zhang, H.; Zhu, B.; Xu, M. Effects of glyphosate exposure on sperm concentration in rodents: A systematic review and meta-analysis. Environ. Toxicol. Pharmacol. 2017, 55, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.M.; Varela Junior, A.S.; Corcini, C.D.; da Silva, A.C.; Guazzelli, V.G.; Tavares, G.; da Rosa, C.E. Effect of glyphosate on the sperm quality of zebrafish Daniorerio. Aquat. Toxicol. 2014, 155, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Akiri, O.F.; Durojaiye, M.A.; Adenike, A. Combined effects of repeated administration of Bretmont Wipeout (glyphosate) and Ultrazin (atrazine) on testosterone, oxidative stress and sperm quality of Wistar rats. Toxicol. Mech. Methods 2015, 25, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.A.; Varela, A.S.; Corcini, C.D.; da Silva, J.C.; Primel, E.G.; Caldas, S.; Klein, R.D.; Martins, C.M. Effects of Roundup formulations on biochemical biomarkers and male sperm quality of the livebearing Jenynsia multidentata. Chemosphere 2017, 177, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Benkhalifa, M.; Ferreira, Y.J.; Chahine, H.; Louanjli, N.; Miron, P.; Merviel, P.; Copin, H. Mitochondria: Participation to infertility as source of energy and cause of senescence. Int. J. Biochem. Cell Biol. 2014, 55, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, K.; Dulaurent, S.; Gaulier, J.M.; Moesch, C.; Lachâtre, G. Determination of glyphosate and AMPA in blood and urine from humans: About 13 cases of acute intoxication. Forensic. Sci. Int. 2013, 226, e20–e25. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Klaunig, J.E. Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol. Sci. 2000, 53, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Chiaradoma, F.; Gaglio, D.; Vanoni, M.; Alberghina, L. Expression of transforming K-Ras oncogene affects mitochondrial function and morphology in mouse fibroblasts. Biochim. Biophys. Acta 2006, 175, 1338–1356. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Ramalho-Santos, J. Assessment of mitochondrial potential: Implications for the correct monitoring of human sperm function. Int. J. Androl. 2010, 33, e180–e186. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Oku, M.; Tsuda, M.; Hoseki, J.; Sakai, Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci. Rep. 2014, 4, 5896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.J.; Kwon, H.K.; Lee, J.H.; Gui, X.; Achek, A.; Kim, J.H.; Choi, S. Doxorubicin-induced necrosis is mediated by poly-(ADP-ribose) polymerase 1 (PARP1) but is independent of p53. Sci. Rep. 2015, 5, 15798. [Google Scholar] [CrossRef] [PubMed]
- Scheller, K.; Sekeris, C.E.; Krohne, G.; Hock, R.; Hansen, I.A.; Scheer, U. Localization of glucocorticoid hormone receptors in mitochondria of human cells. Eur. J. Cell Biol 2000, 79, 299–307. [Google Scholar] [CrossRef]
- Solakidi, S.; Psarra, A.M.; Nikolaropoulos, S.; Sekeris, C.E. Estrogen receptors alpha and beta (ERalpha and ERbeta) and androgen receptor (AR) in human sperm: Localization of ERbeta and AR in mitochondria of the midpiece. Hum. Reprod. 2005, 20, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.P.; D’Cruz, S.C. The effect of environmental contaminants on testicular function. Asian J. Androl. 2011, 13, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Watson, R.E.; DeSesso, J.M. Developmental and reproductive outcomes in humans and animals after glyphosate exposure: A critical analysis. J. Toxicol. Environ. Health B Crit. Rev. 2015, 15, 39–96. [Google Scholar] [CrossRef] [PubMed]
- De Liz Oliveira Cavalli, V.L.; Cattani, D.; Heinz Rieg, C.E.; Pierozan, P.; Zanatta, L.; Benedetti Parisotto, E.; Wilhelm Filho, D.; Mena Barreto Silva, F.R.; Pessoa-Pureur, R.; Zamoner, A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013, 65, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Moslemi, S.; Sipahutar, H.; Benachour, N.; Seralini, G.E. Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ. Health Perspect. 2005, 113, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Arch. Toxicol. 2010, 84, 309–317. [Google Scholar] [CrossRef] [PubMed]
- The German Federal Institute for Occupational Safety and Health. GLH Report for Glyphosate. Proposal for Harmonised Classification and Labelling. Based on Regulation (EC) No 1272/2008. (CLP Regulation), Annex VI, Part 2. Version 2.0, May 2016; The German Federal Institute for Occupational Safety and Health: Dortmund, Germany.
