Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Microorganisms
2.2. Yogurt-Like Beverages
2.3. Microbiological Analysis
2.4. Determination of pH, Total Titratable Acidity (TTA) and Kinetics of Acidification
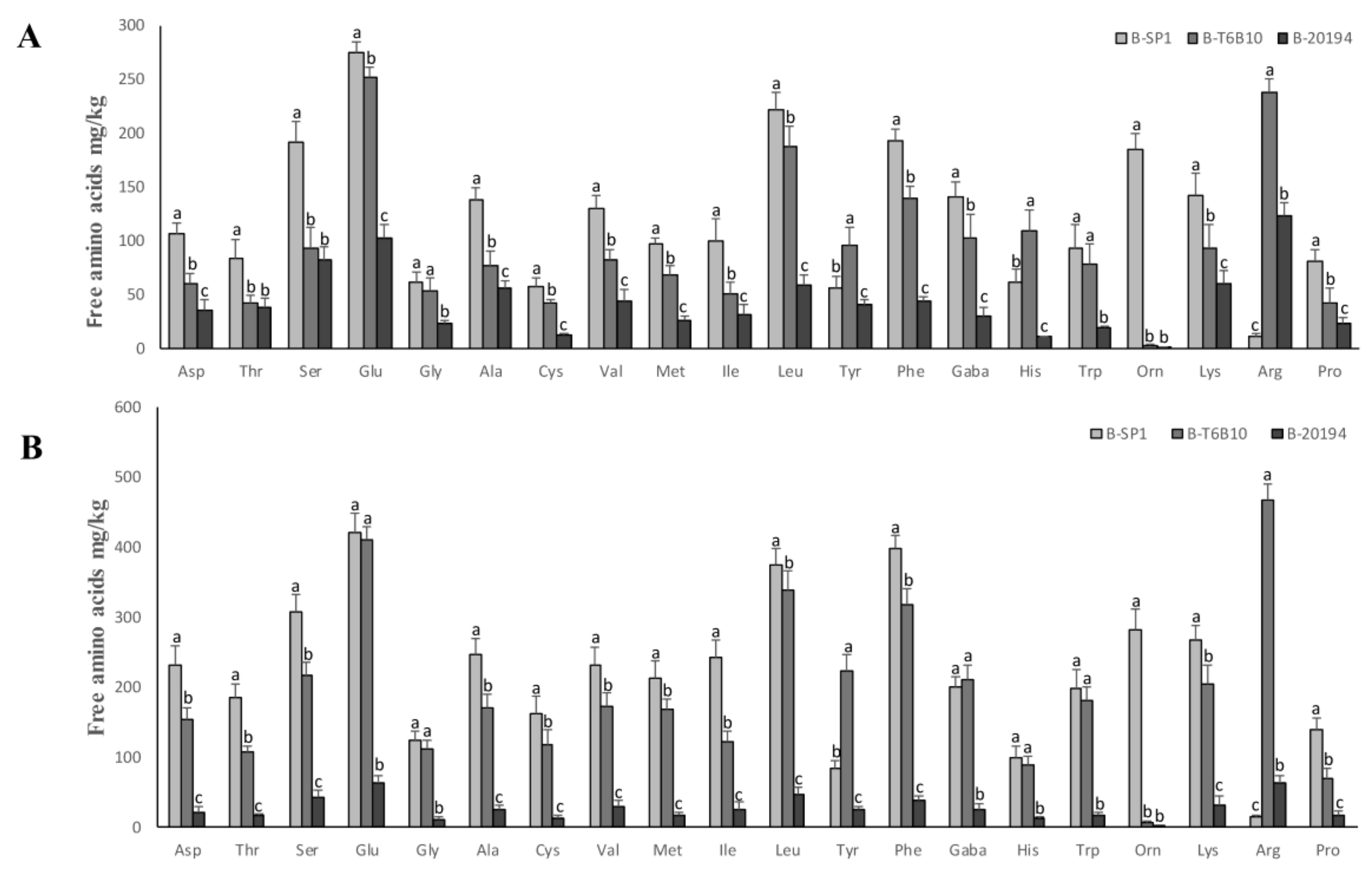
2.5. Organic Acids and Free Amino Acids
2.6. Total Phenols and Antioxidant Activity
2.7. Water Holding Capacity, Viscosity, Total Dry Matter and Color
2.8. Nutritional Characterization
2.9. Starch Hydrolysis Index and Predicted Glycaemic Index
2.10. Sensory Analysis
2.11. Statistical Analysis
3. Results
3.1. Beverage Manufacturing and LAB Fermentation
3.2. Total Phenols and Antioxidant Activity
3.3. Technological Characterization
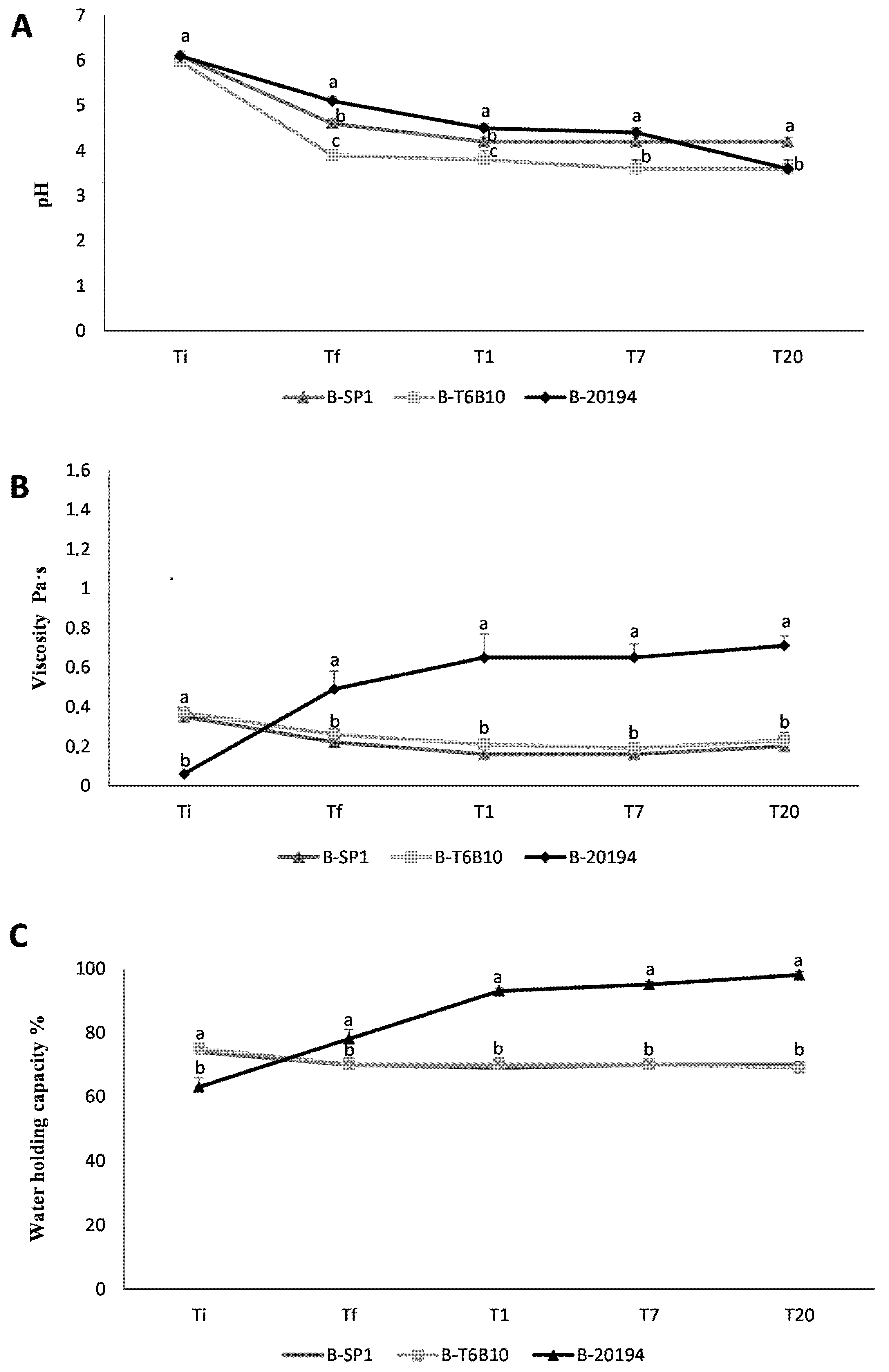
3.4. Shelf-Life Assessment
3.5. Nutritional Characterization
3.6. Sensory Analysis
4. Discussion
4.1. Quinoa Bioprocessing through LAB Fermentation
4.2. Biochemical and Functional Characterization
4.3. Viscosity and EPS Production
4.4. Nutritional Features
4.5. Organoleptic Profile
4.6. Storage Effects
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.B.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and nondairy probiotic food development: Trends, concepts and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and nondairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Prado, F.C.; Parada, J.L.; Pandey, A.; Soccol, C.R. Trends in non-dairy probiotic beverages. Food Res. Int. 2008, 41, 111–123. [Google Scholar] [CrossRef]
- Soccol, C.R.; De Dea Lindner, J.; Yamaguishi, C.T.; Spier, M.R.; Porto De Souza Vandenberghe, L.; Soccol, V.T. Probiotic non-dairy beverages. In Handbook of Plant-Based Fermented Food and Beverage Technology; Hui, Y.H., Ed.; Taylor & Francis Group: Florence, Italy, 2012; ISBN 9781439849040. [Google Scholar]
- Nionelli, L.; Coda, R.; Curiel, J.A.; Kaisa, P.; Gobbetti, M.; Rizzello, C.G. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int. J. Food Microbiol. 2014, 185, 17–26. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Trani, A.; Gobbetti, M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011, 28, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Hekmat, S.; Ahmadi, L. Microbial and sensory evaluation of probiotic yoghurt supplemented with cereal/pseudo-cereal grains and legumes. Int. J. Dairy Thecnol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Do Amaral Santos, C.C.A.; da Silva Libeck, B.; Schwan, R.F. Co-culture fermentation of peanut-soy milk for the development of a novel functional beverage. Int. J. Food Microbiol. 2014, 186, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Matejćeková, Z.; Liptá ková, D.; Valík, Z. Evaluation of the potential of amaranth flour for lactic acid fermentation. J. Pharm. Pharm. Nutr. Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Kürşat Demir, M. Use of quinoa flour in the production of gluten-free Tarhana. Food Sci. Technol. Res. 2014, 20, 1087–1092. [Google Scholar] [CrossRef]
