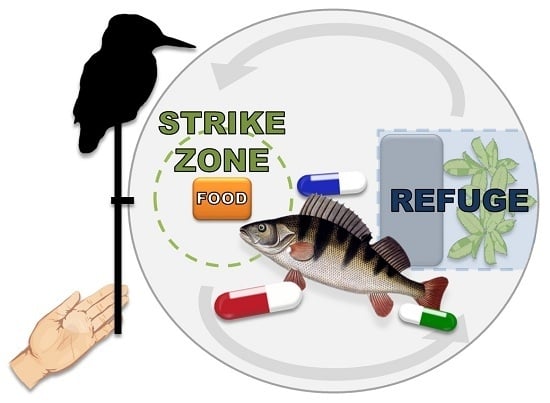
Assessing Potential Vulnerability and Response of Fish to Simulated Avian Predation after Exposure to Psychotropic Pharmaceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Collection
2.2. Chemical Exposures
2.3. Behavioral Assays
2.4. Video and Data Analyses
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SSRI | selective serotonin reuptake inhibitor |
| GABA | gamma-aminobutyric acid |
| Cmax | human therapeutic plasma concentrations |
References
- Barbosa Júnior, A.; Alves, F.L.; Fim Pereira, A.D.S.; Ide, L.M.; Hoffmann, A. Behavioral characterization of the alarm reaction and anxiolytic-like effect of acute treatment with fluoxetine in piauçu fish. Physiol. Behav. 2012, 105, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodin, T.; Fick, J.; Jonsson, M.; Klaminder, J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 2013, 339, 814–815. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, V.; Mehinto, A.C.; Denslow, N.D.; Schlenk, D. Effects of exposure to the β-blocker propranolol on the reproductive behavior and gene expression of the fathead minnow, Pimephales promelas. Aquat. Toxicol. 2012, 116–117, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sackerman, J.; Donegan, J.J.; Cunningham, C.S.; Nguyen, N.N.; Lawless, K.; Long, A.; Benno, R.H.; Gould, G.G. Zebrafish behavior in novel environments: Effects of acute exposure to anxiolytic compounds and choice of Danio rerio line. Int. J. Comp. Psychol. 2010, 23, 43–61. [Google Scholar] [PubMed]
- Valenti, T.W.; Gould, G.G.; Berninger, J.P.; Connors, K.A.; Keele, N.B.; Prosser, K.N.; Brooks, B.W. Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI) sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows. Environ. Sci. Technol. 2012, 46, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.S. Neural mechanisms underlying the evolvability of behaviour. Philos. Trans. R. Soc. B. 2011, 366, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, L.; Jauhiainen, A.; Kristiansson, E.; Nerman, O.; Larsson, D.G.J. Evolutionary conservation of human drug targets in organisms used for environmental risk assessments. Environ. Sci. Technol. 2008, 42, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.M. An endocrine disrupter increases growth and risky behavior in threespined stickleback (Gasterosteus aculeatus). Horm. Behav. 2004, 45, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems—Impacts through behavioural alterations. Philos. Trans. R. Soc. B. 2014, 369, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hedgespeth, M.L.; Nilsson, P.A.; Berglund, O. Ecological implications of altered fish foraging after exposure to an antidepressant pharmaceutical. Aquat. Toxicol. 2014, 151, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the european pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Magnhagen, C.; Magurran, A.E. Decision-making and trade-offs in fish behaviour. In Fish Behaviour; Magnhagen, C., Braithwaite, V.A., Forsgren, E., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 499–523. [Google Scholar]
