Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD
Abstract
:1. Overview
2. Prevalence of Biomass Smoke Exposure
3. Biomass Smoke as a Toxic Air Pollutant
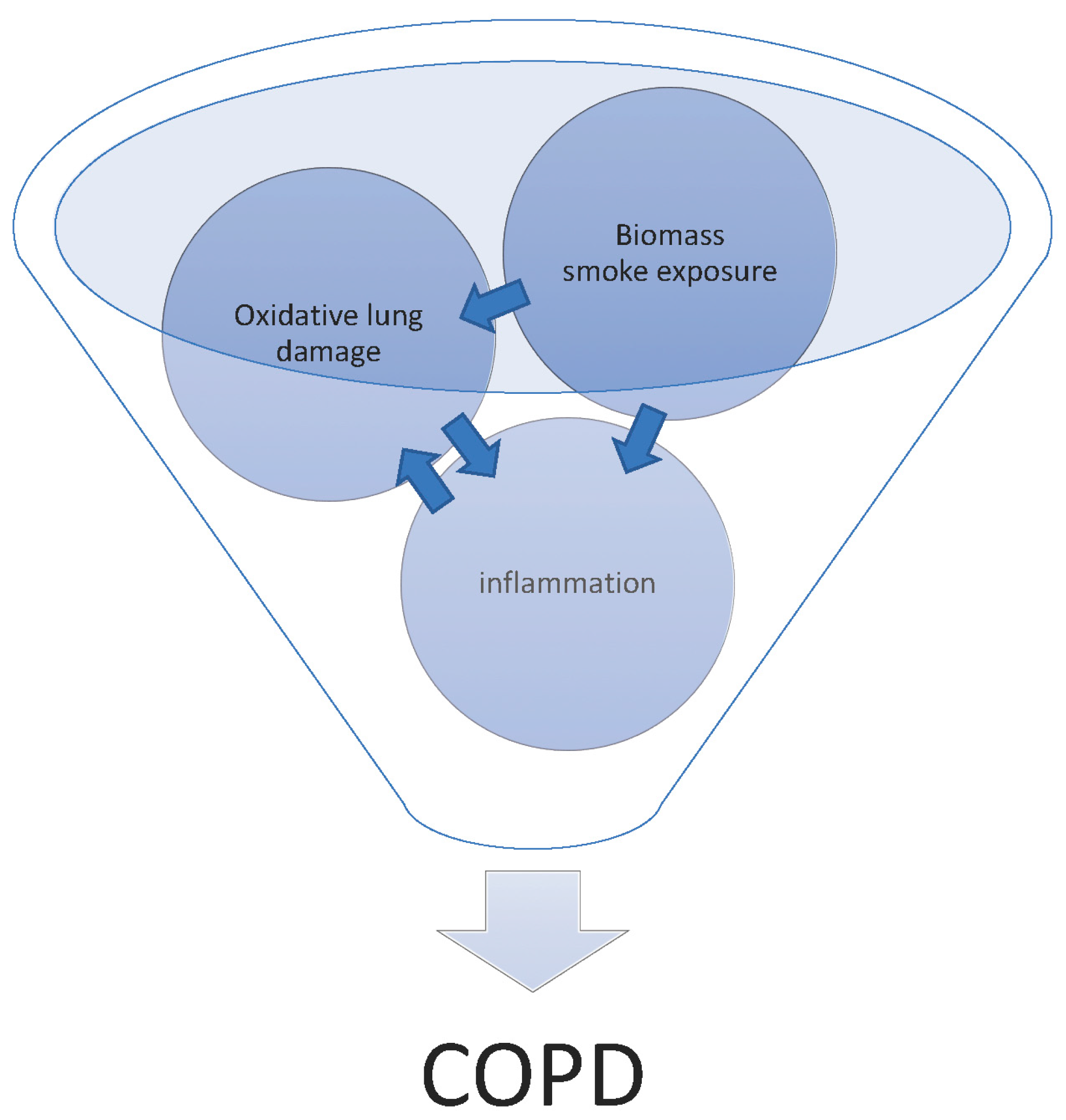
4. Biomass Smoke-Induced COPD
5. Life-Long Exposure to Biomass Smoke in COPD Patients
6. How Does Biomass Smoke Exposure Contribute to the Development of COPD?
6.1. In Vitro Studies
6.2. Animal Studies
6.3. Controlled Human Exposure
7. Is Oxidative Damage the Major Mechanism of Biomass Smoke Induced COPD?
8. Other Potential Mechanisms
9. Biomass as a Risk Factor for COPD Exacerbations
10. How Should In Vitro and In Vivo Models of Biomass Smoke Induced COPD Be Carried Out?
11. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ito, K.; Ito, M.; Elliott, W.M.; Cosio, B.; Caramori, G.; Kon, O.M.; Barczyk, A.; Hayashi, S.; Adcock, I.M.; Hogg, J.C.; et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005, 352, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Brehm, J.M.; Manichaikul, A.; Cho, M.H.; Boutaoui, N.; Yan, Q.; Burkart, K.M.; Enright, P.L.; Rotter, J.I.; Petersen, H.; et al. A genome-wide association study of chronic obstructive pulmonary disease in hispanics. Ann. Am. Thorac. Soc. 2015, 12, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.T.; Elliott, J.E.; Laurie, S.S.; Beasley, K.M.; Gust, C.E.; Mangum, T.S.; Gladstone, I.M.; Duke, J.W. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann. Am. Thorac. Soc. 2014, 11, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Decramer, M.; Janssens, W.; Miravitlles, M. Chronic obstructive pulmonary disease. Lancet 2012, 379, 1341–1351. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ge, Q.; Tjin, G.; Alkhouri, H.; Deng, L.; Brandsma, C.A.; Adcock, I.; Timens, W.; Postma, D.; Burgess, J.K.; et al. Effects of cigarette smoke extract on human airway smooth muscle cells in COPD. Eur. Respir. J. 2014, 44, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Robertoni, F.S.Z.; Olivo, C.R.; Lourenço, J.D.; Gonçalves, N.G.; Velosa, A.P.P.; Lin, C.J.; Fló, C.M.; Saraiva-Romanholo, B.M.; Sasaki, S.D.; Martins, M.A.; et al. Collagenase mrna overexpression and decreased extracellular matrix components are early events in the pathogenesis of emphysema. PLoS ONE 2015, 10, e0129590. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; Simpson, J.L.; Gibson, P.G. Innate immune responses are increased in chronic obstructive pulmonary disease. PLoS ONE 2011, 6, e18426. [Google Scholar] [CrossRef] [PubMed]