- Fleming, J.E.; Galwey, N.W. Quinoa (Chenopodium quinoa). In Cereals and Pseudocereals; Williams, J.T., Ed.; Chapman & Hall: London, UK, 1995; pp. 3–83. ISBN 978-0-412-46570-3. [Google Scholar]
- Caperuto, L.C.; Amaya-Farfan, J.; Camargo, C.R.O. Performance of quinoa (Chenopodium quinoa Willd) flour in the manufacture of gluten-free spaghetti. J. Sci. Food Agric. 2000, 81, 95–101. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.; Verni, M.; Montemurro, M.; Coda, R.; Gobbetti, M.; Rizzello, C.G. Use of fermented quinoa flour for pasta making and evaluation of the technological and nutritional features. LWT-Food Sci. Technol. 2017, 78, 215–221. [Google Scholar] [CrossRef]
- Urquizo, F.E.L.; Torres, S.M.G.; Tolonen, T.; Jaakkola, M.; Maria Pena-Niebuhr, G.; Wright, A.; Valencia, R.R.C.; Korhonen, H.; Ferrer, C.P. Development of a fermented quinoa-based Beverage. Food Sci. Nutr. 2016, 5, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Magala, M.; Kohajdová, Z.; Karovicá, J.; Greifová, M.; Hojerová, J. Application of lactic acid bacteria for production of fermented beverages based on rice flour. Czech J. Food Sci. 2015, 5, 458–463. [Google Scholar] [CrossRef]
- Coda, R.; Montemurro, M.; Rizzello, C.G. Yogurt like beverages made with cereals. In Yogurt in Healty and Deasease Prevention; Shah, N.P., Ed.; Academic Press: London, UK, 2017; pp. 183–196. ISBN 9780128051344. [Google Scholar]
- Gobbetti, M.; Di Cagno, R.; De Angelis, M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010, 50, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.M.; Mauch, A.; Coffey, A.; Arendt, E.K.; Zannini, E. Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, J. Traditional biotechnology for new foods and beverages. Curr. Opin. Biotechnol. 2013, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W. Use of starter cultures in fermentation on a household scale. Food Control 1997, 8, 241–258. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]
- De Angelis, M.; Damiano, N.; Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Sourdough fermentation as a tool for the manufacture of low-glycemic index white wheat bread enriched in dietary fibre. Eur. Food Res. Technol. 2009, 229, 593–601. [Google Scholar] [CrossRef]
