Concentrations of Polybrominated Diphenyl Ethers (PBDEs) in Water from Asunle Stream, Ile-Ife, Nigeria
Abstract
:1. Introduction
2. Materials and Methods
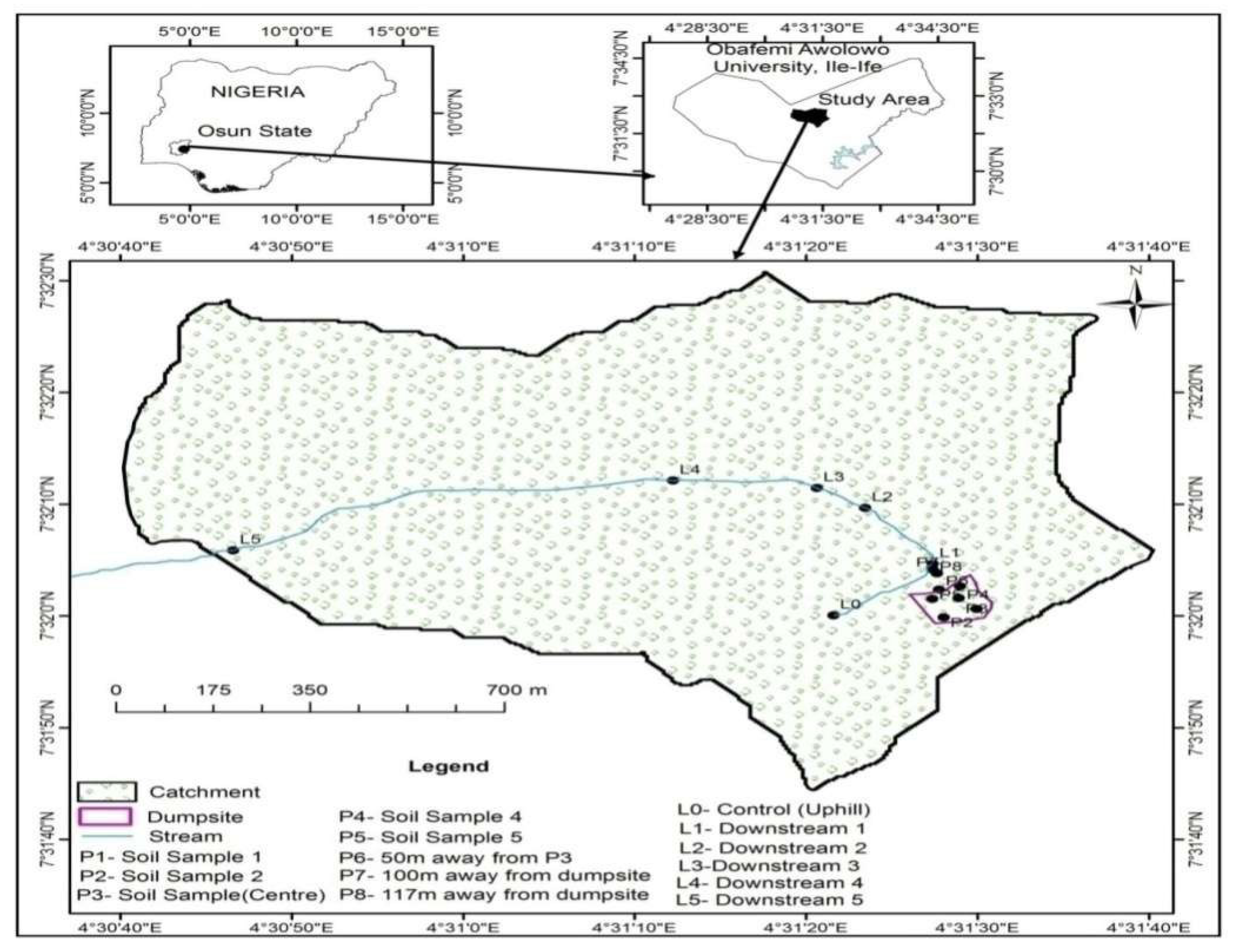
2.1. Study Area
2.2. Reagents and Chemicals
2.3. Sample Collection, Preparation, and Instrumental Analysis
2.4. Statistical Analysis of the Data
3. Results and Discussion
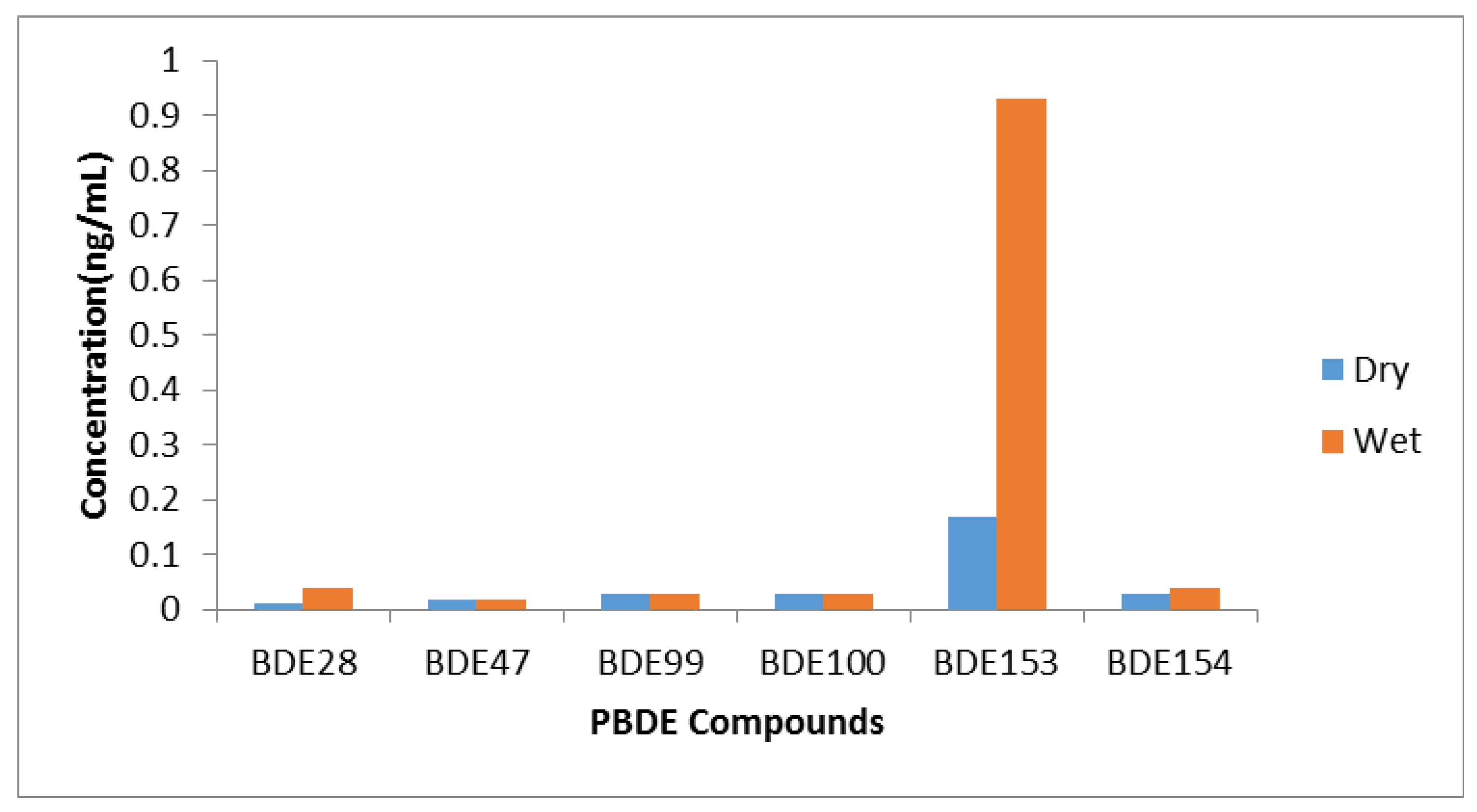
3.1. Concentrations of PBDEs in Stream Water
3.2. Comparison of PBDE Levels in Water Samples with Levels in Various Countries of the World
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheung, K.C.; Poon, B.H.T.; Lan, C.Y.; Wong, M.H. Assessment of metal and nutrient concentrations in River Waternand sediment collected from the cities in the Pearl River Delta, South China. Chemosphere 2003, 52, 1431–1440. [Google Scholar] [CrossRef]
- Guo, W.; He, M.; Yang, Z.; Lin, C.; Quan, X.; Wang, H. Distribution of polycyclicaromatic hydrocarbons in water suspended particulate matters and sediment from Daliao River Watershed, China. Chemosphere 2007, 68, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Daso, A.P.; Fatoki, O.S.; Odendaal, J.P. Occurrence of polybrominated diphenyl ethers (PBDEs) and 2,2’,4,4’,5,5’-hexabromobiphenyl (BB-153) in water samples from the Diep River, Cape Town, South Africa. Environ. Sci. Pollut. Res. 2013, 20, 5168–5176. [Google Scholar] [CrossRef] [PubMed]
- Odebunmi, E.O.; Olutona, G.O.; Akintunde, E.A.; Oluwaniyi, O.O. Characteristics and quality assessment of drinking water in some major towns of Osun State, southwestern Nigeria. Niger. Soc. Exp. Biol. 2014, 14, 51–55. [Google Scholar]
- American Water Works Association (AWWA). Water Quality Treatment, 3rd ed.; McGrawhill Book Company: London, UK, 1971. [Google Scholar]
- Akindele, E.O.; Olutona, G.O. Water physico-chemistry and zooplankton fauna of Aiba reservoir headwaters stream, Iwo, Nigeria. J. Ecosyst. 2014, 2014, 11. [Google Scholar] [CrossRef]
- De Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Frederiksen, M.; Vorkamp, K.; Thomsen, M.; Knudsen, L.E. Human Internalandexternal exposure to PBDEs—A review of levels and sources. Int. Hyg. Environ. Health 2009, 212, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Alaee, M.; Eljarrat, E.; Barcel, D. Introduction to brominated flame retardants: Commercially productions, applications and physicochemical properties. In The Handbook of Environmental Chemistry, Vol 16: Brominated Flame Retardants; Ethel, E., Damia, B., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2011. [Google Scholar]
