Risk Mitigation Measures: An Important Aspect of the Environmental Risk Assessment of Pharmaceuticals
Abstract
:1. Introduction
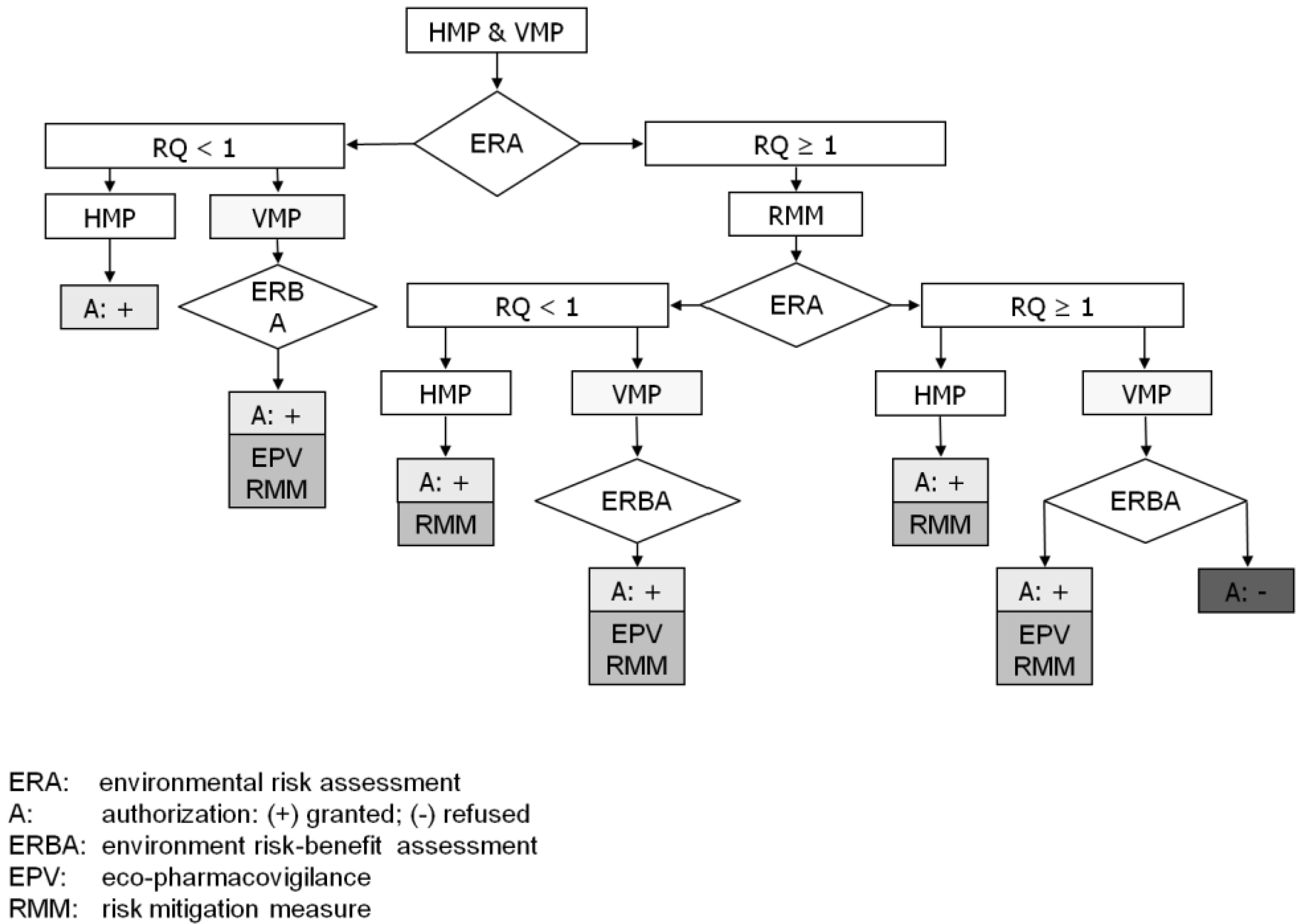
2. Sources of RMMs
2.1. Compilation of Existing RMM from Authorized VMP and Scientific Literature
- The product should not enter surface waters;
- Treated animals should not have access to watercourses;
- The long-term effect of the product on the population dynamics of dung organisms is unknown; therefore, it is advisable not to treat animals on the same pasture every season.