- Page, C.; O’Shaughnessy, B.; Barnes, P. Pathogenesis of COPD and asthma. In Pharmacology and Therapeutics of Asthma and COPD; Springer: New York, NY, USA, 2017; pp. 1–21. [Google Scholar]
- Wedzicha, J.A. The heterogeneity of chronic obstructive pulmonary disease. Thorax 2000, 55, 631. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Vargas, C.; Cano, I.; Selivanov, V.; Barreiro, E.; Maier, D.; Falciani, F.; Wagner, P.; Cascante, M.; Garcia-Aymerich, J.; et al. Chronic obstructive pulmonary disease heterogeneity: Challenges for health risk assessment, stratification and management. J. Transl. Med. 2014, 12, S3. [Google Scholar] [CrossRef] [PubMed]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Barnes, P.J. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest 2010, 138, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J., 2nd; Glass, R.I.; Balbus, J.M.; Collins, F.S. A major environmental cause of death. Science 2011, 334, 180–181. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Indoor Air Pollution and Health; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Apte, K.; Salvi, S. Household air pollution and its effects on health. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Bank, T.W. Household Cookstoves, Environment, Health and Climate Change: A New Look at an Old Problem; The Wrold Bank: Washington, DC, USA, 2011. [Google Scholar]
- Rehfuess, E.; Mehta, S.; Prüss-Üstün, A. Assessing household solid fuel use: Multiple implications for the millennium development goals. Environ. Health Perspect. 2006, 114, 373. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ray, S.; Ganesan, K.; Aklin, M.; Cheng, C.-Y.; Urpelainen, J. Council on energy, environment and water. In Access to Clean Cooking Energy and Electricity: Survey of States in India (ACCESS); Council on Energy, Environment and Water: New Delhi, India, 2016; Volume 1. [Google Scholar]