- Angelov, A.; Gotcheva, V.; Kuncheva, R.; Hristozova, T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006, 112, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Cox, S.; Abu-ghannam, N. Process optimization for the development of a functional beverage based on lactic acid fermentation of oats. Biochem. Eng. J. 2010, 52, 199–204. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Falk, K.C.; Tyler, R.T. Characteristics of starch from eight quinoa lines. Cereal Chem. 2005, 82, 216–222. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). ISO 3219 Determination of Viscosity of Polymers and Resins in the Liquid State or as Emulsions or Dispersions Using a Rotational Viscometer with Defined Shear Rate; DIN EN ISO 3219; International Organization for Standardization - ISO Central Secretariat: Geneva, Switzerland, 1993. [Google Scholar]
- Di Cagno, R.; De Angelis, M.; Limitone, A.; Minervini, F.; Carnevali, P.; Corsetti, A.; Gaenzle, M.; Ciati, R.; Gobbetti, M. Glucan and fructan production by sourdough Weissella cibaria and Lactobacillus plantarum. J. Agric. Food Chem. 2006, 54, 9873–9881. [Google Scholar] [CrossRef] [PubMed]
- Amatayakul, T.; Halmos, A.L.; Sherkat, F.; Shah, N.P. Physical characteristics of yoghurts made using exopolysaccharide-producing starter cultures and varying casein to whey protein ratios. Int. Dairy J. 2006, 16, 40–51. [Google Scholar] [CrossRef]
- Purwandari, U.; Shah, N.P.; Vasiljevic, T. Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. Int. Dairy J. 2007, 17, 1344–1352. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongeberger, I.; Roumbouts, F.M.; van’t Riet, K. Modelling of bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [PubMed]
- Weiss, W.; Vogelmeier, C.; Gorg, A. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers’ asthma. Electrophoresis 1993, 14, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, G.; Conterno, L.; Gerbi, V. Determination of organic acids, sugars, diacetyl, and acetoin in cheese by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Gentès, M.C.; St-Gelais, D.; Turgeon, S.L. Gel formation and rheological properties of fermented milk with in situ exopolysaccharide production by lactic acid bacteria. Dairy Sci. Technol. 2011, 91, 645. [Google Scholar] [CrossRef]
- AOAC Official Methods of the Association of Official Analytical Chemists. Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1985. [Google Scholar]
- Akeson, W.R.; Stahmann, M.A. A pepsin pancreatin digest index of protein quality evaluation. J. Nutr. 1964, 83, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Coda, R.; Limitone, A.; Katina, K.; Raulio, M.; Giuliani, G.; Rizzello, C.G.; Gobbetti, M. Manufacture and characterization of pasta made with wheat flour rendered gluten-free using fungal proteases and selected sourdough lactic acid bacteria. J. Cereal Sci. 2014, 59, 79–87. [Google Scholar] [CrossRef]
