Extraction and Optimization of Potato Starch and Its Application as a Stabilizer in Yogurt Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Starch from Yellow Skin Potato
2.2. Preparation of Yogurt
2.3. Analysis of Yogurt
2.4. Acidity of Yogurt
2.5. pH of Yogurt
2.6. Syneresis of Yogurt
2.7. Microbiological Analysis of Yogurt
2.8. Sensory Evaluation
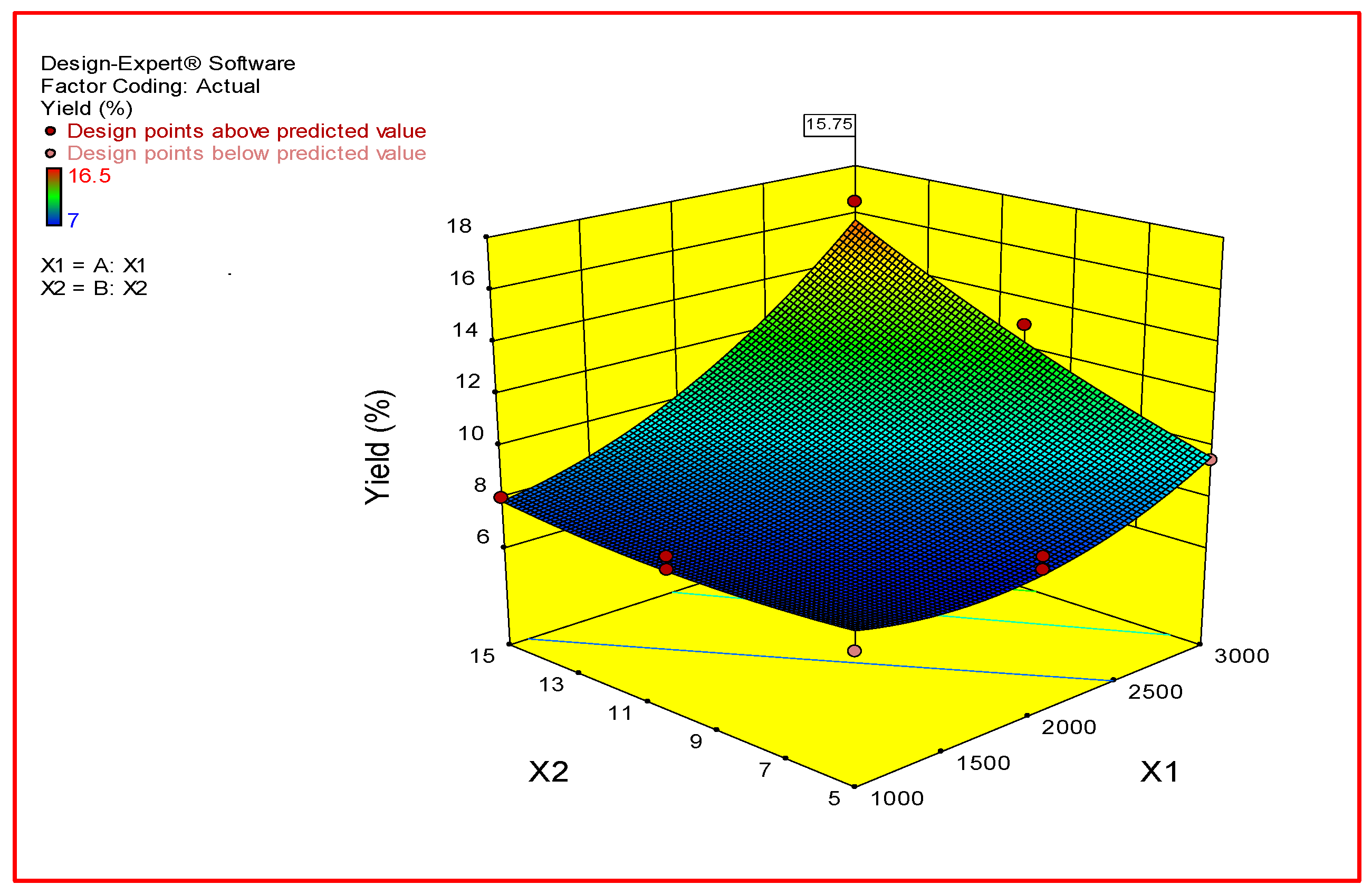
2.9. Experimental Design and Data Analysis
3. Results and Discussion
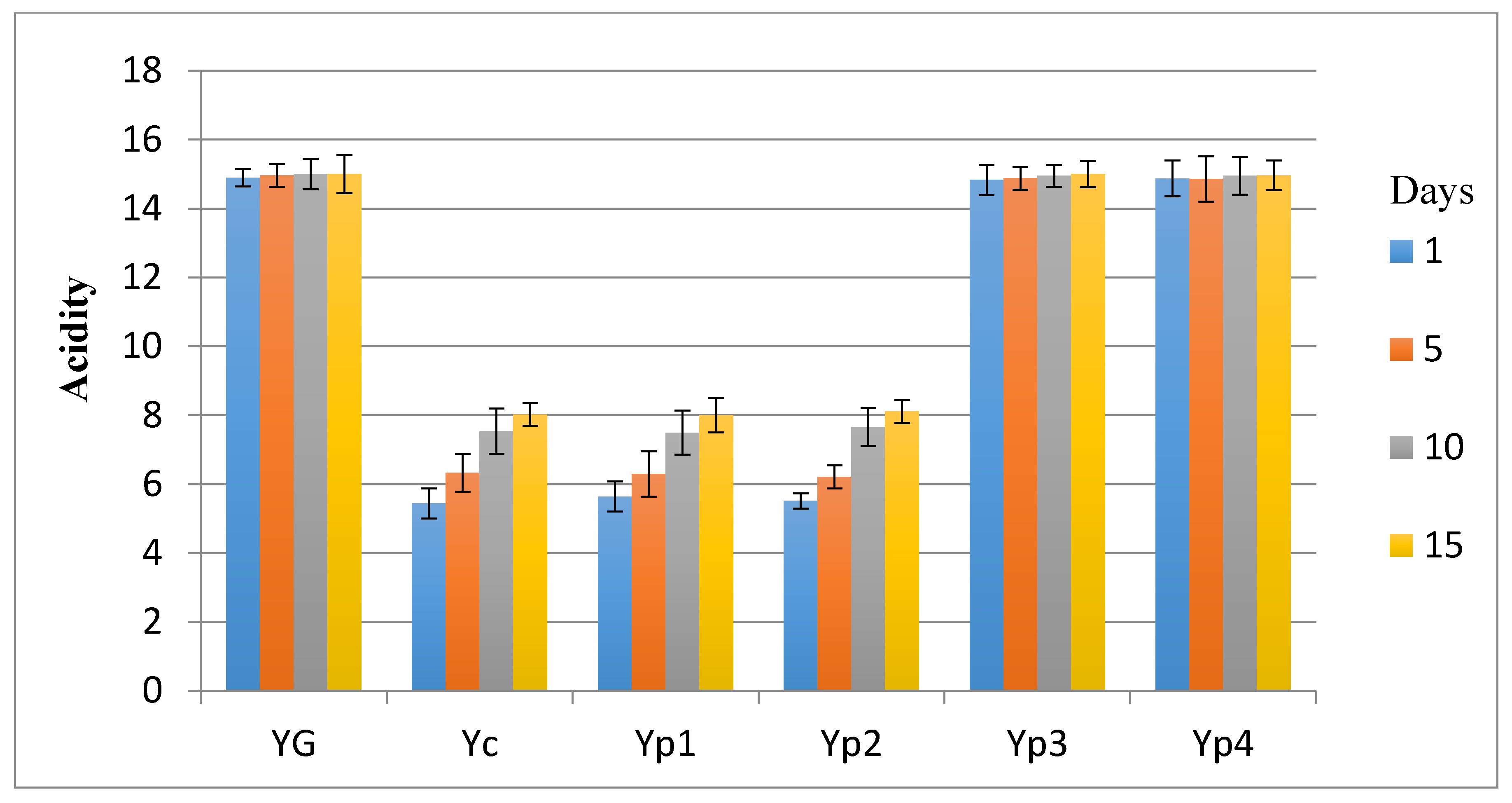
3.1. Total Acidity in Yogurt Samples
3.2. pH of Yogurt Samples
3.3. Syneresis of Yogurt Samples
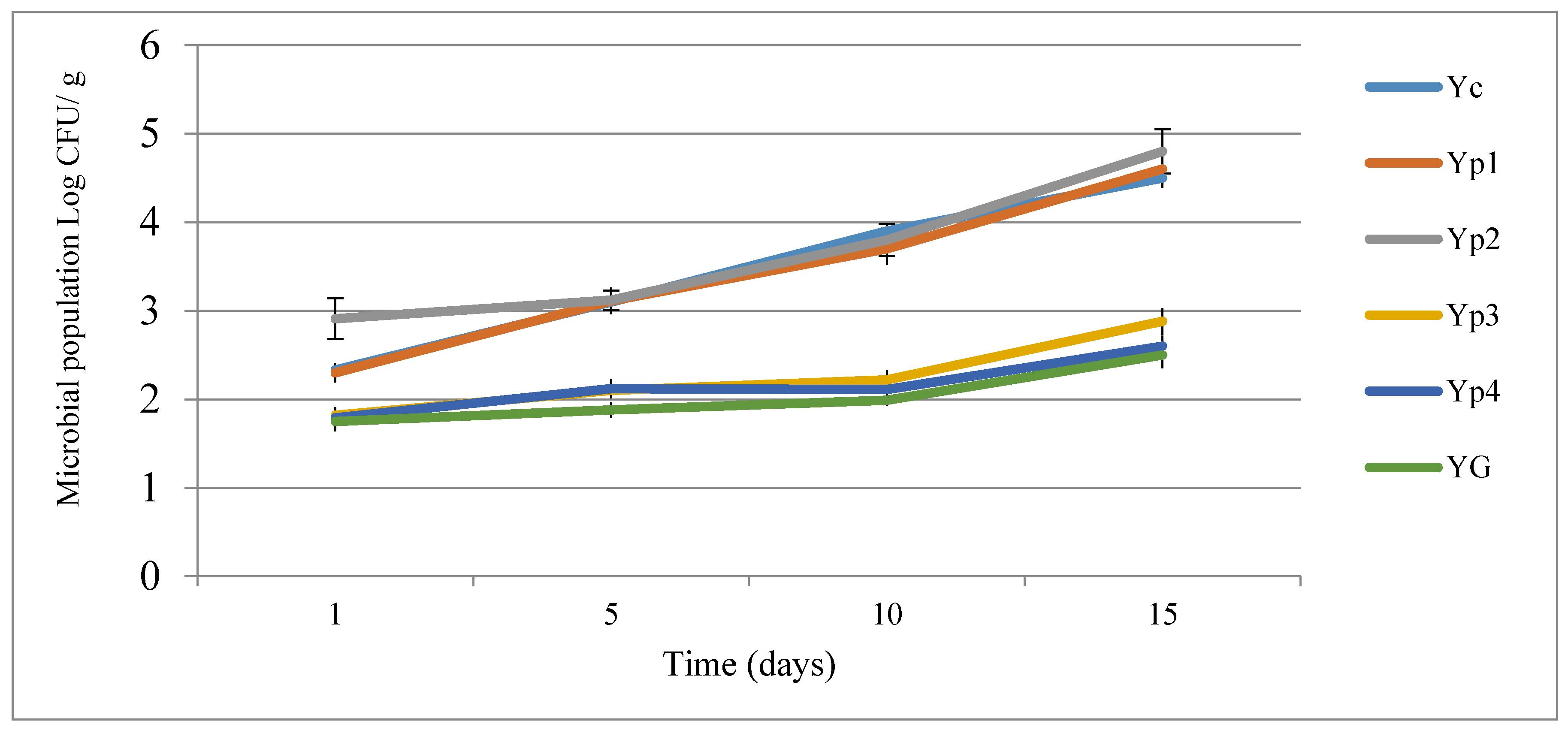
3.4. Microbiological Analysis of Yogurt
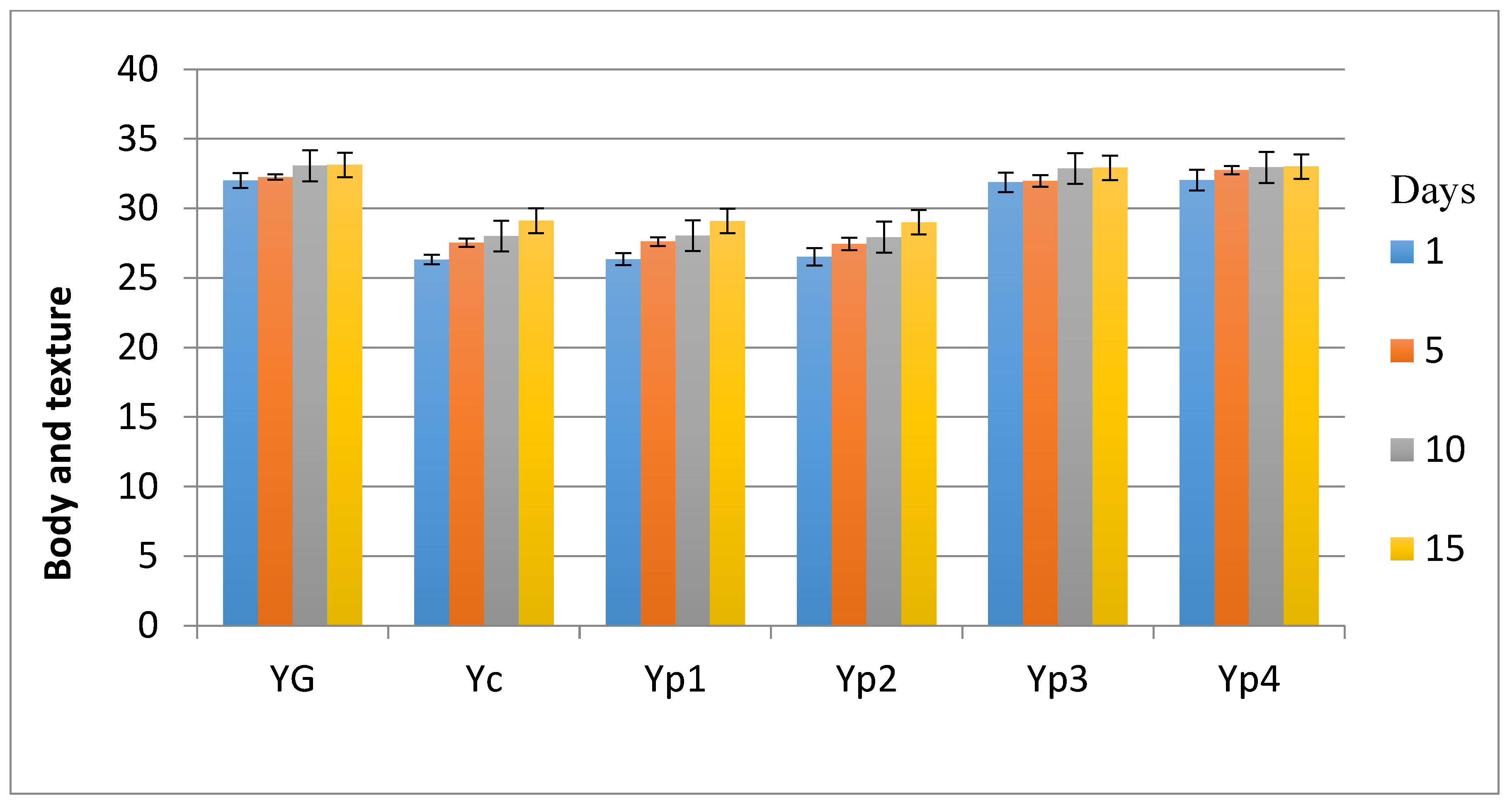
3.5. Sensory Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gawai, K.M.; Mudgal, S.P.; Prajapati, J.B. Stabilizers, Colorants, and Exopolysaccharides in Yogurt. In Yogurt in Health and Disease Prevention; Academic Press: London, UK, 2017; p. 49. [Google Scholar]