- Short-term measures; e.g., improved disposal and sewage treatment techniques, refusal of the spreading of contaminated manure;
- Mid-term measures; e.g., modified risk perception and risk communication of producers and consumers of medicinal products;
- Long-term measures; e.g., decisions that foster the concept of sustainable pharmacy.
2.2. Derivation of RMM Based on Exposure Models

| PECsoil initial | Predicted environmental concentration in soil | - | (µg kg−1) |
|---|---|---|---|
| D | Daily dose of the active ingredient | From SmPC | (mg kgbw−1 d−1) |
| Ad | Number of days of treatment | From SmPC | (d) |
| BW | Animal body weight | Default [4] | (kgbw) |
| SD | Stocking density | Default [4] | (animal ha−1) |
| Fh | Fraction of herd treated | Between zero and one; default [4] | (--) |
| 1500 | Bulk density of dry soil | [4] | (kg m−3) |
| 10,000 | Area of one hectare | [4] | (m2 ha−1) |
| 0.05 | Depth of penetration into soil | [4] | (m) |
| 1000 | Conversion factor | [4] | (μg mg−1) |
- Reduction of the fraction of herd treated;
- Reduction of the maximum applicable amount of nitrogen per hectare and year;
- Reduction of the fraction of dip entering dirty water;
- Reduction of the spreading rate for dirty water;
- Reduction of the animal turnover rate per place per year in intensively reared animals;
- Reduction of the stocking density per hectare and year in pasture animals;
- Increase of the housing factor per year from 0.5 to one for intensively reared cattle and horses;
- Increase of the depth of penetration of dung into soil (>5 cm).
- Reduction of the highest fraction of the dose excreted in dung in one day;
- Increase of the length of time that manure is stored;
- Increase of the number of spreading events of dung;
- Avoidance of the removal of topically applied ectoparasiticides;
- Avoidance of the release to soil with a low adsorption capacity.
3. Selection of Appropriate RMMs
3.1. Evaluation Criteria for RMM
- Exposure of the drug to the environment is effectively reduced (effectiveness);
- The measure has a long-lasting effect (sustainability);
- The effectiveness of the measure is verifiable (verifiability), e.g., by means of re-assessment of the exposure, taking the measure into account;
- The measure is explicitly directed to the appropriate addressee (addressing);
- Action is in accordance with good agricultural practices (practicality, for VMP);
- The measure is proportionate (proportionality principle);
- The measure is consistent with the relevant law(s) (legitimacy).