- Covaci, A.; Voorspoels, S.; de Boer, J. Determination of brominated flame retardants, with emphasis on polybrominated diphenyl ethers (PBDEs) in environmental and human samples—A review. Environ. Int. 2003, 29, 735–756. [Google Scholar] [CrossRef]
- Sudaryanto, K.N.; Takahashi, S.; Muawanah, T.S. Geographical distribution and accumulation features of PBDEs in human breast milk from Indonesia. Environ. Pollut. 2008, 151, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Olutona, G.O.; Oyekunle, J.A.O.; Ogunfowokan, A.O.; Fatoki, O.S. Assessment of polybrominated diphenyl ethers in sediment of Asunle Stream of the Obafemi Awolowo University, Ile-Ife, Nigeria. Environ. Sci. Pollut. Res. 2016, 23, 21195–21205. [Google Scholar] [CrossRef] [PubMed]
- De Wit, C.A.; Alaee, M.; Muir, D.C. Levels and trends of brominated flame retardants in the Arctic. Chemosphere 2006, 64, 209–233. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O.; Eriksen, G.S.; Johannesson, T.; Larsen, P.B.; Viluksela, M. Polybrominated diphenyl ethers: Occurrence, dietary exposure and toxicology. Environ. Health Perspect. 2001, 109, 49–68. [Google Scholar] [CrossRef] [PubMed]
- William, R.J.; Jurgens, M.D.; Johnson, A.C. Initial predictions of the concentrations and distributions of 17- oestradiol, oestrone and ethinyl oestradiol in three English rivers. Water Res. 1999, 33, 1663. [Google Scholar] [CrossRef]
- Adewuyi, G.O.; Adeleye, A.O. Evaluation of polybrominated diphenyl ethers in sedimentoflagos Lagoon, Nigeria. Afr. Environ. Sci. Technol. 2013, 7, 686–693. [Google Scholar]
- Bodin, N.; N’Gom Ka, R.; Le Loc’h, F.; Raffray, J.; Budzinski, H.; Peluhet, L.; Tito de, M. Are exploited mangrove molluscs exposed to persistent organic pollutant contamination in Senegal, West Africa? Chemosphere 2011, 84, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Olukunle, O.; Okonkwo, J.; Odusanya, O. Accelerated Solvent Extraction of Common Polybrominated Diphenyl Ethers from River Sediment; Tshwane University of Technology and Department of Water Affairs: Tshwane, South Africa, 2011; pp. 1–9. [Google Scholar]
- Gonzalez, M.; Miglioranza, K.S.B.; Grondona, S.I.; Barni, M.F.S.; Martineze, D.E.; Pena, A. Organic pollutant levels in an agricultural watershed:the importance of analyzing multiple matrices for assessing streamwater pollution. Environ. Sci. Processes Impacts 2013, 15, 739–750. [Google Scholar] [CrossRef]