- El-Sayed, E.M.; Abd El-Gawad, I.A.; Murad, H.A.; Salah, S.H. Utilization of laboratory produced xanthan gum in the manufacture of yoghurt and soy yogurt. Eur. Food Res. Technol. 2002, 215, 298–304. [Google Scholar]
- Uba, A.; Izuagie, T.; Hassan, L.G.; Achor, M.; Sahabi, D.M. Physicochemical Properties of Starch Isolated from Seeds of Chrysophyllum albidum. Int. J. Chem. Sci. 2011, 4, 264–270. [Google Scholar]
- Heyman, M. Effect of lactic acid bacteria on diarrheal diseases. J. Am. Coll. Nutr. 2000, 19, 137S–146S. [Google Scholar] [CrossRef] [PubMed]
- Chandan, R.C. An Overview of Yogurt Production and Composition. In Yogurt in Health and Disease Prevention; Academic Press: London, UK, 2017; p. 31. [Google Scholar]
- Kolars, J.C. Yoghurt—An auto digesting source of lactose. N. Engl. J. Med. 1984, 310, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Anjum, F.M.; Sameen, A.; Khan, M.I.; Sohaib, M. Extraction of starch from Water Chestnut (Trapa bispinosa Roxb) and its application in yoghurt as a stabilizer. Pak. J. Food Sci. 2012, 22, 209–218. [Google Scholar]
- Imeson, A. Thickening and Gelling Agents for Foods, 2nd ed.; Aspen Publ. Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Alakali, J.S.; Okonkwo, T.M.; Iordye, E.M. Effect of stabilizers on the physico-chemical and sensory attributes of thermized yoghurt. Afr. J. Biotechnol. 2008, 7, 158–163. [Google Scholar]
- Pang, Z.; Deeth, H.; Yang, H.; Prakash, S.; Bansal, N. Evaluation of tilapia skin gelatin as a mammalian gelatin replacer in acid milk gels and low-fat stirred yogurt. J. Dairy Sci. 2017, 100, 3436–3447. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Han, Y.P.; Zhao, X.H. Quality attributes of set-style skimmed yoghurt affected by the addition of a cross-linked bovine gelatin. CyTA-J. Food 2017, 15, 320–325. [Google Scholar] [CrossRef]