- Pinter-Szakács, M.; Molnán-Perl, I. Determination of tryptophan in unhydrolyzed food and feed stuff by the acid ninhydrin method. J. Agric. Food Chem. 1990, 38, 720–726. [Google Scholar] [CrossRef]
- Millward, D.J. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr. 2012, 108, S31–S43. [Google Scholar] [CrossRef] [PubMed]
- Block, R.J.; Mitchel, H.H. The correlation of the amino acid composition of protein with their nutritive value. Nutr. Abstr. Rev. 1946, 16, 249–278. [Google Scholar]
- Oser, B.L. An integrated essential amino acid index for predicting the biological value of proteins. In Protein and Amino Acid Acids in Nutrition; Albanese, A.A., Ed.; Academic Press: New York, NY, USA, 1959; pp. 281–291. ISBN 9780323144452. [Google Scholar]
- Ihekoronye, A.I. A Rapid Enzymatic and Chromatographic Predictive Model for the In-vivo Rat-based Protein Efficiency Ratio. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 1981. [Google Scholar]
- Crisan, E.V.; Sands, A. Nutritional value. In The Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayes, W.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 137–165. ISBN 978-0-12-168050-3. [Google Scholar]
- Capriles, V.D.; Arêas, J.A.G. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lapvetelainen, A.; Rannikko, H. Quantitative sensory profiling of cooked oatmeal. LWT-Food Sci. Technol. 2000, 33, 374–379. [Google Scholar] [CrossRef]
- Luckow, T.; Delahunty, C. Which juice is healthier? A consumer study of probiotic non-dairy juice drinks. Food Qual. Preference 2004, 15, 751–759. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdel-Aal, E.S.M.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Giuliani, A.; Hintermann, F.; Rojas, W.; Padulosi, S. Biodiversity of Andean Grains: Balancing Market Potential and Sustainable Livelihoods; Biodiversity International: Rome, Italy, 2012. [Google Scholar]
- Dixit, A.A.; Azar, K.M.J.; Gardner, C.D.; Palaniappan, L.P. Incorporation of whole, ancient grains into a modern Asian Indian diet to reduce the burden of chronic disease. Nutr. Rev. 2011, 69, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Apostolidis, E.; Genovese, M.I.; Lajolo, F.M.; Shetty, K. Evaluation of indigenous grains from the Peruvian Andean region for antidiabetes and antihypertension potential using in vitro methods. J. Med. Food 2009, 12, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, N.; Singhal, R.; Kulkarni, P.; Pal, R. Physicochemical and functional properties of Chenopodium quinoa starch. Carbohydr. Polym. 1996, 31, 99–103. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Chenopodium quinoa—An Indian perspective. Ind. Crops Prod. 2006, 23, 73–87. [Google Scholar] [CrossRef]
