Can Zymomonas mobilis Substitute Saccharomyces cerevisiae in Cereal Dough Leavening?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Maintenance
2.2. Biomass Production
2.3. Dough Production and Analytical Determinations
- -
- 400 g, inserted into a 1 L graduate cylinder to evaluate the dough volume increase up to 24 h of leavening;
- -
- 25 g, inserted into a double chamber flask connected with a graduate burette filled with acidified water, to evaluate the total amount of CO2 produced during leavening [16];
- -
- The remaining sample was left to leaven into a Becker; samples were taken at appropriate intervals to determine dough pH, microbial counts and to carry out HPLC (high performance liquid chromatography) analysis.
2.4. Evaluation of Dough Volume Increase and Total CO2 Production
2.5. Determination of the Microbial Populations in Doughs
2.6. HPLC Analyses and pH Monitoring
2.7. Statistical Analysis
3. Results and Discussion
3.1. Trials with Glucose Addition into Dough
3.2. Bacterial Association Z. mobilis-L. sanfranciscensis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gobbetti, M. The sourdough microflora: Interactions of lactic acid bacteria and yeasts. Food Sci. Technol. 1998, 9, 267–274. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vrancken, G.; Ravyts, F.; Rimaux, T.; Weckx, S. Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol. 2009, 26, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, C.; D’Anchise, F.; Foschino, R. PCR detection of Lactobacillus sanfranciscensis in sourdough and Panettone baked product. Eur. Food Res. Technol. 2006, 222, 330–335. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci. Technol. 2005, 16, 57–69. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Bello, F.D. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Angioloni, A.; Romani, S.; Gaetano Pinnavaia, G.; Dalla Rosa, M. Characteristics of bread making doughs: Influence of sourdough fermentation on the fundamental rheological properties. Eur. Food Res. Technol. 2006, 222, 54–57. [Google Scholar] [CrossRef]