- Wepener, V.; Smith, N.; Covaci, A. Seasonal bioaccumulation of organohalogens inTigerfish Hydrocynus vittatus Castelnau, from Lake Pongolapoort, South Africa. Bull. Environ. Contam. Toxicol. 2012, 88, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Law, K.; Halldorson, T.; Danell, R.; Stern, G.; Gewurtz, S.; Alaee, M. Bioaccumulation and trophic transfer of some brominated flame retardants in a lake Winnipeg (Canada) food web. Environ. Toxicol. Chem. 2006, 25, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Kulalowski, P. Priority and hazardous substances in water environment. Results of analytical methods implementation on the base of the research being carried out within the framework of the project p10302. Tarnow 2009. [Google Scholar]
- Windi, B. Environmental threats of natural water contamination with polybrominated diphenylethers (PBDE). Pollut. Environ. Stud. 2015, 24, 47–55. [Google Scholar]
- Helleday, T.; Tuominen, K.L.; Bergman, A.; Jenssen, D. Brominated flame retardant induce intragenic recombination in mammalian cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 439, 137–147. [Google Scholar] [CrossRef]
- Lana, N.B.; Fontana, A.R.; Ciocco, N.F.; Altamirano, J.C. Determination of polybrominated diphenylethers in water samples from Mendoza River Basin by HS-SPME-GC-MS/MS. Rev. FCA UNCuyo. 2010, 42, 85–98. [Google Scholar]
- Kavlock, R.J.; Datston, G.P.; DeRosa, C.; Fenner-Crisp, P.; Gray, L.E.; Kaattari, S.; Lucier, G.; Luster, M.; Mac, M.J.; Maczka, C.; et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the US EPA- sponsored workshop. Environ. Health Persp. 1996, 104, 715–740. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Ward, T.R. Differential effects of commercial polybrominated diphenylether and polychlorinated biphenyls mixtures on intracellular signaling in rat brain in vitro. Toxicol. Sci. 2005, 85, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R.S.; Derr-Yellin, E.C. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on [H-3] arachidonic acid release in rat cerebellar granules neurons. Toxicol. Sci. 2002, 68, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ikonomou, M.G.; Rayne, S.; Addison, R.F. Exponential increase of the brominated flame retardants, polybrominated diphenylethers in the Canadian Arctic from 1981 to 2000. Environ. Sci. Technol. 2002, 36, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Ogunfowokan, A.O.; Oyekunle, J.A.O.; Olutona, G.O.; Atoyebi, A.O.; Lawal, A. Speciation study of heavy metals in water and sediment from Asunle River of the Obafemi Awolowo University, Ile-Ife, Nigeria. Int. Environ. Protect. 2013, 3, 6–16. [Google Scholar]