- Van Straalen, N.M. Biodiversity of ecotoxicological responses in animals. Neth. J. Zool. 1994, 44, 112–129. [Google Scholar] [CrossRef]
- Bean, T.G.; Boxall, A.B.; Lane, J.; Herborn, K.A.; Pietravalle, S.; Arnold, K.E. Behavioural and physiological responses of birds to environmentally relevant concentrations of an antidepressant. Philos. Trans. R. Soc. B. 2014, 369, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.S.; Rattner, B.A.; Brooks, B.W.; Du, B.; McGowan, P.C.; Blazer, V.S.; Ottinger, M.A. Exposure and food web transfer of pharmaceuticals in ospreys (Pandion haliaetus): Predictive model and empirical data. Integr. Environ. Assess. Manag. 2015, 11, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Breed, M.D.; Moore, J. Chapter 2—Neurobiology and endocrinology for animal behaviorists. In Animal Behavior; Breed, M.D., Moore, J., Eds.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Conrad, J.L.; Weinersmith, K.L.; Brodin, T.; Saltz, J.B.; Sih, A. Behavioural syndromes in fishes: A review with implications for ecology and fisheries management. J. Fish Biol. 2011, 78, 395–435. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Makara, G.B.; Kruk, M.R. Catecholaminergic involvement in the control of aggression: Hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci. Biobehav. Rev. 1997, 22, 85–97. [Google Scholar] [CrossRef]
- Mennigen, J.A.; Stroud, P.; Zamora, J.M.; Moon, T.W.; Trudeau, V.L. Pharmaceuticals as neuroendocrine disruptors: Lessons learned from fish on prozac. J. Toxicol. Environ. Health B 2011, 14, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Pereira, A.M.P.T.; Meisel, L.M.; Lino, C.M.; Pena, A. Reviewing the serotonin reuptake inhibitors (SSRIs) footprint in the aquatic biota: Uptake, bioaccumulation and ecotoxicology. Environ. Pollut. 2015, 197, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Trudeau, V.L.; Moon, T.W. B-blockers as endocrine disruptors: The potential effects of human β-blockers on aquatic organisms. J. Exp. Zool. A 2011, 315A, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.F.; Giltrow, E.; Huggett, D.B.; Hutchinson, T.H.; Saye, J.; Winter, M.J.; Sumpter, J.P. Comparative physiology, pharmacology and toxicology of β-blockers: Mammals versus fish. Aquat. Toxicol. 2007, 82, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.; Hui, M.; Li, V.; Lorenzi, V.; de la Paz, N.; Cheng, S.H.; Lai-Chan, L.; Schlenk, D. Effects of propranolol on heart rate and development in Japanese medaka (Oryzias latipes) and zebrafish (Danio rerio). Aquat. Toxicol. 