3.2. Legal Boundaries for the Application of RMMs
- The problem of the addressee: The addressee of the authorization to market pharmaceuticals is the pharmaceutical industry (marketing authorization holder). If an unacceptable environmental risk was identified within the marketing authorization procedure (i.e., RQ ≥ 1), which could be reduced due to THE RMM (RQ < 1), the marketing authorization holder receives the permission with the condition of THE RMM. However, the addressee of the RMM in practice is, in most cases, not the marketing authorization holder itself, but the user of the VMP. Thus, the RMM restricts the user in its freedom of exercise of profession, e.g., this would be the case for the RMM. “U-22: Animals [animal group] from free-range husbandry must be kept indoors during treatment and x days following treatment.” In Germany, a restriction of the freedom of exercise of profession by the government must be based on an authorization. This is not provided in the European Directive 2001/82/EC [7] and not in the AMG [8]. Thus, in such a case, the RMM is only applicable if another European (German) regulation authorizes the state for such restrictions;
- Principle of proportionality: The restriction of the freedom of exercise of profession by the state due to RMM needs to be proportional, i.e.: (1) the measure needs to be suitable for reaching the goal; (2) a milder measurement to reach the goal is not available; and (3) the measure is reasonable. The principle of proportionality was originally required in the case of restrictions of German fundamental rights by the state. In the meantime, it is also implemented in other regulations and in European legislation [22];
- Periods when fertilization is prohibited;
- Minimum storage capacity for livestock manure (at least six months; use only when the crop needs nutrients) according to national administrative regulations [26] and local ordinances;
- Rules to control the spread of nutrients near water or on slopes, to reduce the risk of contamination, e.g., by immediate incorporation of the applied slurry into the soil and under consideration of nitrogen (N) balance between nitrogen added to the soil (e.g., mineral fertilizer, livestock manure, etc.) and nitrogen removed from the soil in crops. The prevention of excessive levels of nutrients on farmland is a binding principle of the Good Agricultural Practices as laid down in the DüV [25]. A breach of this requirement would be considered as an administrative offence;
- A limit of 170 kg nitrogen per hectare per year (in justified exceptional cases, higher amounts are possible upon application).
4. Proposed RMM for Use within the Authorization of VMP
| Precautions for disposal | |
|---|---|
| D-01 | The user (e.g., veterinarian or livestock owner) has to ensure that any unused product or waste materials derived from the product, such as empty containers, do not contaminate water courses, surface waters or other parts of the environment. Veterinary pharmaceutical products must not be disposed of via sewage, but should be disposed of preferentially via local return systems for hazardous waste. If disposed with household waste, it should be taken care that no misuse of these wastes could occur. |
| D-02 | The user (e.g., veterinarian or livestock owner) has to ensure that any unused product or the rest of the dip do not contaminate water courses, surface waters or other parts of the environment. Dips must not be disposed of via sewage, but should be disposed of via local return systems for hazardous waste. |
| Precautions for use in aquacultures | |
| U-01 | Constraint to the user (fish owner): Prior to the use of the product, a discharge certificate is required from the relevant authority for the release of this product into the aquatic environment. |
| U-02 | Constraint to the user (fish owner): Use only if the flow rate of untreated waters allows for an x-fold dilution of the volume of treated water before discharge into surface waters. Where the appropriate dilution of treated water cannot be achieved, the farm must have a discharge process to limit the release of product into the environment to within the parameters described. This can be achieved by the use of holding tanks and ponds, discharge lagoons and biofilters to clean treated water. Where this applies, the user must monitor the discharge concentration to ensure that the parameters are not exceeded. |
| Precautions for use in intensively reared animals | |
| U-11 | Constraint to the farmer: Before spreading slurry (manure) from treated animals, it has to be stored for at least x days/months. |
| U-12 | Constraint to the farmer: Slurry (manure) from treated animals must not be spread on areas where run-off could occur (slope > 10%). |
| U-13 | Constraint to the farmer: Slurry (manure) from treated animals must only be spread on arable land if it is x-fold diluted with slurry (manure) from untreated animals. |