- Fullerton, D.G.; Bruce, N.; Gordon, S.B. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, O.P.; Lam, K.B.; Ayres, J.G. Indoor air pollution and the lung in low-and medium-income countries. Eur. Respir. J. 2012, 40, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.B.; Bruce, N.G.; Grigg, J.; Hibberd, P.L.; Kurmi, O.P.; Lam, K.B.; Mortimer, K.; Asante, K.P.; Balakrishnan, K.; Balmes, J.; et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014, 2, 823–860. [Google Scholar] [CrossRef]
- Smith, K.R.; Bruce, N.; Balakrishnan, K.; Adair-Rohani, H.; Balmes, J.; Chafe, Z.; Dherani, M.; Hosgood, H.D.; Mehta, S.; Pope, D.; et al. Millions dead: How do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu. Rev. Public Health 2014, 35, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Sussan, T.E.; Ingole, V.; Kim, J.-H.; McCormick, S.; Negherbon, J.; Fallica, J.; Akulian, J.; Yarmus, L.; Feller-Kopman, D.; Wills-Karp, M.; et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. Am. J. Respir. Cell Mol. Biol. 2014, 50, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Krimmer, D.; Ichimaru, Y.; Burgess, J.; Black, J.; Oliver, B. Exposure to biomass smoke extract enhances fibronectin release from fibroblasts. PLoS ONE 2013, 8, e83938. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, C.A.; Tang, Y.; Momen, M.; Makambi, K.; Radwan, G.N.; Aboul-Foutoh, A. Pm2.5 as a marker of exposure to tobacco smoke and other sources of particulate matter in Cairo, Egypt. Int. J. Tuberc. Lung Dis. 2016, 20, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duque, C.; Maldonado, D.; Perez-Padilla, R.; Ezzati, M.; Viegi, G.; Forum of International Respiratory Studies Task Force on Health Effects of Biomass Exposure. Biomass fuels and respiratory diseases: A review of the evidence. In Proceedings of the American Thoracic Society, New York, NY, USA, 3 April 2008; Volume 5, pp. 577–590. [Google Scholar]
- World Health Organization; Joint United Nations Programme on HIV/AIDS. Air Quality Guidelines: Global Update 2005; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Environmental Issues: Energy Use and Conservation, March 2011; Australian Bureau of Statistics: Canberra, Australia, 2017.