- Diaz, J.M.R.; Kirjoranta, S.; Tenitz, S.; Penttila, P.A.; Serimaa, R.; Lampi, A.; Jouppila, K. Use of amaranth, quinoa and kaniwa in extruded corn-based snacks. J. Cereal Sci. 2013, 58, 59–67. [Google Scholar] [CrossRef]
- Zannini, E.; Jeske, S.; Lynch, K.; Arendt, E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018, 268, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Nout, M.J.R. Rich nutrition from the poorest—Cereal fermentations in Africa and Asia. Food Microbiol. 2009, 26, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Tanwar, B. Quinoa beverages: Formulation, processing and potential health benefits. Rom. J. Diabetes Nutr. Metab. Dis. 2016, 23, 215–225. [Google Scholar] [CrossRef]
- Pineli, L.L.O.; Botelho, R.B.A.; Zandonadi, R.P.; Solorzano, J.L.; de Oliveira, G.T.; Reis, C.E.G.; Teixeira, D.S. Low glycemic index and increased protein content in a novel quinoa milk. LWT-Food Sci. Technol. 2015, 63, 1261–1267. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Maina, N.H.; Tenkanen, M.; Maaheimo, H.; Juvonen, R.; Virkki, L. NMR spectroscopic analysis of exopolysaccharides produced by Leuconostoc citreum and Weissella confusa. Carbohydr. Res. 2008, 343, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Maina, N.H.; Juvonen, R.; Flander, L.; Johansson, L.; Virkki, L.; Tenkanen, M.; Laitila, A. In situ production and analysis of Weissella confusa dextran in wheat sourdough. Food Microbiol. 2009, 26, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Kajala, I.; Mäkelä, J.; Coda, R.; Shukla, S.; Shi, Q.; Maina, N.H.; Juvonen, R.; Ekholm, P.; Goyal, A.; Tenkanen, M.; et al. Rye bran as fermentation matrix boosts in situ dextran production by Weissella confusa compared to wheat bran. Appl. Microbiol. Biotechnol. 2016, 100, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martinez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa willd.): Composition, chemistry, nutritional, and functional properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.X.; Zhou, H.M.; Qian, H.F. Proteins extracted from defatted wheat germ: Nutritional and structural properties. Cereal Chem. 2006, 83, 69–75. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci. Technol. 2005, 16, 57–69. [Google Scholar] [CrossRef]
- Inoue, K.; Shirai, T.; Ochiai, H.; Kassao, M.; Hayakawa, K.; Rimura, M.; Sansawa, H. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensive. Eur. J. Clin. Nutr. 2003, 57, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Quìlez, J.; Rafecasa, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Food 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors—A randomized trial. Olive Oil Polyphenols and Heart Disease Risk. Ann. Int. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Arendt, E.; Liukkonen, K.H.; Autio, K.; Flander, L.; Poutanen, K. Potential of sourdough for healthier cereal products. Trends Food Sci. Technol. 2005, 16, 104–112. [Google Scholar] [CrossRef]