| U-14 | Constraint to the farmer: When spreading slurry (manure) from treated animals onto arable land, a safety margin of x meters to the water’s edge has to be maintained. |
| U-15 | Constraint to the farmer: When spreading slurry (manure) from treated animals onto arable land, the maximum nitrogen spreading limit must not exceed x kg N ha−1 yr−1. |
| U-16 | Constraint to the farmer: Slurry (manure) from treated animals must only be spread on arable land in x portions of the maximum nitrogen spreading limit with minimum time intervals of y days. |
| U-17 | Constraint to the farmer: Slurry (manure) from treated animals must not be spread on soils with an organic C content < x%. |
| U-18 | Constraint to the farmer: After spreading of slurry (manure) from treated animals, soil must be ploughed to a depth of at least x cm (>5 cm). |
| Precautions for use in pasture animals | |
| U-21 | Constraint to the veterinarian/animal holder: Strategic treatment of stock is only allowed after the fly or dung beetle season in autumn or in early spring. |
| U-22 | Constraint to the animal holder: Animals [animal group] from free-range husbandry must be kept indoor during treatment and x days following treatment. |
| U-23 | Constraint to the animal holder: During treatment and x hours/days following treatment animals [animal group] must be kept away from watercourses. |
| U-24 | Constraint to the animal holder: [Product] is toxic to dung organism (flies, beetles). Therefore, animals [animal group] must not be kept on the same pasture every season. |
| Precautions for use in intensively reared and pasture animals | |
| U-31 | Constraint to the veterinarian/animal holder: Only treat affected animals [animal group] when required. For correct diagnosis and development of an appropriate treatment schedule, a veterinarian should be consulted. Fecal worm (worm egg) counts can be used as an indicator of whether treatment is needed or not. |
| U-32 | Constraint to the user of the product: During the use of the teat dipping or spraying, dripping residues must be collected and disposed of separately (cf. special precautions for disposal, SmPC, Section 6.6). |
| U-33 | Constraint to the farmer: Dirty water must only be spread with a maximum spreading rate of x L (<50,000) ha−1 onto arable land or pastures. |
5. Evaluation of RMMs for HMPs
6. Discussion and Conclusions
Abbreviations
| Ad | number of days of treatment |
| AMG | Arzneimittelgesetz (German Pharmaceuticals Act) |
| BVL | Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (German Federal Office of Consumer Protection and Food Safety) |
| BW | animal body weight |
| D | daily dose of the active ingredient |
| DüV | Düngeverordnung (German Fertilization Ordinance; implementation of the Nitrate Council Directive in German law) |
| EEC | European Economic Community |
| EMA | European Medicines Agency |
| EPAR | European public assessment report |
| EPV | eco-pharmacovigilance |
| ERA | environmental risk assessment |
| ERBA | environmental risk-benefit assessment |
| EU | European Union |
| Fh | fraction of herd treated |
| HMP | human medicinal product |
| PEC | predicted environmental concentration |
| PNEC | predicted no effect concentration |
| RMM | risk mitigation measure |
| RQ | risk quotient |
| SD | stocking density |
| SmPC | summary of product characteristics |
| VICH | International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products |
| VMP | veterinary medicinal product |
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Guideline on Environmental Risk Assessment of Medicinal Products for Human Use; EMEA/CHMP/SWP/4447/00; EMA: London, UK, 2006. [Google Scholar]
- VICH—International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products. Environmental Impact Assessment (EIAs) for Veterinary Medicinal Products (VMPs)—Phase I. VICH GL 6, Ecotoxicity Phase I; Canary Wharf: London, UK, 2000. [Google Scholar]
- VICH—International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products. Environmental Impact Assessment for Veterinary Medicinal Products Phase II Guidance. VICH GL 38, Ecotoxicity Phase II; Canary Wharf: London, UK, 2004. [Google Scholar]
- EMA (European Medicines Agency). Revised Guideline on Environmental Impact Assessment for Veterinary Medicinal Products in Support of the VICH Guidelines GL6 and GL38. Committee for Medicinal Products for Veterinary Use (CVMP), EMEA: London, UK, 2008; EMEA/CVMP/ERA/418282/2005-Rev.1. [Google Scholar]
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Off. J. Eur. Communities 2001, L311, 67–128.
- Liebig, M.; Alonso Fernandez, Á.; Blübaum-Gronau, E.; Boxall, A.; Brinke, M.; Carbonell, G.; Egeler, P.; Fenner, K.; Fernandez, C.; Fink, G.; et al. Environmental risk assessment of ivermectin—A case study. Integr. Environ. Assess. Manag. 2010, 6, 567–587. [Google Scholar] [CrossRef]
- Directive 2001/82/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products. Off. J. Eur. Communities 2001, L311, 1–66.