- Saffari, A.; Daher, N.; Samara, C.; Voutsa, D.; Kouras, A.; Manoli, E.; Karagkiozidou, O.; Vlachokostas, C.; Moussiopoulos, N.; Shafer, M.M.; et al. Increased biomass burning due to the economic crisis in greece and its adverse impact on wintertime air quality in thessaloniki. Environ. Sci. Technol. 2013, 47, 13313–13320. [Google Scholar] [CrossRef] [PubMed]
- Rogalsky, D.K.; Mendola, P.; Metts, T.A.; Martin, W.J. Estimating the number of low-income americans exposed to household air pollution from burning solid fuels. Environ. Health Perspect. 2014, 122, 806. [Google Scholar] [CrossRef] [PubMed]
- Reisen, F.; Brown, S.K. Australian firefighters’ exposure to air toxics during bushfire burns of autumn 2005 and 2006. Environ. Int. 2009, 35, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H.; Hanigan, I.C.; Henderson, S.B.; Morgan, G.G. Evaluation of interventions to reduce air pollution from biomass smoke on mortality in launceston, australia: Retrospective analysis of daily mortality, 1994–2007. BMJ 2013, 346, e8446. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.P.; Lakshmanan, K. Using levoglucosan as a molecular marker for the long-range transport of biomass combustion aerosols. Environ. Sci. Technol. 2000, 34, 4560–4564. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Schauer, J.J.; Nolte, C.G.; Oros, D.R.; Elias, V.O.; Fraser, M.P.; Rogge, W.F.; Cass, G.R. Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles. Atmos. Environ. 1999, 33, 173–182. [Google Scholar] [CrossRef]
- Timothy, V.L.; Jane, Q.K. A Summary of the Emissions Characterization and Noncancer Respiratory Effects of Wood Smoke; Departments of Civil Engineering and Environmental Health University: Washington, WA, USA, 1993. [Google Scholar]
- Hu, G.; Zhou, Y.; Tian, J.; Yao, W.; Li, J.; Li, B.; Ran, P. Risk of COPD from exposure to biomass smoke: A metaanalysis. Chest 2010, 138, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Mehta, S.; Maeusezahl-Feuz, M. Indoor air pollution room household use of solid fuels. In Comparative Quantification of Health Risks. Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; Ezzati, M., Lopez, A.D., Rogers, A., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004; pp. 1453–1493. [Google Scholar]
- Balmes, J.R. When smoke gets in your lungs. Proc. Am. Thorac. Soc. 2010, 7, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Dherani, M.; Pope, D.; Mascarenhas, M.; Smith, K.R.; Weber, M.; Bruce, N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: A systematic review and meta-analysis. Bull. World Health Organ. 2008, 86, 390C–398C. [Google Scholar]
- Perez-Padilla, R.; Schilmann, A.; Riojas-Rodriguez, H. Respiratory health effects of indoor air pollution. Int. J. Tuberc. Lung Dis. 2010, 14, 1079–1086. [Google Scholar] [PubMed]
- Rivera, R.M.; Cosio, M.G.; Ghezzo, H.; Salazar, M.; Perez-Padilla, R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int. J. Tuberc. Lung Dis. 2008, 12, 972–977. [Google Scholar] [PubMed]
- Kim, Y.J.; Jung, C.Y.; Shin, H.W.; Lee, B.K. Biomass smoke induced bronchial anthracofibrosis: Presenting features and clinical course. Respir. Med. 2009, 103, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, S.H.; Im, S.A.; Kim, Y.K.; Lee, K.Y. CT differentiation of anthracofibrosis from endobronchial tuberculosis. AJR Am. J. Roentgenol. 2008, 191, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Zuk, M.; Rojas, L.; Blanco, S.; Serrano, P.; Cruz, J.; Angeles, F.; Tzintzun, G.; Armendariz, C.; Edwards, R.D.; Johnson, M.; et al. The impact of improved wood-burning stoves on fine particulate matter concentrations in rural mexican homes. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Riojas-Rodriguez, H.; Marron-Mares, A.T.; Schilmann, A.; Perez-Padilla, R.; Masera, O. Improved biomass stove intervention in rural mexico: Impact on the respiratory health of women. Am. J. Respir. Crit. Care Med. 2009, 180, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Raiyani, C.V.; Jani Jp Fau-Desai, N.M.; Desai Nm Fau-Shah, S.H.; Shah Sh Fau-Shah, P.G.; Shah Pg Fau-Kashyap, S.K.; Kashyap, S.K. Assessment of indoor exposure to polycyclic aromatic hydrocarbons for urban poor using various types of cooking fuels. Bull. Environ. Contam. Toxicol. 1993, 50, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, C.; Rao, G.U. Emission factors of carbon monoxide and size-resolved aerosols from biofuel combustion. Environ. Sci. Technol. 2001, 35, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Togo, S.; Holz, O.; Fau-Liu, X.; Liu, X.; Fau-Sugiura, H.; Sugiura, H.; Fau-Kamio, K.; Kamio, K.; Fau-Wang, X.; Wang, X.; et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am. J. Respir. Crit. Care Med. 2008, 178, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Happo, M.S.; Kelz, J.; Brunner, T.; Hakulinen, P.; Mäki-Paakkanen, J.; Hukkanen, A.; Jokiniemi, J.; Obernberger, I.; Hirvonen, M.-R. In vitro toxicological characterization of particulate emissions from residential biomass heating systems based on old and new technologies. Atmos. Environ. 2012, 50, 24–35. [Google Scholar] [CrossRef]
- Rylance, J.; Fullerton, D.G.; Scriven, J.; Aljurayyan, A.N.; Mzinza, D.; Barrett, S.; Wright, A.K.; Wootton, D.G.; Glennie, S.J.; Baple, K. Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am. J. Resp. Cell Mol. Biol. 2015, 52, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Happo, M.S.; Hirvonen, M.-R.; Uski, O.; Kasurinen, S.; Kelz, J.; Brunner, T.; Obernberger, I.; Jalava, P.I. Particulate emissions from modern and old technology wood combustion induce distinct time-dependent patterns of toxicological responses in vitro. Toxicol. In Vitro 2017, 44, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Fidan, F.; Unlu, M.; Sezer, M.; Sahin, O.; Tokyol, C.; Esme, H. Acute effects of environmental tobacco smoke and dried dung smoke on lung histopathology in rabbits. Pathology 2006, 38, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Campen, M.J.; Gigliotti, A.P.; Harrod, K.S.; McDonald, J.D.; Seagrave, J.C.; Mauderly, J.L.; Seilkop, S.K. Health effects of subchronic exposure to environmental levels of hardwood smoke. Inhal. Toxicol. 2006, 18, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli-Rocha, F.; Oliveira, V.R.; Barcellos, B.C.; Moreira, D.K.M.; Saldiva, P.H.N.; Faffe, D.S.; Zin, W.A. Time-dependency of mice lung recovery after a 4-week exposure to traffic or biomass air pollutants. Resp. Physiol. Neurobiol. 2016, 230, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Mehra, D.; Geraghty, P.M.; Hardigan, A.A.; Foronjy, R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS ONE 2012, 7, e52889. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.R.; Wang, C.Y.; Lai, C.J. Role of vagal afferents in the acute ventilatory responses to inhaled wood smoke in rats. J. Appl. Physiol. 1995, 78, 2070–2078. [Google Scholar] [PubMed]
- Hu, G.; Zhou, Y.; Hong, W.; Tian, J.; Hu, J.; Peng, G.; Cui, J.; Li, B.; Ran, P. Development and systematic oxidative stress of a rat model of chronic bronchitis and emphysema induced by biomass smoke. Exp. Lung Res. 2013, 39, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, F.; Liao, B.; Zhou, Y.; Li, B.; Ran, P. Exposure to ambient particulate matter alters the microbial composition and induces immune changes in rat lung. Resp. Res. 2017, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.H.; Lai, Y.L.; Kou, Y.R. Acetylcholine and tachykinin receptor antagonists attenuate wood smoke-induced bronchoconstriction in guinea pigs. Eur. J. Pharmacol. 1998, 360, 175–183. [Google Scholar] [CrossRef]