2012, 122–123, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.M.; Moon, T.W. Behavioral and biochemical adjustments of the zebrafish Danio rerio exposed to the β-blocker propranolol. Comp. Biochem. Phys. B 2015. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.M.; Markussen, B.; Baun, A.; Halling-Sørensen, B. Probabilistic environmental risk characterization of pharmaceuticals in sewage treatment plant discharges. Chemosphere 2009, 77, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.F.; Huggett, D.B.; Hutchinson, T.H.; Hetheridge, M.J.; Kinter, L.B.; Ericson, J.F.; Sumpter, J.P. Uptake of propranolol, a cardiovascular pharmaceutical, from water into fish plasma and its effects on growth and organ biometry. Aquat. Toxicol. 2009, 93, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Occurrence of drugs in german sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Johnson, D.J.; Sanderson, H.; Brain, R.A.; Wilson, C.J.; Bestari, K.T.; Solomon, K.R. Exposure assessment and microcosm fate of selected selective serotonin reuptake inhibitors. Regul. Toxicol. Pharm. 2005, 42, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Cook, J.C.; Ericson, J.F.; Williams, R.T. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess. 2003, 9, 1789–1799. [Google Scholar] [CrossRef]
- Boström, M.J.; Ugge, G.; Jönsson, J.A.; Berglund, O. Bioaccumulation and trophodynamics of the antidepressants sertraline and fluoxetine in laboratory-constructed, three-level aquatic food chains. Environ. Toxicol. Chem. submitted. 2016. [Google Scholar]
- Gaworecki, K.M.; Klaine, S.J. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat. Toxicol. 2008, 88, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-blockers in the environment: Part i. Mobility and hydrolysis study. Sci. Total Environ. 2014, 493, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Øverli, Ø.; Pottinger, T.G.; Carrick, T.R.; Øverli, E.; Winberg, S. Differences in behaviour between rainbow trout selected for high-and low-stress responsiveness. J. Exp. Biol. 2002, 205, 391–395. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, 3.1.0; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Winder, V.L.; Pennington, P.L.; Hurd, M.W.; Wirth, E.F. Fluoxetine effects on sheepshead minnow (Cyprinodon variegatus) locomotor activity. J. Environ. Sci. Health B 2012, 47, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Nakamichi, R.; Krause, J.; Butlin, R.K. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio). Behav. Genet. 2006, 36, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Magnhagen, C.; Borcherding, J. Risk-taking behaviour in foraging perch: Does predation pressure influence age-specific boldness? Anim. Behav. 2008, 75, 509–517. [Google Scholar] [CrossRef]