- AMG (Arzneimittelgesetz; German Pharmaceuticals Act), 2005; Version dated 12 December 2005 (BGBl. I S. 3394); as most recently amended by Article 4, Paragraph 11 of the law of August 7, 2013 (BGBl. I p. 3154).
- Daughton, C.G.; Ruhoy, I.S. The afterlife of drugs and the role of PharmEcovigilance. Drug Saf. 2008, 31, 1069–1082. [Google Scholar]
- Holm, G.; Snape, J.R.; Murray-Smith, R.; Talbot, J.; Taylor, D.; Sörme, P. Implementing ecopharmacovigilance in practice: Challenges and potential opportunities. Drug Saf. 2013, 36, 533–546. [Google Scholar] [CrossRef]
- Directive 2004/27/EC of the European Parliament and of the Council of 31 March 2004 amending Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off. J. 2004, L136, 34–57.
- EC (European Commission). Notice to Applicants-Veterinary Medicinal Products: Volume 6C—Summary of the Product Characteristics, SPC-Pharmaceuticals: Guideline on the Summary of Product Characteristics for Pharmaceutical Veterinary Medicinal Products; DG ENTR/F/2/KK D(2006). Revision 2; European Commission, Enterprise Directorate-General, Consumer Goods, Pharmaceuticals: Brussels, Belgium, July 2006. [Google Scholar]
- EC (European Commission). Notice to Applicants—A Guideline on Summary of Product Characteristics (SmPC); European Commission, Enterprise and Industry Directorate-General: Brussels, Belgium, September 2009. [Google Scholar]
- Montforts, M.H.M.M.; van Rijswick, H.F.M.W.; Udo de Haes, H.A. Legal constraints in EU product labelling to mitigate the environmental risk of veterinary medicines at use. Regul. Toxicol. Pharmacol. 2004, 40, 327–335. [Google Scholar] [CrossRef]
- Marketing authorization procedure (decentralized, centralized, mutual recognition or national): For marketing of a product exclusively in only one country, a national licensing procedure in order to obtain a marketing authorisation for that product might be adequate. In order to gain a marketing authorisation for several EU countries at the same time, the pharmaceutical entrepreneur can initiate a so-called Decentralised Procedure (DCP) or submit an application for Mutual Recognition (MRP). A Centralised Licensing Procedure is necessary in order to receive a marketing authorisation for the entire European Economic Area (EEA). In such procedure, the marketing authorisation for the medicinal product is not granted by a national licensing authority but by the Commission in Brussels. The organisational aspect of such procedures is coordinated by the European Medicines Agency (EMA) in London. For more information about the different marketing authorisation procedures in the European Community please refer to EMA (http://www.ema.europa.eu/ema/).
- Liebig, M.; Floeter, C.; Hahn, T.; Wenzel, A.; Knacker, T. Development of efficient mitigation measures to reduce environmental risks posed by veterinary and human pharmaceuticals. Umweltbundesamt (Federal Environment Agency). Germany, 2011; Final Report, UBA-FB 3709 65 403 (German report with English abstract). [Google Scholar]
- European Medicines Agency. EudraPharm. Available online: http://www.eudrapharm.eu (accessed on 25 January 2014).
- European Medicines Agency. EPAR – European Public Assessment Reports. Available online: http://www.ema.europa.eu (accessed on 25 January 2014).
- Bundesamt für Verbraucherschutz und Lebensmittelsicherheit—The German Federal Office of Consumer Protection and Food Safety. Available online: http://www.bvl.bund.de (accessed on 25 January 2014).
- Council Regulation (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off. J. Eur. Communities 1990, L224, 1–8.