- Messens, W.; De Vuyst, L. Inhibitory substances produced by Lactobacilli isolated from sourdoughs—A review. Int. J. Food Microbiol. 2002, 72, 31–43. [Google Scholar] [CrossRef]
- Mansueto, P.; Montalto, G.; Pacor, M.L.; Esposito-Pellitteri, M.; Ditta, V.; Lo Bianco, C.; Leto-Barone, S.M.; Di Lorenzo, G. Food allergy in gastroenterologic diseases: Review of literature. World J. Gastroenterol. 2006, 12, 7744–7752. [Google Scholar] [CrossRef] [PubMed]
- Salamati, S.; Martins, C.; Kulseng, B. Baker’s yeast (Saccharomyces cerevisiae) antigen in obese and normal weight subjects. Clin. Obes. 2015, 5, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Perricone, R.; Blank, M.; Perricone, C.; Shoenfeld, Y. Anti-Saccharomyces cerevisiae autoantibodies in autoimmune diseases: From bread baking to autoimmunity. Clin. Rev. Allergy Immunol. 2013, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sicard, D.; Legras, J.-L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. Comptes Rendus Biol. 2011, 334, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Israeli, E.; Grotto, I.; Gilburd, B.; Balicer, R.D.; Goldin, E.; Wiik, A.; Shoenfeld, Y. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut 2005, 54, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Muratori, P.; Muratori, P.; Muratori, L.; Guidi, M.; Maccariello, S.; Pappas, G.; Ferrari, R.; Gionchetti, P.; Campieri, M.; Bianchi, F.B. Anti-Saccharomyces cerevisiae antibodies (ASCA) and autoimmune liver diseases. Clin. Exp. Immunol. 2003, 132, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.A.; Tadros, S.; Bansal, A.S. Beer, Cider, and Wine Allergy. Case Rep. Immunol. 2017, 2017, 7958924. [Google Scholar] [CrossRef] [PubMed]
- Sahm, H.; Bringer-Meyer, S.; Sprenger, G.A. Proteobacteria: Alpha and Beta Subclasses. In The Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Springer: Berlin, Germany, 2006; Volume 5, ISBN 978-0-387-25476-0. [Google Scholar]