- Hallgreen, S.; Sinjari, T.; Hakansson, H.; Darnerud, P. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch. Toxicol. 2001, 75, 200–208. [Google Scholar] [CrossRef]
- Hale, R.C.; La Guardia, M.J.; Harvey, E.; Gaylor, M.O.; Mainor, T.M. Brominated flame retardant concentrations and trends in abiotic media. Chemosphere 2006, 64, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Streets, S.S.; Henderson, S.A.; Stoner, A.D.; Carlson, D.L.; Simcik, M.F.; Swackhamer, D.L. Partitioning and bioaccumulation of PBDEs and PCBs in lake Michigan. Environ. Sci. Technol. 2006, 40, 7263–7269. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.; Cousins, I.T.; Mackay, D.; Tysklind, M.; Metcalfe, C.; Alaee, M. Assessingthe environmental fate of chemicals of emerging concern: A case study of the polybrominated diphenyl ethers. Environ. Pollut. 2002, 117, 195–213. [Google Scholar] [CrossRef]
- Oros, D.R.; Hoover, D.; Rodigari, F.; Crane, D.; Sericano, J. Levels and distribution of polybrominated diphenyl ethers in water, surface sediment and bivalves from the San Francisco Estuary. Environ. Sci. Technol. 2005, 39, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat, E.; Marsh, G.; Labandeira, A.; Barcelo, D. Effects of sewage sludge contaminated with polybrominated diphenyl ethers on agricultural soils. Chemosphere 2008, 71, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Voorspoels, S.; Covaci, A.; Neels, H.; Schepens, P. Dietary PBDE intake: A Market- Basket study in Belgium. Environ. Intern. 2007, 33, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, S.; Christe, P. Plants uptake and dissipation of PBDEs in the soils of electronic waste recycling sites. Environ. Pollut. 2011, 159, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Booij, K.; Zegers, B.N.; Boon, J.P. Levels of some polybrominated diphenyl ethers (PBDEs) flame retardants along the Dutch Coast as derived from their accumulation in SPMDs and Blue Massels (Mytilus edulis). Chemosphere 2002, 46, 683–688. [Google Scholar] [CrossRef]
- North, K. Tracking polybrominated diphenyl ethers releases inawastewater treatment plant effluent, Palo Alto, Califonia. Environ. Sci. Technol. 2004, 38, 4484–4486. [Google Scholar] [CrossRef] [PubMed]
- Luckey, F.; Fowler, B.; Litten, S. Establishing baseline levels of polybrominated dipheynl ethers in Lake Ontario surface waters. In Proceedings of the Second International Workshop on Brominated Flame Retardants, the Swedish Chemical Society, Stockholm, Sweden, 2001. [Google Scholar]