- Korakli, M.; Rossmann, A.; Gänzle, M.G.; Vogel, R.F. Sucrose metabolism and exopolysaccharide production in wheat and rye sourdoughs by Lactobacillus sanfranciscensis. J. Agric. Food Chem. 2001, 49, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Korakli, M.; Gänzle, M.G.; Vogel, R.F. Metabolism by bifidobacteria and lactic acid bacteria of polysaccharides from wheat and rye, and exopolysaccharides produced by Lactobacillus sanfranciscensis. J. Appl. Microbiol. 2002, 92, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Tieking, M.; Gänzle, M.G. Exopolysaccharides from cereal associated lactobacilli. Trends Food Sci. Technol. 2005, 16, 79–84. [Google Scholar] [CrossRef]
- De Angelis, M.; Rizzello, C.G.; Alfonsi, G.; Arnault, P.; Cappelle, S.; Tossut, P.; Di Cagno, R.; Gobbetti, M. Use of sourdough lactobacilli and oat fibre to decrease the glycemic index of white wheat bread. Br. J. Nutr. 2007, 98, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.; Eliasson, A.C.; Björck, I. An examination of the possibility of lowering the glycemic index of oat and barley flakes by minimal processing. J. Nutr. 2000, 130, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
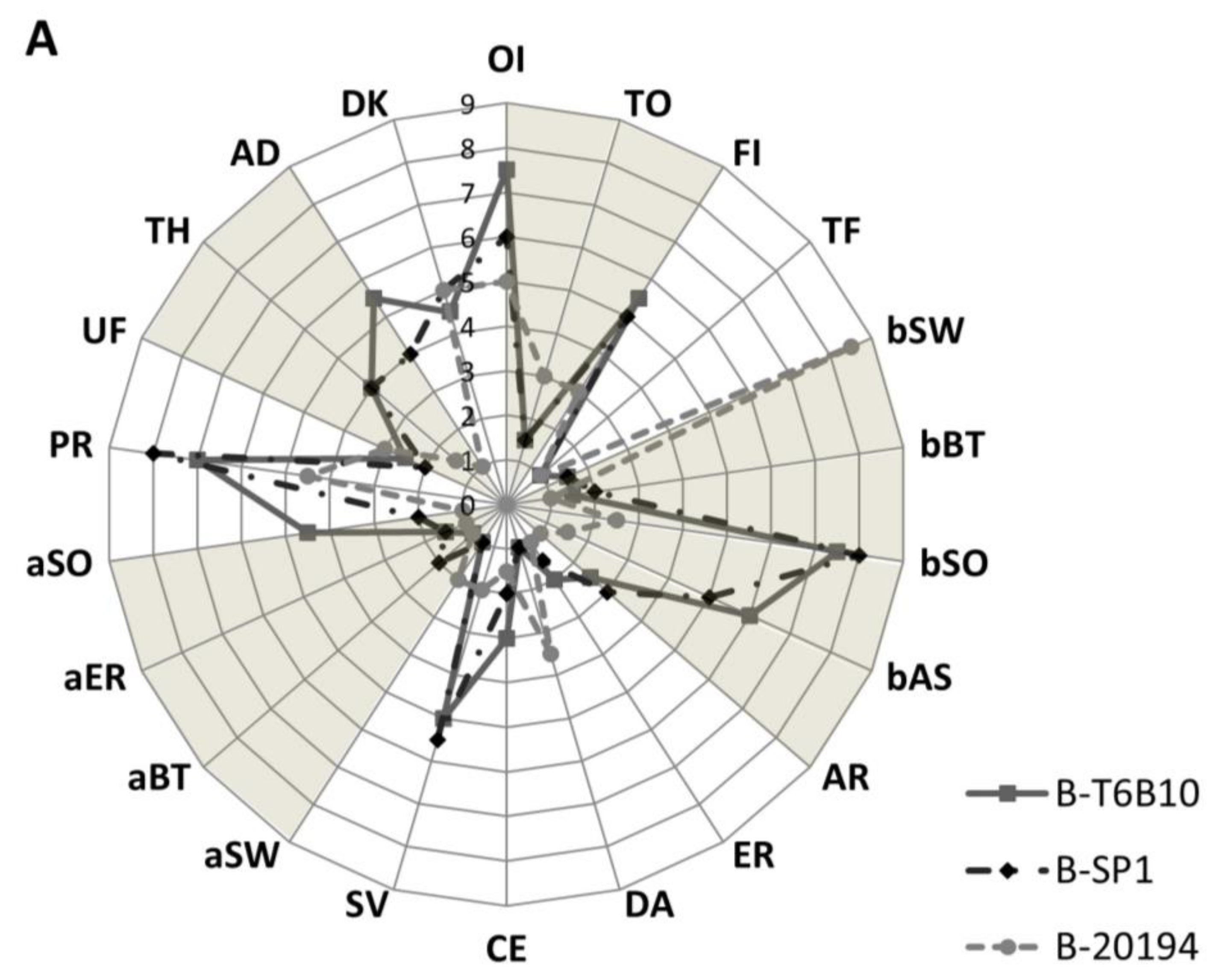
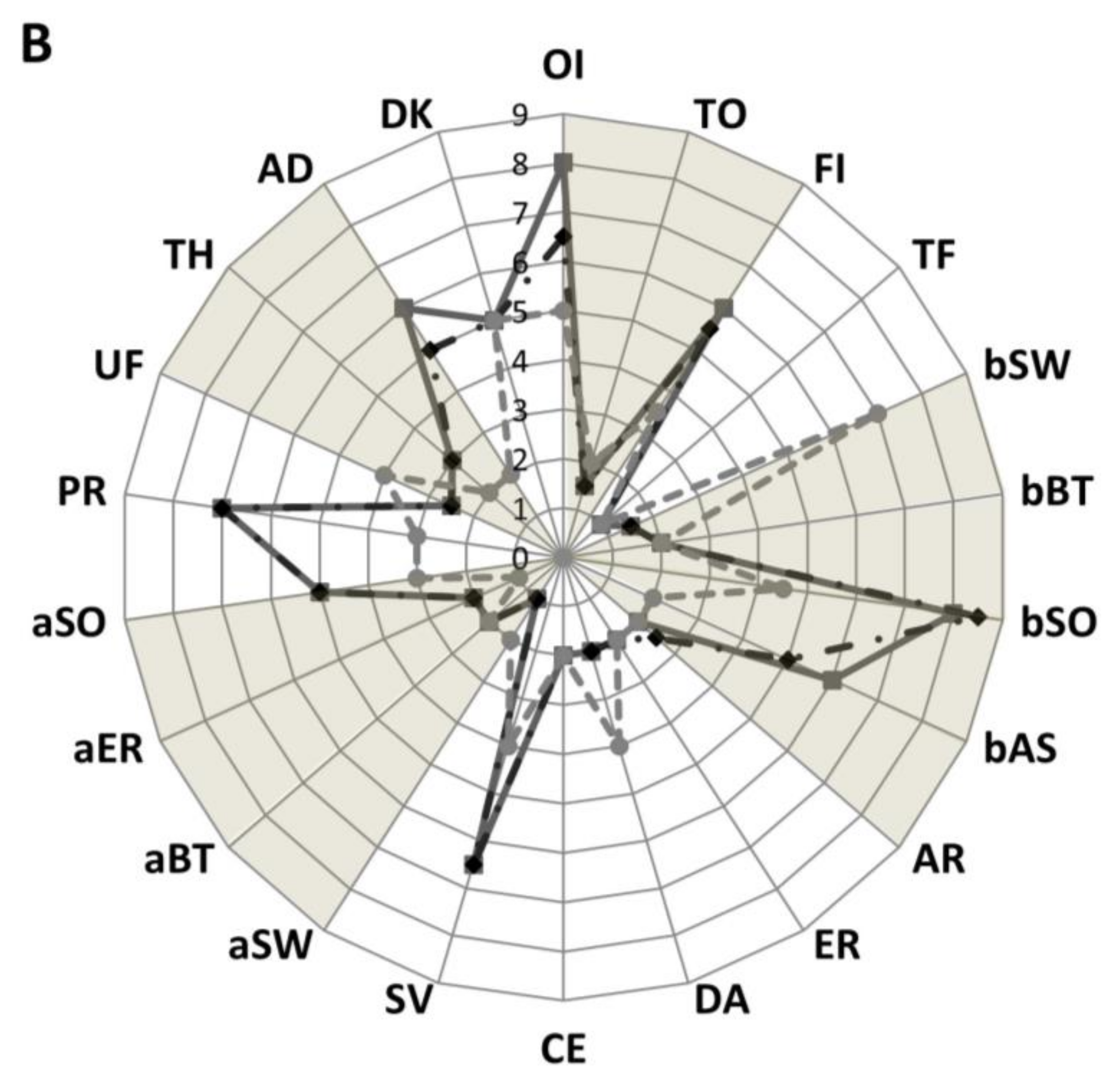
| Characteristic | Abbreviation | Definition |
|---|---|---|
| Odor | ||
| Overall intensity of odor | OI | The odor perceived immediately |
| Toasted odor | TO | The odor related to toasted/coke cereal evacuate before mixing |
| Flavor | ||
| Overall intensity of flavor | FI | The flavor evaluated orally after mixing the sample with a spoon |
| Toasted flavor | TF | The flavor related to toasted cereal |
| Basic tastes | ||
| Sweet | bSW | Taste on the tongue stimulated by sugars |
| Bitter | bBT | Taste associated with caffeine and quinine |
| Sour/Acid | bSO | Taste associated with lactic acid |
| Astringent | bAS | Mouth drying. The complex of drying, puckering and shrinking sensations in the lower oral cavity causing contractions of the body tissue in the mouth |
| Others | ||
| Artificial | AR | Non-specific, often used to describe imitation products |
| Earthy | ER | Tasting dirty and musty |
| Dairy | DA | A flavor of condensed, sweet milk |
| Cereal | CE | A flavor of cereal |
| Savory | SV | A meaty, fleshy, beany flavor |
| After taste | ||
| Sweet | aSW | A lingering sweet syrupy flavor |
| Bitter | aBT | A lingering bitter flavor that hits the top of the tongue |