- Wilson, D.S.; Clark, A.B.; Coleman, K.; Dearstyne, T. Shyness and boldness in humans and other animals. Trends Ecol. Evol. 1994, 9, 442–446. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Dzieweczynski, T.L.; Kane, J.L.; Campbell, B.A.; Lavin, L.E. Fluoxetine exposure impacts boldness in female siamese fighting fish, Betta splendens. Ecotoxicology 2016, 25, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.K.; Ramirez, A.J.; Chambliss, C.K.; Brooks, B.W. Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate. Chemosphere 2007, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lu, G.; Li, S.; Nie, Y.; Ma, B.; Liu, J. Behavioral and biochemical responses in freshwater fish Carassius auratus exposed to sertraline. Chemosphere 2015, 135, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.; Zhang, W.; Carter, P.; Shaw, J.; Chernet, E.; Phebus, L.; Wong, D.; Perry, K. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology 2002, 160, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Van Harten, J. Clinical pharmacokinetics of selective serotonin reuptake inhibitors. Clin. Pharmacokinet. 1993, 24, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Kreke, N.; Dietrich, D.R. Physiological endpoints for potential SSRI interactions in fish. Crit. Rev. Toxicol. 2008, 37, 215–247. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Buerkley, M.A.; Julius, M.L.; Vajda, A.M.; Norris, D.O.; Barber, L.B.; Furlong, E.T.; Schultz, M.M.; Schoenfuss, H.L. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2009, 28, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J., II; Klaper, R. Environmental concentrations of the selective serotonin reuptake inhibitor fluoxetine impact specific behaviors involved in reproduction, feeding and predator avoidance in the fish Pimephales promelas (fathead minnow). Aquat. Toxicol. 2014, 151, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Domenici, P. Context-dependent variability in the components of fish escape response: Integrating locomotor performance and behavior. J. Exp. Zool. A 2010, 313A, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J. Fluoxetine inhibits predator avoidance behavior in tadpoles. Toxicol. Environ. Chem. 2014, 96, 641–649. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Fredriksson, S.; Sandblom, E.; Paxeus, N.; Axelsson, M. Is heart rate in fish a sensitive indicator to evaluate acute effects of β-blockers in surface water? Environ. Toxicol. Pharmacol. 2006, 22, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Giltrow, E.; Eccles, P.D.; Winter, M.J.; McCormack, P.J.; Rand-Weaver, M.; Hutchinson, T.H.; Sumpter, J.P. Chronic effects assessment and plasma concentrations of the β-blocker propranolol in fathead minnows (Pimephales promelas). Aquat. Toxicol. 