- Guan, Y.F.; Wang, J.Z.; Zeng, E.Y. Riverine inputs of polybrominated diphenyl ethers from de Pearl River Delta (China) to the coastal ocean. Environ. Sci. Technol. 2007, 41, 6007–6013. [Google Scholar] [CrossRef] [PubMed]
- Odusanya, D.O.; Okonkwo, J.O.; Botha, B. Polybrominated diphenyls in leachates from selected landfill sites in South Africa. Waste Manag. 2009, 29, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Daso, A.P.; Fatoki, O.S.; Odendaal, J.P. Development of analytical procedures for the simultaneous determination of tri-to heptabrominated diphenyl ethers and hexabrominated biphenyl (BB153) in sediment samples. Water SA 2011, 37, 331–337. [Google Scholar] [CrossRef]
- Moon, H.B.; Choi, M.; Yu, J.; Jung, R.H.; Choi, H.G. Contamination and potential sources of polybrominated diphenyl ethers (PBDEs) in water and sediment from the artificial lake Shihwa, Korea. Chemosphere 2012, 88, 837–843. [Google Scholar] [CrossRef] [PubMed]
| Analytes | BDE28 | BDE 47 | BDE100 | BDE99 | BDE153 | BDE154 | BDE77 | PCNB |
|---|---|---|---|---|---|---|---|---|
| Retention time (min) | 14.72 | 17.37 | 19.29 | 19.90 | 21.38 | 22.18 | 18.74 | 7.13 |
| Quantitative ions (m/z) | 405 | 487 | 404 | 404 | 485 | 485 | 485 | 294 |
| Qualifier ions (m/z) | 405, 247, 246 | 487, 327, 325 | 405, 137, 137 | 404, 297, 295 | 485, 483, 295 | 485, 485, 376 | 405, 325, 246 | 264, 248, 236, 176 |
| Collision energy | 16, 14 | 18, 16 | 30, 30, 10 | 32, 28 | 32, 32, 10 | 32, 10 | NA | NA |
| Month | BDE28 | BDE47 | BDE99 | BDE100 | BDE153 | BDE154 | Σ6PBDE |
|---|---|---|---|---|---|---|---|
| Nov. | Nd | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.01 ± 0.02 | 0.05 ± 0.08 | 0.06 ± 0.03 | 0.03 |
| Jan. | 0.03 ± 0.04 | 0.01 ± 0.02 | 0.05 ± 0.04 | 0.04 ± 0.06 | 0.35 ± 0.45 | 0.02 ± 0.02 | 0.08 |
| Feb. | 0.01 ± 0.02 | 0.02 ± 0.03 | 0.01 ± 0.01 | 0.04 ± 0.04 | 0.12 ± 0.24 | 0.02 ± 0.01 | 0.04 |
| May. | 0.03 ± 0.04 | 0.02 ± 0.02 | 0.03 ± 0.04 | 0.06 ± 0.06 | 0.23 ± 0.34 | 0.02 ± 0.04 | 0.07 |
| Jun. | 0.03 ± 0.05 | 0.03 ± 0.02 | 0.02 ± 0.04 | Nd | 0.79 ± 1.79 * | 0.03 ± 0.04 | 0.18 |
| Jul. | 0.06 ± 0.05 | 0.03 ± 0.02 | 0.04 ± 0.04 | 0.04 ± 0.06 | 2.49 ± 3.76 * | 0.03 ± 0.03 | 0.45 |
| Aug. | 0.03 ± 0.05 | 0.02 ± 0.03 | 0.02 ± 0.04 | 0.01 ± 0.03 | 0.23 ± 0.34 | 0.06 ± 0.02 | 0.06 |
| Location | BDE28 | BDE47 | BDE99 | BDE100 | BDE153 | BDE154 | Σ6PBDEs |
|---|---|---|---|---|---|---|---|