| Earthy | aER | A lingering dirty, earthy, musty flavor |
| Sour/Acid | aSO | A lingering acidic, tangy flavor |
| Oral texture | ||
| Particles | PR | Particles presence and bits |
| Uniformity of mass | UF | The uniformity of mass after drink |
| Manual texture | ||
| Thickness | TH | The force required to stir the sample with spoon |
| Adherence to spoon | AD | The amount of the sample adhering to the spoon evacuate by taking a spoonful of sample and turning the spoon over |
| Appearance | ||
| Darkness of the color | DK |
| B-SP1 | B-T6B10 | B-20194 | |
|---|---|---|---|
| ΔpH (pH units) | 1.56 ± 0.25 b | 2.35 ± 0.30 a | 1.85 ± 0.10 b |
| Vmax (ΔpH/h) | 0.17 ± 0.04 a | 0.18 ± 0.02 a | 0.15 ± 0.03 a |
| λ (h) | 0.93 ± 0.10 b | 0.22 ± 0.09 c | 1.71 ± 0.030 a |
| B-SP1 | B-T6B10 | B-20194 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ti | Tf | T20 | Ti | Tf | T20 | Ti | Tf | T20 | |
| LAB cfu/mL | 6.8 ± 0.1 f | 8.8 ± 0.1 d | 8.9 ± 0.2 d | 7.3 ± 0.1 e | 9.8 ± 0.1 a | 9.5 ± 0.1 b | 6.6 ± 0.1 f | 8.7 ± 0.2 d | 9.1 ± 0.1 c |
| Yeasts cfu/mL | - | - | - | - | - | - | - | - | - |
| pH | 6.1 ± 0.1 a | 4.6 ± 0.1 c | 4.2 ± 0.1 d | 5.9 ± 0.1 a | 3.9 ± 0.1 e | 3.6 ± 0.2 f | 6.1 ± 0.1 a | 5.1 ± 0.1 b | 3.6 ± 0.2 f |
| TTA | 5.5 ± 0.1 f | 12.6 ± 1 d | 16.5 ± 1 c | 5.3 ± 0.2 f | 18.9 ± 1 b | 24 ± 1.5 a | 4.5 ± 1 f | 7.8 ± 1 e | 14.8 ± 1 c |
| Lactic acid (mmol/Kg) | 1.3 ± 0.1 f | 25.7 ± 0.2 d | 48.8 ± 1.0 c | 1.4 ± 0.2 f | 84.37 ± 2 b | 115.4 ± 3 a | 0.3 ± 0.1 f | 15.36 ± 0.9 e | 30.6 ± 2 d |
| Acetic acid | 0.4 ± 0.2 e | 0.7 ± 0.2 d | 0.7 ± 0.3 d | 0.8 ± 0.1 d | 1.8 ± 0.5 c | 2.6 ± 0.8 b | 0.5 ± 0.1 d | 4.8 ± 0.9 a | 5.3 ± 0.7 a |
| Total free amino acids (mg/kg) | 1265 ± 40 e | 2550 ± 58 c | 4654 ± 55 a | 1289 ± 25 e | 2009 ± 64 d | 4067 ± 63 b | 776 ± 19 f | 1019 ± 17 e | 1752 ± 21 de |
| Total phenols (mmol/kg) | 5.3 ± 0.1 e | 5.8 ± 0.1 d | 9.6 ± 0.6 a | 5.2 ± 0.2 e | 8.4 ± 0.8 b | 9.3 ± 0.5 a | 4.0 ± 0.1 f | 5.9 ± 0.1 d | 7.9 ± 0.2 c |
| Antioxidant activity | 25 ± 1 d | 32 ± 1 c | 49 ± 2 a | 24 ± 1 d | 37 ± 2 b | 44 ± 2 a | 29 ± 2 d | 32 ± 3 c | 38 ± 2 b |
| Viscosity (Pa·s) | 0.35 ± 0.03 c | 0.22 ± 0.02 e | 0.20 ± 0.02 e | 0.37 ± 0.02 c | 0.26 ± 0.01 d | 0.23 ± 0.03 e | 0.06 ± 0.01 f | 0.49 ± 0.09 b | 0.70 ± 0.05 a |
| Dry matter (g/100g) | 33.3 ± 0.1 a | 33.3 ± 0.1 a | 33.4 ± 0.1 a | 33.9 ± 0.5 a | 34.2 ± 0.1 a | 34.4 ± 0.1 a | 31.1 ± 0.1 b | 31.6 ± 0.1 b | 30.3 ± 0.1 b |
| Water holding capacity (%) | 74 ± 1 c | 70 ± 1 d | 70 ± 1 d | 75 ± 1 c | 70 ± 2 d | 69 ± 1 d | 63 ± 3 e | 78 ± 3 b | 98 ± 1 a |
| Color analysis | |||||||||
| L | 65 ± 0.3 a | 64.7 ± 0.3 a | 65.1 ± 0.1 a | 64.8 ± 0.9 a | 65.0 ± 0.5 a | 64.9 ± 0.1 a | 65.5 ± 0.2 a | 65.6 ± 0.2 a | 65.7 ± 0.2 a |