- Adler, N.E.; Koschorreck, J.; Rechenberg, B. Environmental impact assessment and control of pharmaceuticals: The role of environmental agencies. Water Sci. Technol. 2008, 57, 91–97. [Google Scholar] [CrossRef]
- Sachs, M. Grundgesetz. Kommentar, Seit 1996, 6th ed.; Verlag, C.H., Ed.; Beck: München, Germany, 2011. [Google Scholar]
- Kern, K. Die Apotheke im Gewässer. Z. für Umweltrecht 2011, 1, 9–15. [Google Scholar]
- Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources (Nitrate Directive). Off. J. Eur. Communities 1991, L375, 1–8.
- DÜV (Düngeverordnung; Fertiliser Ordinance), 2007. Version dated February 27, 2007 (BGBl. I, p. 221), as most recently amended by Article 18 Paragraph of the law of July 31, 2009 (BGBl. I p. 2585).
- WasgefStAnlV (Verordnung über Anlagen zum Umgang mit wassergefährdenden Stoffen; Ordinance on Installations for the Handling of Substances Hazardous to Water) 2010. Version dated March 31, 2010. BGBl I, 2010, p. 377.
- BBodSchG (Bundes-Bodenschutzgesetz; Federal Soil Protection Act), 1998. Version dated March 17, 1998 (BGBl. I, p. 502), as most recently amended by Article 5, Paragraph 30 of the law of February 24, 2012 (BGBl. I, p. 212).
- Water Framework Directive. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, L327, 1–73. [Google Scholar]
- Groundwater Directive. Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. Off. J. Eur. Union 2006, L372, 19–31.
- Drinking Water Directive. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, L330, 32–54. [Google Scholar]
- TierSchG (Tierschutzgesetz; Animal Health and Welfare Act), 2006. Version dated May18, 2006 (BGBl. I, p. 1206, 1313), as most recently amended by 20 of the law of December 9, 2010 (BGBl. I, p. 1934).
- TierSchNutztV (Tierschutz-Nutztierhaltungsverordnung; Order on the protection of animals and the keeping of production animals, 2006. Version dated August 22, 2006 (BGBl. I, p. 2043), as most recently amended by Article 1 of the ordinance of Oktober 1, 2009 (BGBl. I, p. 3223).
- EC (European Commission). 2009b. Notice to Applicants-Veterinary Medicinal Products: Volume 6C. Guidance on the assessment of environmental risks of veterinary medicinal products. European Commission, Enterprise Directorate-General, June 2009. [Google Scholar]
- Götz, C.W.; Stamm, C.; Fenner, K.; Singer, H.; Schärer, M.; Hollender , J. Targeting aquatic microcontaminants for monitoring: Exposure categorization and application to the Swiss situation. Environ. Sci. Pollut. Res. 2010, 17, 341–354. [Google Scholar] [CrossRef]
- Kase, R.; Eggen, R.I.L.; Junghans, M.; Götz, C.; Hollender, J. Assessment of Micropollutants from Municipal Wastewater-Combination of Exposure and Ecotoxicological Effect Data for Switzerland. In Waste-Water-Evaluation and Management; García Einschlag, F.S., Ed.; Tech Open Science: Rijeka, Croatia, 2011. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liebig, M.; Floeter, C.; Hahn, T.; Koch, W.; Wenzel, A.; Römbke, J. Risk Mitigation Measures: An Important Aspect of the Environmental Risk Assessment of Pharmaceuticals. Toxics 2014, 2, 35-49. https://doi.org/10.3390/toxics2010035
Liebig M, Floeter C, Hahn T, Koch W, Wenzel A, Römbke J. Risk Mitigation Measures: An Important Aspect of the Environmental Risk Assessment of Pharmaceuticals. Toxics. 2014; 2(1):35-49. https://doi.org/10.3390/toxics2010035
Chicago/Turabian StyleLiebig, Markus, Carolin Floeter, Thorsten Hahn, Wolfgang Koch, Andrea Wenzel, and Jörg Römbke. 2014. "Risk Mitigation Measures: An Important Aspect of the Environmental Risk Assessment of Pharmaceuticals" Toxics 2, no. 1: 35-49. https://doi.org/10.3390/toxics2010035