- Musatti, A.; Rollini, M.; Sambusiti, C.; Manzoni, M. Zymomonas mobilis: Biomass production and use as a dough leavening agent. Ann. Microbiol. 2015, 65, 1583–1589. [Google Scholar] [CrossRef]
- Musatti, A.; Mapelli, C.; Foschino, R.; Picozzi, C.; Rollini, M. Unconventional bacterial association for dough leavening. Int. J. Food Microbiol. 2016, 237, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-Hernández, R.J.; Rodríguez-Álvarez, J.A.; Valenzuela-Encinas, C.; Gutiérrez-Miceli, F.A.; Castañón-González, H.; Marsch, R.; Ayora-Talavera, T.; Dendooven, L. The bacterial community in “taberna” a traditional beverage of Southern Mexico. Lett. Appl. Microbiol. 2010, 51, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Giles-Gómez, M.; Hernández, G.; Córdova-Aguilar, M.S.; López-Munguía, A.; Gosset, G.; Bolívar, F. Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int. J. Food Microbiol. 2008, 124, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.N.; Ibekwe, V.I.; Anyanwu, B.N. Investigation of some physicochemical and microbial succession parameters of palm wine. J. Food Technol. 2006, 4, 308–312. [Google Scholar]
- Valadez-Blanco, R.; Bravo-Villa, G.; Santos-Sánchez, N.F.; Velasco-Almendarez, S.I.; Montville, T.J. The Artisanal Production of Pulque, a Traditional Beverage of the Mexican Highlands. Probiotics Antimicrob. Proteins 2012, 4, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Vogelmann, S.A.; Hertel, C. Impact of ecological factors on the stability of microbial associations in sourdough fermentation. Food Microbiol. 2011, 28, 583–589. [Google Scholar] [CrossRef] [PubMed]
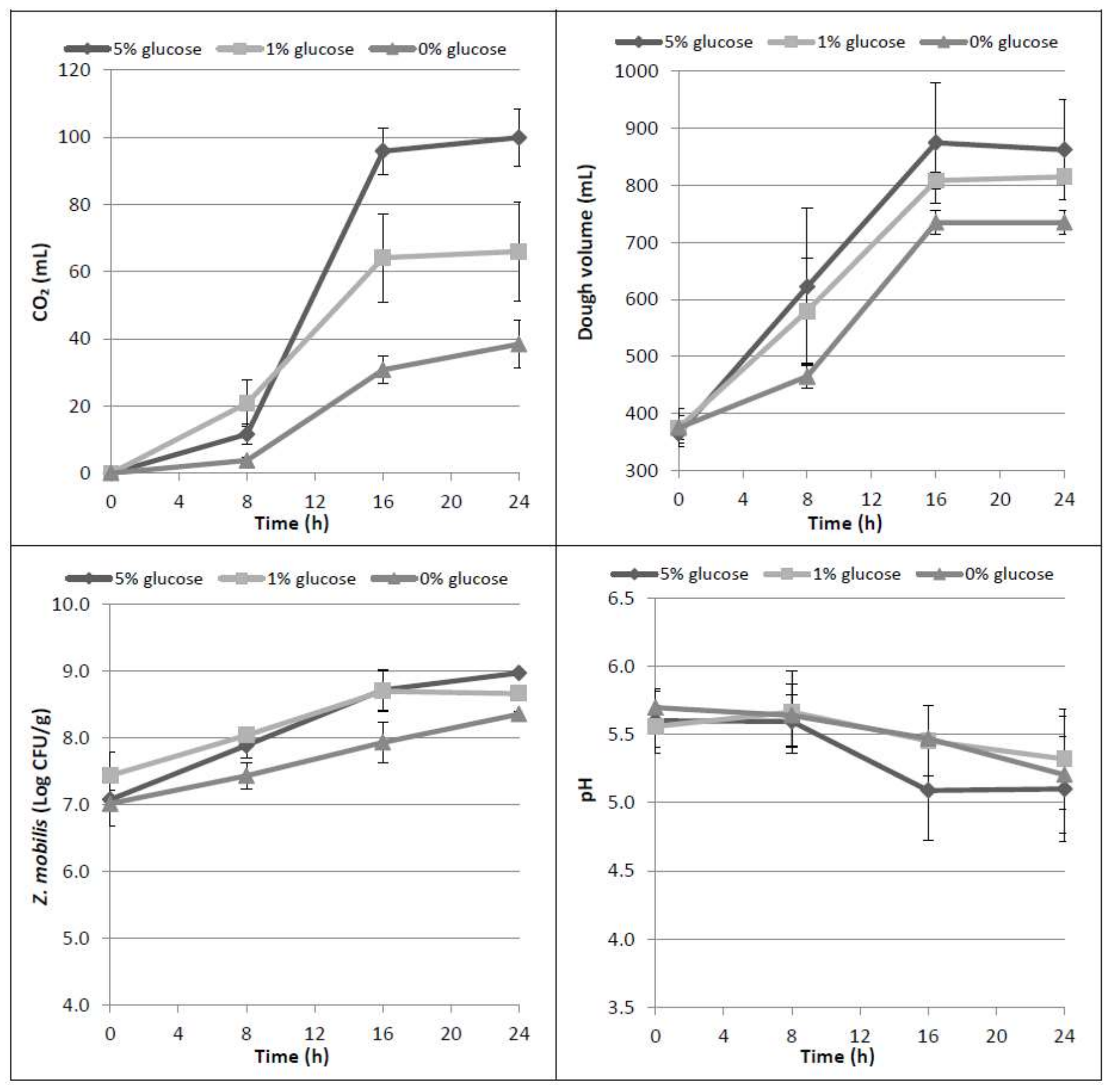
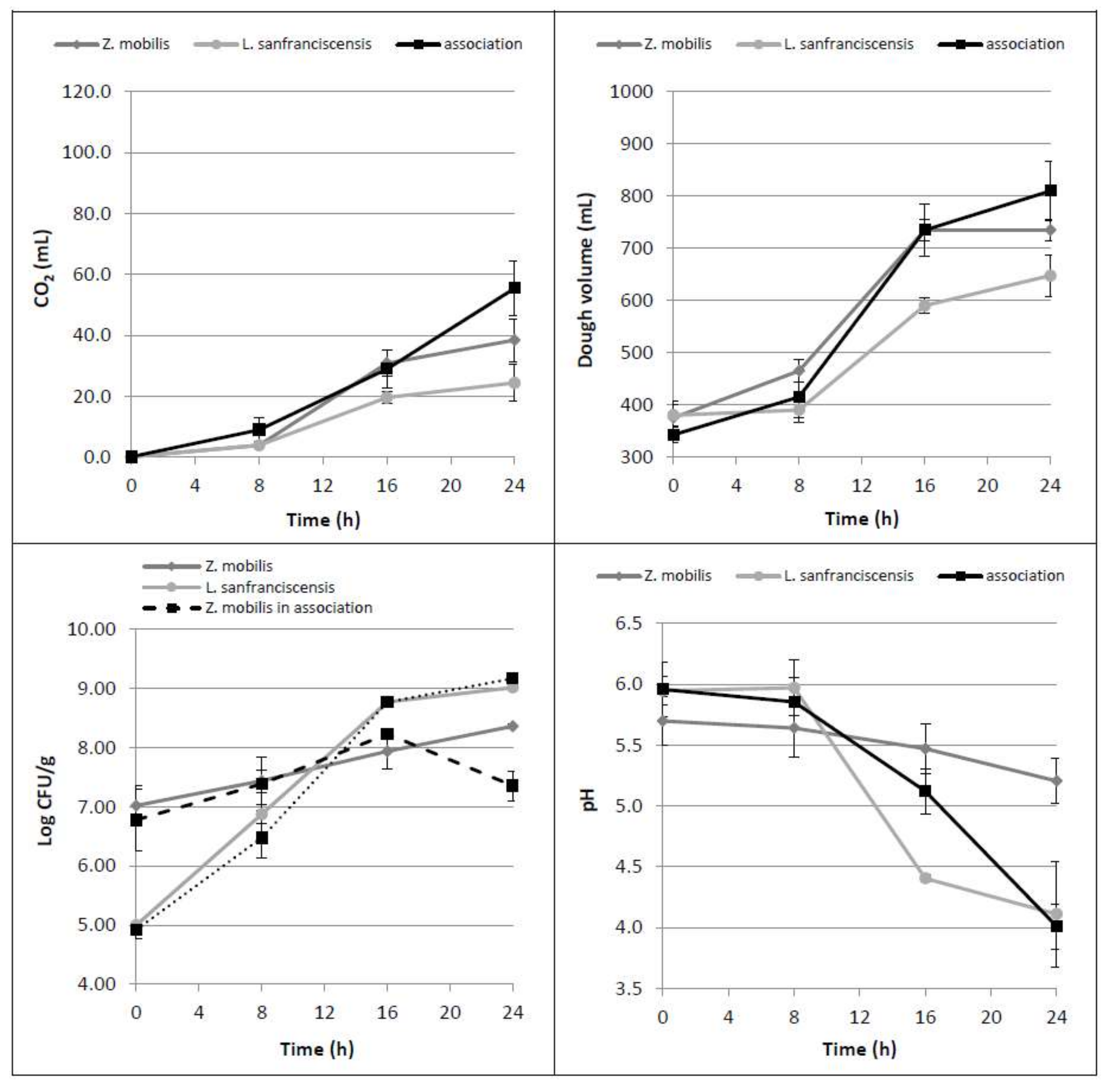
| Glucose (% w/w flour) | Time (h) | Maltose (mg/g) | Glucose (mg/g) | Ethanol (mg/g) | |||
|---|---|---|---|---|---|---|---|
| Mean | St. dev. | Mean | St. dev. | Mean | St. dev. | ||
| 0 | 0 | 10.50 | 0.96 | 1.96 | 0.06 | 0.00 | 0.00 |
| 8 | 14.59 | 1.67 | 1.12 | 0.13 | 1.14 | 0.13 | |
| 16 | 17.71 | 3.16 | 0.23 | 0.33 | 2.79 | 0.49 | |
| 24 | 15.69 | 2.65 | 0.00 | 0.00 | 3.38 | 0.33 | |