| 0 | 0.01 ± 0.03 | 0.02 ± 0.02 | 0.02 ± 0.04 | 0.01 ± 0.03 | 1.31 ± 3.05 | 0.03 ± 0.04 | 0.23 |
| 1 | 0.05 ± 0.04 | 0.02 ± 0.01 | 0.03 ± 0.04 | 0.04 ± 0.05 | 1.65 ± 2.64 | 0.04 ± 0.04 | 0.31 |
| 2 | 0.02 ± 0.03 | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.05 ± 0.06 | 0.24 ± 0.21 | 0.03 ± 0.02 | 0.07 |
| 3 | 0.04 ± 0.05 | 0.02 ± 0.03 | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.20 ± 0.41 | 0.05 ± 0.03 | 0.07 |
| 4 | 0.02 ± 0.04 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.03 | 0.06 ± 0.07 | 0.05 ± 0.03 | 0.03 |
| 5 | 0.01 ± 0.03 | 0.02 ± 0.02 | 0.04 ± 0.03 | 0.03 ± 0.05 | 0.18 ± 0.31 | 0.02 ± 0.03 | 0.05 |
| BDE28 | BDE47 | BDE99 | BDE100 | BDE153 | BDE154 | |
|---|---|---|---|---|---|---|
| BDE28 | 1.00 | 0.351 * | 0.406 ** | 0.179 | 0.350 * | −0.117 |
| BDE47 | 1.00 | 0.119 | 0.104 | 0.015 | 0.084 | |
| BDE99 | 1.00 | 0.392 * | 0.514 ** | −0.417 ** | ||
| BDE100 | 1.00 | 0.163 | −0.451 ** | |||
| BDE153 | 1.00 | −0.319 * | ||||
| BDE154 | 1.00 |
| Location | ΣPBDEs ( tri-hept) | BDE 47 | BDE 99 | Reference |
|---|---|---|---|---|
| Mendoza River, Argentine | Nd-1.9 b | - | - | [26] |
| Scheldt Estuary and North Sea, Dutch Coast | <0.1–5.6 a | 1 | 0.5 | [40] |
| Waste Water Treatment Plant, California | 29,023 a | 10,467 | 11,200 | [41] |
| Lake Ontario, North America | 4–13 a | 0.11–3.83 | - | [42] |
| San Francisco Estuary, California | 3–513 a | <16.1–179.5 | <13.1–90.7 | [35] |
| Pearl River, South China | 0.344–0.68 b | 3–143 | <1–200 | [43] |
| Leachate, Pretoria, South Africa | 9.79 b | - | - | [44] |
| Diep/Kuils Rivers, South Africa | 0.25–21,200 b | - | - | [45] |
| Lake Shihwa, Korea | 1.1–11(creek) b 0.25–2.1(in shore) b 0.16–0.37 (off shore) b 0.1 | - | - | [46] |
| Asunle Stream, Ile-Ife | 0.03–0.45 a | - | - | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olutona, G.O.; Oyekunle, J.A.O.; Ogunfowokan, A.O.; Fatoki, O. . Concentrations of Polybrominated Diphenyl Ethers (PBDEs) in Water from Asunle Stream, Ile-Ife, Nigeria. Toxics 2017, 5, 13. https://doi.org/10.3390/toxics5020013
Olutona GO, Oyekunle JAO, Ogunfowokan AO, Fatoki O . Concentrations of Polybrominated Diphenyl Ethers (PBDEs) in Water from Asunle Stream, Ile-Ife, Nigeria. Toxics. 2017; 5(2):13. https://doi.org/10.3390/toxics5020013
Chicago/Turabian StyleOlutona, Godwin O., John A.O. Oyekunle, Aderemi O. Ogunfowokan, and Olalekan S. Fatoki. 2017. "Concentrations of Polybrominated Diphenyl Ethers (PBDEs) in Water from Asunle Stream, Ile-Ife, Nigeria" Toxics 5, no. 2: 13. https://doi.org/10.3390/toxics5020013