2009, 95, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of select beta adrenergic receptor-blocking pharmaceuticals (b-blockers) on aquatic organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, P.N.; Fernandez, J.D.; Hoffman, A.D.; Butterworth, B.C.; Nichols, J.W. Branchial elimination of superhydrophobic organic compounds by rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2001, 55, 23–34. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Xie, Z.; Zhang, Z.; Li, S.; Yan, Z. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci. Total Environ. 2015, 511, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Eklöv, P.; Diehl, S. Piscivore efficiency and refuging prey: The importance of predator search mode. Oecologia 1994, 98, 344–353. [Google Scholar] [CrossRef]
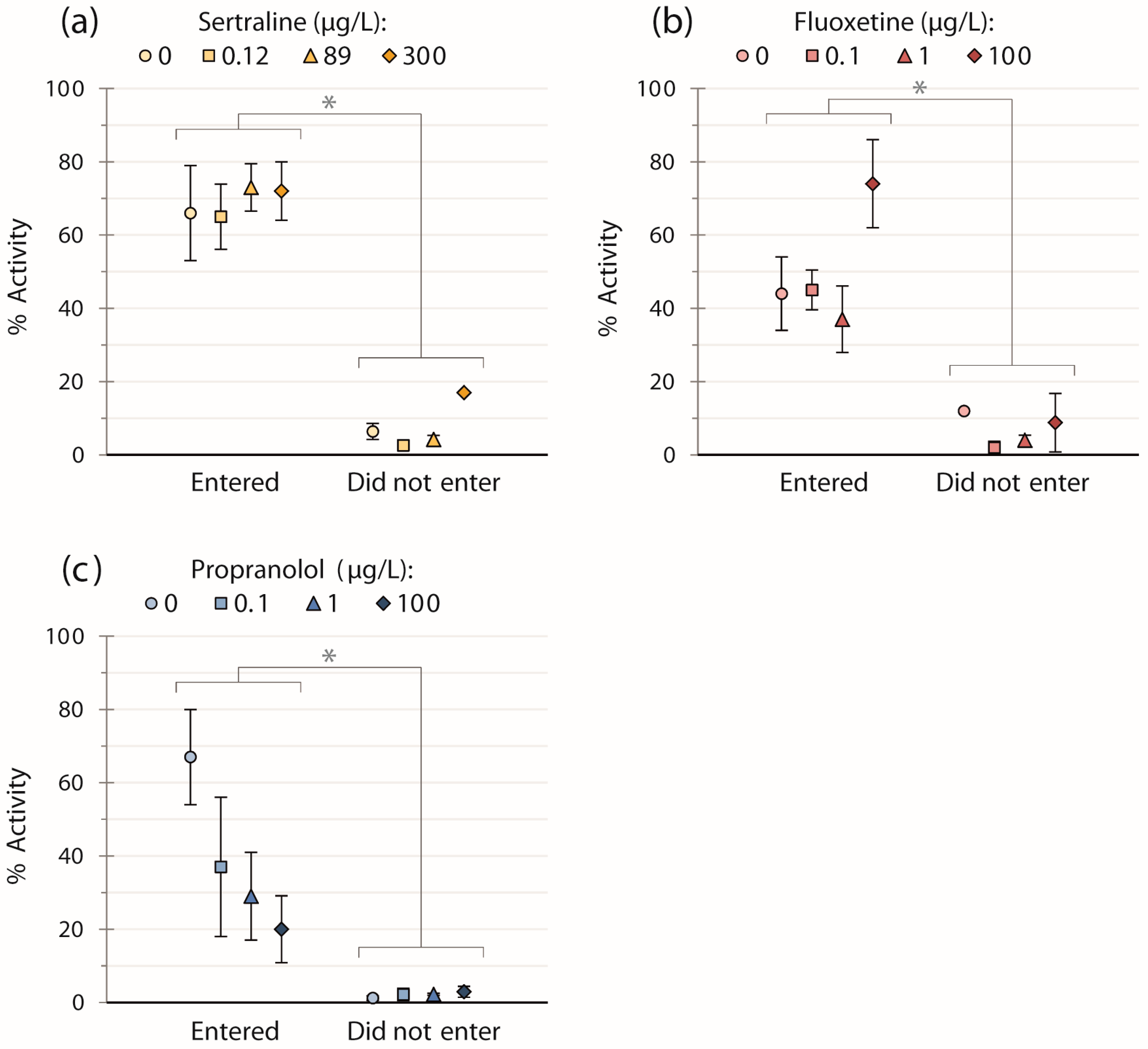
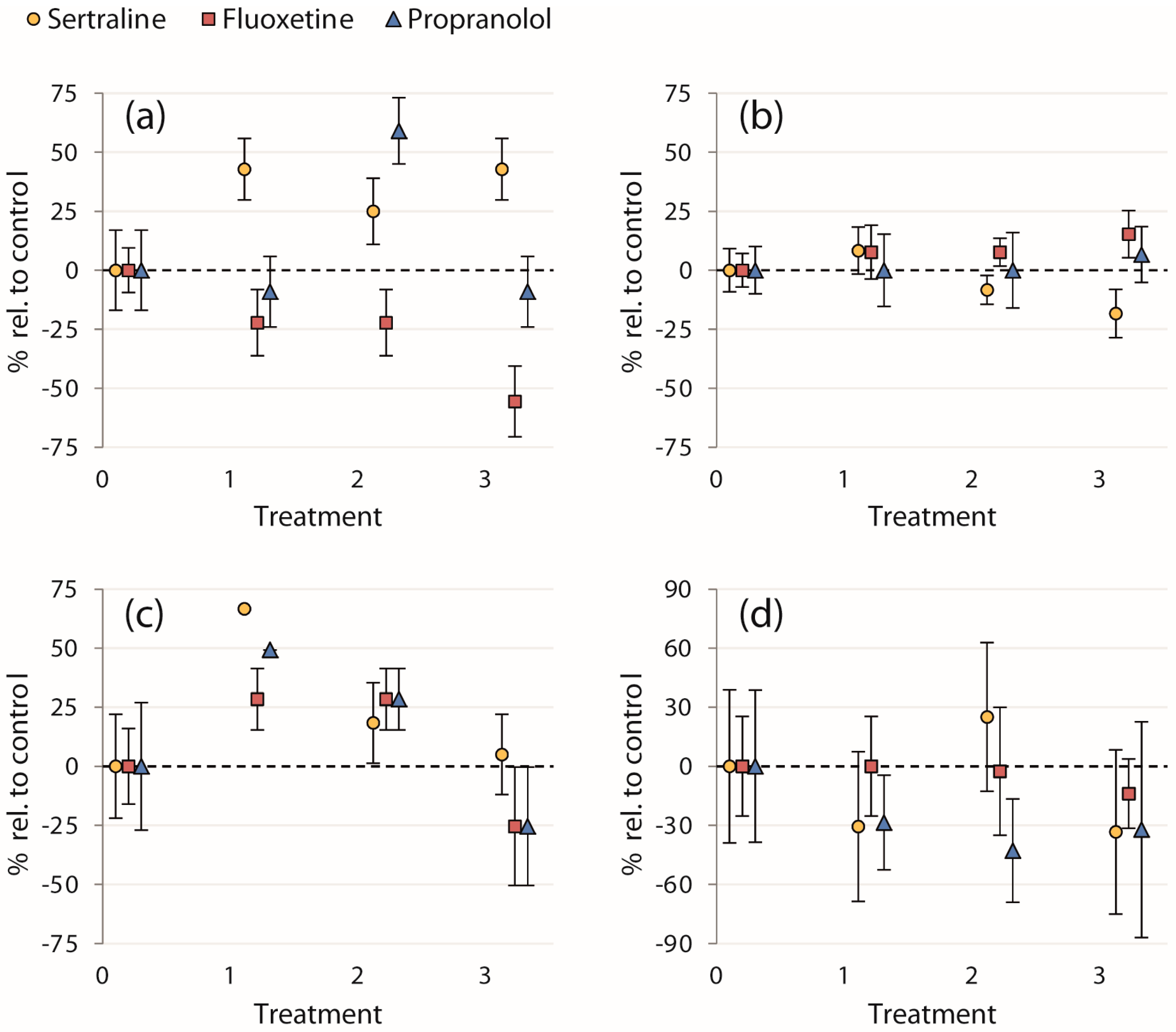
| Chemical | (μg/L) | Proportion Entering Strike Zone (%) | Initial Escape Velocity (BL/s) | Proportion Entering Cover (%) | Time to Reach Cover (s) |
|---|---|---|---|---|---|
| Sertraline | 0 | 56 ± 17 (9) | 12 ± 1.1 (5) | 60 ± 22 (5) | 0.72 ± 0.28 (3) |
| 0.12 | 80 ± 13 (10) | 13 ± 1.3 (8) | 100 ± 0.0020 (8) | 0.50 ± 0.19 (8) | |
| 89 | 70 ± 14 (10) | 11 ± 0.67 (7) | 71 ± 17 (7) | 0.90 ± 0.34 (5) | |
| 300 | 80 ± 13 (10) | 9.8 ± 1.0 (8) | 63 ± 17 (8) | 0.48 ± 0.20 (5) | |
| Fluoxetine | 0 | 90 ± 9.5 (10) | 13 ± 0.93 (9) | 67 ± 16 (9) | 0.79 ± 0.20 (6) |
| 0.1 | 70 ± 14 (10) | 14 ± 1.6 (6) | 86 ± 13 (7) | 0.79 ± 0.20 (6) | |
| 1 | 70 ± 14 (10) | 14 ± 0.83 (7) | 86 ± 13 (7) | 0.77 ± 0.25 (6) | |
| 100 | 40 ± 15 (10) | 15 ± 1.5 (3) | 50 ± 25 (4) | 0.68 ± 0.12 (2) | |
| Propranolol | 0 | 44 ± 17 (9) | 15 ± 1.5 (3) | 67 ± 27 (3) | 1.4 ± 0.54 (2) |
| 0.1 | 40 ± 15 (10) | 15 ± 2.3 (3) | 100 ± 0.0028 (4) | 1.0 ± 0.24 (4) | |
| 1 | 70 ± 14 (10) | 15 ± 2.4 (6) | 86 ± 13 (7) | 0.80 ± 0.21 (6) | |
| 100 | 40 ± 15 (10) | 16 ± 1.9 (4) | 50 ± 25 (4) | 0.95 ± 0.52 (2) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedgespeth, M.L.; Nilsson, P.A.; Berglund, O. Assessing Potential Vulnerability and Response of Fish to Simulated Avian Predation after Exposure to Psychotropic Pharmaceuticals. Toxics 2016, 4, 9. https://doi.org/10.3390/toxics4020009
Hedgespeth ML, Nilsson PA, Berglund O. Assessing Potential Vulnerability and Response of Fish to Simulated Avian Predation after Exposure to Psychotropic Pharmaceuticals. Toxics. 2016; 4(2):9. https://doi.org/10.3390/toxics4020009
Chicago/Turabian StyleHedgespeth, Melanie L., Per Anders Nilsson, and Olof Berglund. 2016. "Assessing Potential Vulnerability and Response of Fish to Simulated Avian Predation after Exposure to Psychotropic Pharmaceuticals" Toxics 4, no. 2: 9. https://doi.org/10.3390/toxics4020009