- Granados-Castro, L.F.; Rodríguez-Rangel, D.S.; Montaño, M.; Ramos, C.; Pedraza-Chaverri, J. Wood smoke exposure induces a decrease in respiration parameters and in the activity of respiratory complexes i and iv in lung mitochondria from guinea pigs. Environ. Toxicol. 2015, 30, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Hamdouchi, C.; de Blas, J.; del Prado, M.; Gruber, J.; Heinz, B.A.; Vance, L. 2-amino-3-substituted-6-[(e)-1-phenyl-2-(n-methylcarbamoyl)vinyl]imid azo[1,2-a]pyridines as a novel class of inhibitors of human rhinovirus: Stereospecific synthesis and antiviral activity. J. Med. Chem. 1999, 42, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.A.; Shortnacy, A.T.; Arnett, G.; Shannon, W.M. 2-substituted derivatives of 9-alpha-d-arabinofuranosyladenine and 9-alpha-d-arabinofuranosyl-8-azaadenine. J. Med. Chem. 1977, 20, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, C.T.; Mauderly, J.L. Biomass smoke exposures: Toxicology and animal study design. Inhal. Toxicol. 2010, 22, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Muala, A.; Rankin, G.; Sehlstedt, M.; Unosson, J.; Bosson, J.A.; Behndig, A.; Pourazar, J.; Nyström, R.; Pettersson, E.; Bergvall, C.; et al. Acute exposure to wood smoke from incomplete combustion-indications of cytotoxicity. Part. Fibre Toxicol. 2015, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Soukup, J.M.; Case, M.; Dailey, L.A.; Richards, J.; Berntsen, J.; Devlin, R.B.; Stone, S.; Rappold, A. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup. Environ. Med. 2012, 69, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Burchiel, S.W.; Lauer, F.T.; MacKenzie, D.; McClain, S.; Kuehl, P.J.; McDonald, J.D.; Harrod, K.S. Changes in hpbmc markers of immmune function following controlled short-term inhalation exposures of humans to hardwood smoke. Inhal. Toxicol. 2016, 28, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Karottki Dg Fau-Christensen, J.M.; Christensen Jm Fau-Bonlokke, J.H.; Bonlokke Jh Fau-Sigsgaard, T.; Sigsgaard, T.; Fau-Glasius, M.; Glasius M Fau-Loft, S.; Loft S Fau-Moller, P.; Moller, P. Biomarkers of oxidative stress and inflammation after wood smoke exposure in a reconstructed viking age house. Environ. Mol. Mutagen. 2014, 55, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, R.; Bozinovski, S.; Jones, J.E.; Powell, J.; Gras, J.; Lilja, A.; Hansen, M.J.; Gualano, R.C.; Irving, L.; Anderson, G.P. Differential protease, innate immunity, and nf-kappab induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L931–L945. [Google Scholar] [CrossRef] [PubMed]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J.; et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, O.P.; Dunster, C.; Fau-Ayres, J.G.; Ayres Jg Fau-Kelly, F.J.; Kelly, F.J. Oxidative potential of smoke from burning wood and mixed biomass fuels. Free Radic Res. 2013, 47, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Van Berlo, D.; Wessels, A.; Boots, A.W.; Wilhelmi, V.; Scherbart, A.M.; Gerloff, K.; van Schooten, F.J.; Albrecht, C.; Schins, R.P. Neutrophil-derived ros contribute to oxidative DNA damage induction by quartz particles. Free Radic Biol. Med. 2010, 49, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Mondal, N.K.; Das, D.; Ray, M.R. Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation 2012, 35, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Dutta, A.; Roychoudhury, S.; Ray, M.R. Chronic inhalation of biomass smoke is associated with DNA damage in airway cells: Involvement of particulate pollutants and benzene. J. App. Toxicol. 2013, 33, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Roychoudhury, S.; Chowdhury, S.; Ray, M.R. Changes in sputum cytology, airway inflammation and oxidative stress due to chronic inhalation of biomass smoke during cooking in premenopausal rural indian women. Int. J. Hyg. Environ. Health 2013, 216, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Ray, M.R.; Banerjee, A. Systemic inflammatory changes and increased oxidative stress in rural indian women cooking with biomass fuels. Toxicol. App. Pharmacol. 