- EFSA. The 2011 European Union report on pesticide residues in food. EFSA J. 2014, 12, 36–94. [Google Scholar]
- De Araujo, J.S.; Delgado, I.F.; Paumgartten, F.J. Glyphosate and adverse pregnancy outcomes, a systematic review of observational studies. BMC Public Health 2016, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R.; Anadon, A.; Carasquilla, G. Coca and poppy eradication in Colombia: Environmental and human health assessment of aerially applied glyphosate. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2007; Volume 190, pp. 43–125. [Google Scholar]
- Solomon, K.R.; Anadon, A.; Brain, R.A.; Cerdeira, A.L.; Crossan, A.N.; Marshall, J.; Satin, L.-H.; Smith, J.L. Comparative hazard assessment of the substances used for production and control of coca and poppy in Colombia. In Rational Environmental Management of Agrochemicals: Risk Assessment, Monitoring, and Remedial Action ACS Symposium Series; Kennedy, I.R., Solomon, K.R., Gee, S., Crossan, A.N., Wang, S., Sanchez-Bayo, F., Eds.; American Chemical Society: Washington, DC, USA, 2007; Chapter 6; Volume 966, pp. 87–99. ISBN 978-0-8412-7420-4. [Google Scholar]
- Solomon, K.R. Glyphosate in the general population and in applicators: A critical review of studies on exposures. Crit. Rev. Toxicol. 2016, 46, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Owagboriaye, F.O.; Dedeke, G.A.; Ademolu, K.O.; Olujimi, O.O.; Ashidi, J.S.; Adeyinka, A.A. Reproductive toxicity of Roundup herbicide exposure in male albino rat. Exp. Toxicol. Pathol. 2017, 69, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Dallegrave, E.; Mantese, F.D.; Oliveira, R.T.; Andrade, A.J.; Dalsenter, P.R.; Langeloh, A. Pre- and postnatal toxicity of the commercial glyphosate formulation in Wistar rats. Arch. Toxicol. 2007, 81, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.E. Activity and androgenic control of enzymes associated with the tricarboxylic acid cycle, lipid oxidation and mitochondrial shuttles in the epididymis and epididymal spermatozoa of the rat. Biochem. J. 1978, 174, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.P.; Shaha, C. Estrogen-induced spermatogenic cell apoptosis occurs via the mitochondrial pathway: Role of superoxide and nitric oxide. J. Biol. Chem. 2005, 280, 6181–6196. [Google Scholar] [CrossRef] [PubMed]
- Rajender, S.; Rahul, P.; Mahdi, A.A. Mitochondria, spermatogenesis and male infertility. Mitochondrion 2010, 10, 419–428. [Google Scholar] [CrossRef] [PubMed]
- De Jager, C.; Farias, P.; Barraza-Villarreal, A.; Avila, M.H.; Ayotte, P.; Dewailly, E.; Dombrowski, C.; Rousseau, F.; Sanchez, V.D.; Bailey, J.L. Reduced seminal parameters associated with environmental DDT exposure and p,p’-DDE concentrations in men in Chiapas, Mexico: A cross-sectional study. J. Androl. 2006, 27, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Amaral, S.; Paiva, C.; Baptista, M.; Ramalho-Santos, J. In vitro exposure to the organochlorine p,p’-DDE affects functional human sperm parameters. Chemosphere 2015, 120, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Shukla, M.; Upadhyay, A.D.; Chaturvedi, P.K.; Saxena, D.K.; Gupta, Y.K. Association between environmental exposure to p,p’-DDE and lindane and semen quality. Environ. Sci. Pollut. Res. Int. 2014, 21, 11009–11016. [Google Scholar] [CrossRef] [PubMed]
- Psarra, A.M.; Sekeris, C.E. Nuclear receptors and other nuclear transcription factors in mitochondria: Regulatory molecules in a new environment. Biochim. Biophys. Acta 2008, 1783, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Cell biology: Metabolic control of cell death. Science 2014, 345, 1250256. [Google Scholar] [CrossRef] [PubMed]