| 1 | 0 | 10.55 | 1.11 | 8.72 | 0.41 | 0.00 | 0.00 |
| 8 | 15.48 | 5.22 | 3.42 | 0.62 | 1.84 | 0.41 | |
| 16 | 19.00 | 1.59 | 1.23 | 0.54 | 4.03 | 1.02 | |
| 24 | 21.43 | 4.49 | 0.92 | 0.44 | 3.96 | 0.77 | |
| 5 | 0 | 8.33 | 0.95 | 35.72 | 2.95 | 0.00 | 0.00 |
| 8 | 15.72 | 3.29 | 34.56 | 1.62 | 0.72 | 1.01 | |
| 16 | 18.57 | 2.84 | 2.66 | 1.48 | 9.73 | 0.13 | |
| 24 | 20.80 | 2.92 | 1.46 | 0.40 | 13.65 | 2.46 | |
| Microorganism | Time(h) | Maltose (mg/g) | Glucose (mg/g) | Ethanol (mg/g) | |||
|---|---|---|---|---|---|---|---|
| Mean | St. dev. | Mean | St. dev. | Mean | St. dev. | ||
| Lactobacillus sanfranciscensis (5 Log CFU/g) | 0 | 11.33 | 1.46 | 2.08 | 1.01 | 0.00 | 0.00 |
| 8 | 16.68 | 0.66 | 3.20 | 0.82 | 0.00 | 0.00 | |
| 16 | 9.43 | 0.60 | 4.01 | 0.28 | 0.00 | 0.00 | |
| 24 | 10.80 | 0.80 | 4.54 | 0.52 | 0.00 | 0.00 | |
| Zymomonas mobilis (7 Log CFU/g) | 0 | 10.50 | 0.96 | 1.96 | 0.06 | 0.00 | 0.00 |
| 8 | 14.59 | 1.67 | 1.12 | 0.13 | 1.14 | 0.13 | |
| 16 | 17.71 | 3.16 | 0.23 | 0.33 | 2.79 | 0.49 | |
| 24 | 15.69 | 2.65 | 0.00 | 0.00 | 3.38 | 0.33 | |
| L. sanfranciscensis coupled with Z. mobilis (5–7 Log CFU/g) | 0 | 8.96 | 1.82 | 1.04 | 0.56 | 0.00 | 0.00 |
| 8 | 14.21 | 2.70 | 0.92 | 0.31 | 0.75 | 0.28 | |
| 16 | 13.58 | 0.94 | 0.56 | 0.79 | 3.73 | 0.70 | |
| 24 | 12.33 | 2.76 | 0.82 | 0.35 | 4.91 | 1.23 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musatti, A.; Mapelli, C.; Rollini, M.; Foschino, R.; Picozzi, C. Can Zymomonas mobilis Substitute Saccharomyces cerevisiae in Cereal Dough Leavening? Foods 2018, 7, 61. https://doi.org/10.3390/foods7040061
Musatti A, Mapelli C, Rollini M, Foschino R, Picozzi C. Can Zymomonas mobilis Substitute Saccharomyces cerevisiae in Cereal Dough Leavening? Foods. 2018; 7(4):61. https://doi.org/10.3390/foods7040061
Chicago/Turabian StyleMusatti, Alida, Chiara Mapelli, Manuela Rollini, Roberto Foschino, and Claudia Picozzi. 2018. "Can Zymomonas mobilis Substitute Saccharomyces cerevisiae in Cereal Dough Leavening?" Foods 7, no. 4: 61. https://doi.org/10.3390/foods7040061