2012, 261, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Kocyigit, A.; Gencer, M.; Aksoy, N.; Selek, S. Increased DNA damage in patients with chronic obstructive pulmonary disease who had once smoked or been exposed to biomass. Resp. Med. 2006, 100, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Sehlstedt, M.; Dove, R.; Boman, C.; Pagels, J.; Swietlicki, E.; Löndahl, J.; Westerholm, R.; Bosson, J.; Barath, S.; Behndig, A.F. Antioxidant airway responses following experimental exposure to wood smoke in man. Part. Fibre Toxicol. 2010, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Montaño, M.; Cisneros, J.; Ramírez-Venegas, A.; Pedraza-Chaverri, J.; Mercado, D.; Ramos, C.; Sansores, R.H. Malondialdehyde and superoxide dismutase correlate with fev1 in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal. Toxicol. 2010, 22, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, O.; Arinola, G.O.; Ana, G.R.; Wiskel, T.; Huo, D.; Olopade, O.I.; Olopade, C.O. Relationship between household air pollution from biomass smoke exposure, and pulmonary dysfunction, oxidant-antioxidant imbalance and systemic inflammation in rural women and children in nigeria. Glob. J. Health Sci. 2013, 5, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kathir, K.; Dennis, J.M.; Croft, K.D.; Mori, T.A.; Lau, A.K.; Adams, M.R.; Stocker, R. Equivalent lipid oxidation profiles in advanced atherosclerotic lesions of carotid endarterectomy plaques obtained from symptomatic type 2 diabetic and nondiabetic subjects. Free Radic. Biol. Med. 2010, 49, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Barregard, L.; Sällsten, G.; Gustafson, P.; Andersson, L.; Johansson, L.; Basu, S.; Stigendal, L. Experimental exposure to wood-smoke particles in healthy humans: Effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal. Toxicol. 2006, 18, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Commodore, A.A.; Zhang, J.J.; Chang, Y.; Hartinger, S.M.; Lanata, C.F.; Mäusezahl, D.; Gil, A.I.; Hall, D.B.; Aguilar-Villalobos, M.; Vena, J.E. Concentrations of urinary 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in women exposed to woodsmoke in a cookstove intervention study in san marcos, peru. Environ. Int. 2013, 60, 112–122. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.E.; Duffney, P.F.; Wyatt, J.D.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Comparison of in vitro toxicological effects of biomass smoke from different sources of animal dung. Toxicol. In Vitro 2017, 43, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Wong, M.; McIntyre, D. Characterization of macrophage subpopulations responsive to activation by endotoxin and lymphokines. J. Immunol. 1981, 126, 2474. [Google Scholar] [PubMed]
- Hansel, N.N.; McCormack, M.C.; Belli, A.J.; Matsui, E.C.; Peng, R.D.; Aloe, C.; Paulin, L.; Williams, D.L.; Diette, G.B.; Breysse, P.N. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.L.; Anderson, H.R.; Bremner, S.A.; Marston, L.; Seemungal, T.A.; Strachan, D.P.; Wedzicha, J.A. Outdoor air pollution and respiratory health in patients with COPD. Thorax 2011, 66, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Naess, O.; Nafstad, P.; Aamodt, G.; Claussen, B.; Rosland, P. Relation between concentration of air pollution and cause-specific mortality: Four-year exposures to nitrogen dioxide and particulate matter pollutants in 470 neighborhoods in oslo, norway. Am. J. Epidemiol. 2007, 165, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Loft, S.; Sorensen, M.; Tjonneland, A.; Overvad, K.; Raaschou-Nielsen, O. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: A cohort study. Am. J. Respir. Crit. Care Med. 2011, 183, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H.; Purdie, S.; Jalaludin, B.; Martin, K.L.; Henderson, S.B.; Morgan, G.G. Air pollution events from forest fires and emergency department attendances in sydney, australia 1996–2007: A case-crossover analysis. Environ. Health 2014, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Hanigan, I.C.; Morgan, G.G.; Henderson, S.B.; Johnston, F.H. Air pollution from bushfires and their association with hospital admissions in sydney, newcastle and wollongong, australia 1994–2007. Aust. N. Z. J. Public Health 2013, 37, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Sheppeard, V.; Khalaj, B.; Ayyar, A.; Lincoln, D.; Jalaludin, B.; Beard, J.; Corbett, S.; Lumley, T. Effects of bushfire smoke on daily mortality and hospital admissions in sydney, australia. Epidemiology 2010, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Widdicombe, J.H. Regulation of the depth and composition of airway surface liquid. J. Anat. 2002, 201, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.A.; Caramori, G.; Casolari, P.; Papi, A.A.; Edwards, M.; Shamji, B.; Triantaphyllopoulos, K.; Hussain, F.; Pinart, M.; Khan, Y.; et al. Oxidative stress-induced antibodies to carbonyl-modified protein correlate with severity of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 184, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Matthew, E.; Warden, G.; Dedman, J. A murine model of smoke inhalation. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 280, L716–L723. [Google Scholar] [PubMed]
- Bellmann, B.; Creutzenberg, O.; Ernst, H.; Muhle, H. Rat inhalation test with particles from biomass combustion and biomass co-firing exhaust. J. Phys. Conf. Ser. 2009, 151, 12067. [Google Scholar] [CrossRef]
- Vlahos, R.; Bozinovski, S. Recent advances in pre-clinical mouse models of COPD. Clin. Sci. 2014, 126, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Mauderly, J.L. Effect of inhaled toxicants on pulmonary function. In Concepts in Inhalation Toxicology; McClellan, R.O., Ed.; Hemisphere Publishing Corporation: New York, NY, USA, 1989; pp. 347–402. [Google Scholar]
| Pollutant | Physical State | Emissions for g/kg Wood |
|---|---|---|
| Carbon Monoxide | vapour | 80–370 |
| Methane | vapour | 14–25 |
| VOCs (C2–C7) | vapour | 7–27 |
| Substituted Furans | vapour | 0.15–1.7 |
| vapour | ||
| Benzene | vapour | 0.6–4.0 |
| vapour | ||
| Alkyl Benzenes (including toluene) | vapour | 1–6 |
| Aldehydes (including Formaldehyde, Acrolein, Propionaldehyde, Butryaldehyde, Acetaldehyde, Furfural) | 0.6–5.4 | |
| Acetic Acid | vapour | 1.8–2.4 |
| Formic Acid | vapour | 0.06–0.08 |
| Nitrogen Oxides (NO,NO2) | vapour | 0.2–0.9 |
| Sulfur Dioxide | vapour | 0.16–0.24 |
| Methyl chloride | vapour | 0.01–0.04 |
| Napthalene | vapour | 0.24–1.6 |
| Substituted Napthalenes | vapour/particulate | 0.3–2.1 |
| Total Particle Mass | particulate | 7–30 |
| particulate | ||
| Particulate Organic Carbon | particulate | 2–20 |
| particulate | ||
| Oxygenated PAHs | vapour/particulate | 0.15–1 |
| Oxygenated Monoaromatics (including Guaiacol (and derivatives), Phenol (and derivatives), Syringol (and derivatives), and Catechol (and derivatives) | 1–7 | |
| PAHs (including Fluorene, Benzo(e)pyrene), Chrysene | vapour particulate | <1 g |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capistrano, S.J.; Van Reyk, D.; Chen, H.; Oliver, B.G. Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD. Toxics 2017, 5, 36. https://doi.org/10.3390/toxics5040036
Capistrano SJ, Van Reyk D, Chen H, Oliver BG. Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD. Toxics. 2017; 5(4):36. https://doi.org/10.3390/toxics5040036
Chicago/Turabian StyleCapistrano, Sarah J., David Van Reyk, Hui Chen, and Brian G. Oliver. 2017. "Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD" Toxics 5, no. 4: 36. https://doi.org/10.3390/toxics5040036
APA StyleCapistrano, S. J., Van Reyk, D., Chen, H., & Oliver, B. G. (2017). Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD. Toxics, 5(4), 36. https://doi.org/10.3390/toxics5040036