- Modesto, K.A.; Martinez, C.B. Roundup causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 2010, 78, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Glusczak, L.; Loro, V.L.; Pretto, A.; Moraes, B.S.; Raabe, A.; Duarte, M.F.; da Fonseca, M.B.; de Menezes, C.C.; Valladão, D. Acute exposure to glyphosate herbicide affects oxidative parameters in piava (Leporinus obtusidens). Arch. Environ. Contam. Toxicol. 2011, 61, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Murussi, C.R.; Costa, M.D.; Leitemperger, J.W.; Guerra, L.; Rodrigues, C.C.; Menezes, C.C.; Severo, E.S.; Flores-Lopes, F.; Salbego, J.; Loro, V.L.; et al. Exposure to different glyphosate formulations on the oxidative and histological status of Rhamdia quelen. Fish. Physiol. Biochem. 2016, 42, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Hu, P.; Tang, J.; Li, Y.; Li, C. Effect of glyphosate on reproductive organs in male rat. Acta Histochem. 2016, 118, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Bounartzi, T.; Messini, C.I.; Dafopoulos, K.; Markandona, R.; Sotiriou, S.; Tzavella, A.; Messinis, I.E. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia 2015, 47, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Bounartzi, T.; Dafopoulos, K.; Anifandis, G.; Messini, C.I.; Koutsonikou, C.; Kouris, S.; Satra, M.; Sotiriou, S.; Vamvakopoulos, N.; Messinis, I.E. Pregnancy prediction by free sperm DNA and sperm DNA fragmentation in semen specimens of IVF/ICSI-ET patients. Hum. Fertil. 2016, 19, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sá, R.; Cunha, M.; Rocha, E.; Barros, A.; Sousa, M. Sperm DNA fragmentation is related to sperm morphological staining patterns. Reprod. Biomed. Online 2015, 31, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Bucar, S.; Gonçalves, A.; Rocha, E.; Barros, A.; Sousa, M.; Sá, R. DNA fragmentation in human sperm after magnetic-activated cell sorting. J. Assist. Reprod. Genet. 2015, 32, 147–154. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| No of samples | 66 |
| Age (years) | 40.21 ± 6.1 |
| BMI (kg/mL) | 28.65 ± 3.47 |
| Semen Volume (mL) | 3.33 ± 1.42 |
| Sperm concentration (106/mL) | 49.61 ± 50.9 |
| PRM (%) | NPM (%) | IM (%) | |
|---|---|---|---|
| Control (0 h) a | 53.54 ± 16.43 | 13.57 ± 7.93 | 32.61 ± 15.85 |
| Control (1 h) b | 46.42 ± 16.19 | 12.21 ± 9.22 | 41.35 ± 15.33 |
| Roundup (1 h) c | 35.26 ± 15.21 | 10.17 ± 7.68 | 54.89 ± 17.42 |
| Control (3 h) a | 36.86 ± 13.42 | 11.09 ± 9.87 | 52.05 ± 13.7 |
| Roundup (3 h) c | 30.53 ± 11.67 | 9.17 ± 8.72 | 60.47 ± 15.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.I.; et al. The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics 2018, 6, 2. https://doi.org/10.3390/toxics6010002
Anifandis G, Amiridis G, Dafopoulos K, Daponte A, Dovolou E, Gavriil E, Gorgogietas V, Kachpani E, Mamuris Z, Messini CI, et al. The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics. 2018; 6(1):2. https://doi.org/10.3390/toxics6010002
Chicago/Turabian StyleAnifandis, George, George Amiridis, Konstantinos Dafopoulos, Alexandros Daponte, Eleni Dovolou, Eleftherios Gavriil, Vyron Gorgogietas, Elli Kachpani, Zissis Mamuris, Christina I. Messini, and et al. 2018. "The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria" Toxics 6, no. 1: 2. https://doi.org/10.3390/toxics6010002
APA StyleAnifandis, G., Amiridis, G., Dafopoulos, K., Daponte, A., Dovolou, E., Gavriil, E., Gorgogietas, V., Kachpani, E., Mamuris, Z., Messini, C. I., Vassiou, K., & Psarra, A.-M. G. (2018). The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics, 6(1), 2. https://doi.org/10.3390/toxics6010002