| a | 0.2 ± 0.1 a | –0.1 ± 0.1 a | −0.2 ± 0.1 a | 0.2 ± 0.1 a | −0.2 ± 0.1 a | −0.23 ± 0.2 a | 0.2 ± 0.1 a | –0.1 ± 0.1 a | −0.5 ± 0.1 b |
| b | 8.2 ± 0.1 b | 8.7 ± 0.3 a | 9 ± 0.1 a | 8.3 ± 0.1 b | 8.3 ± 0.1 b | 8.1 ± 0.1 b | 8.2 ± 0.1 b | 8.4 ± 0.1 b | 8.2 ± 0.1 b |
| ΔE | 28.3 ± 0.3 a | 28.7 ± 0.2 a | 28.4 ± 0.1 a | 28.5 ± 0.5 a | 28.4 ± 0.5 a | 28.3 ± 0.4 a | 27.8 ± 0.3 a | 27.8 ± 0.2 a | 27.7 ± 0.2 a |
| Beverages | B-SP1 | B-T6B10 | B-20194 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ti | Tf | T20 | Ti | Tf | T20 | Ti | Tf | T20 | |
| In vitro protein digestibility (%) | 71 ± 1 d | 86 ± 2 b | 91 ± 1 a | 71 ± 1 d | 84 ± 2 b | 88 ± 2 b | 72 ± 2 d | 80 ± 2 c | 83 ± 1 c |
| Sequence of limiting essential amino acids (EAA) | lysine | cystine | cystine | lysine | cystine | cystine | lysine | cystine | cystine |
| cystine | valine | valine | cystine | valine | valine | cystine | valine | valine | |
| tryptophan | lysine | lysine | tryptophan | lysine | lysine | tryptophan | lysine | lysine | |
| Protein score (%) | 22.7 ± 0.5 d | 30.5 ± 0.4 b | 34.7 ± 0.6 a | 22.7 ± 0.3 d | 31.2 ± 0.4 b | 33.6 ± 0.6 a | 22.7 ± 0.5 d | 27.4 ± 0.2 c | 29.6 ± 0.3 b |
| Essential Amino Acid Index (EAAI) | 43 ± 0.5 d | 47 ± 0.3 b | 51 ± 0.3 a | 43 ± 0.4 d | 46 ± 0.7 c | 50 ± 0.6 a | 43 ± 0.2 d | 46 ± 0.3 c | 48 ± 0.2 b |
| Biological Value (BV) | 38.3 ± 0.2 c | 41.3 ± 0.2 b | 44.3 ± 0.6 a | 38.3 ± 0.5 c | 40.5 ± 0.6 b | 43.8 ± 0.5 a | 37.8 ± 0.5 c | 39.5 ± 0.2 b | 42.8 ± 0.7 a |
| Protein Efficiency Ratio (PER) | 20.5 ± 0.6 c | 23.8 ± 0.5 b | 25.0 ± 0.2 a | 21.3 ± 0.4 c | 23.5 ± 0.2 b | 25.2 ± 0.4 a | 21.2 ± 0.1 c | 22.5 ± 0.2 c | 23.8 ± 0.6 b |
| Nutritional Index (NI) | 2.8 ± 0.1 c | 5.4 ± 0.2 b | 5.8 ± 0.2 a | 2.8 ± 0.2 c | 5.2 ± 0.3 b | 5.6 ± 0.1 a | 1.7 ± 0.3 e | 2.2 ± 0.3 d | 2.4 ± 0.4 c |
| Hydrolysis index | 57 ± 2 b | 52 ± 2 c | 50 ± 2 c | 57 ± 2 b | 53 ± 1 c | 52 ± 2 c | 64 ± 1 a | 60 ± 2 b | 58 ± 2 b |
| Predicted GI | 71 ± 3 b | 68 ± 1 c | 67 ± 2 c | 71 ± 4 b | 69 ± 2 c | 68 ± 2 c | 75 ± 4 a | 73 ± 4 b | 71 ± 2 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, A.; Coda, R.; Montemurro, M.; Rizzello, C.G. Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages. Foods 2018, 7, 51. https://doi.org/10.3390/foods7040051
Lorusso A, Coda R, Montemurro M, Rizzello CG. Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages. Foods. 2018; 7(4):51. https://doi.org/10.3390/foods7040051
Chicago/Turabian StyleLorusso, Anna, Rossana Coda, Marco Montemurro, and Carlo Giuseppe Rizzello. 2018. "Use of Selected Lactic Acid Bacteria and Quinoa Flour for Manufacturing Novel Yogurt-Like Beverages" Foods 7, no. 4: 51. https://doi.org/10.3390/foods7040051