- Sameen, A.; Sattar, M.U.; Javid, A.; Ayub, A.; Khan, M.I. Quality evaluation of yoghurt stabilized with sweet potato (Ipomoea batatas) and taro (Colocassia esculenta) starch. Int. J. Food Allied Sci. 2016, 2, 23–29. [Google Scholar] [CrossRef]
- Sameen, A.; Manzoor, M.F.; Huma, N.; Sahar, A.; Sattar, U. Quality evaluation of ice cream prepared with Sagudana (Meteroxylon sagu) and Sweet Potato (Ipomoea batatas) starch as stabilizing agent. Pak. J. Food Sci. 2017, 27, 1–6. [Google Scholar]
- Kategunya, R.; Sanguansri, C. Physico-chemical properties of jackfruit seed starch (Artocarpus heterophyllus) and its application as a thickener and stabilizer in chilli sauce. LWT-Food Sci. Technol. 2011, 44, 1309–1313. [Google Scholar]
- Januário, J.G.B.; da Silva, I.C.F.; de Oliveira, A.S.; de Oliveira, J.F.; Dionísio, J.N.; Klososki, S.J.; Pimentel, T.C. Probiotic yoghurt flavored with organic beet with carrot, cassava, sweet potato or corn juice: Physicochemical and texture evaluation, probiotic viability and acceptance. Int. Food Res. J. 2017, 24, 359–366. [Google Scholar]
- Ammar, M.S.; Hegazy, A.E.; Bedeir, S.H. Using of taro flour as partial substitute of wheat flour in bread making. World J. Dairy Food Sci. 2009, 4, 94–99. [Google Scholar]
- Khan, K.; Rehman, S.; Khan, M.A.; Anwar, F.; Bhadar, S. Physical and chemical quality appraisal of commercial yoghurt brands sold at Lahore. ARPN J. Agric. Biol. Sci. 2008, 3, 14–20. [Google Scholar]
- Ikenebomeh, M.J.; Omogbai, B.A. Chemical analysis and sensory investigation of soymilk yoghurts. Nigerian J. Microbiol. 2000, 14, 85–93. [Google Scholar]
- Tamime, A.Y.; Robinson, R.K. Yoghurt Science and Technology; Woodhead Publishing: Cambridge, UK, 1985. [Google Scholar]
- Onwuka, G.I. Food Analysis and Instrumentation: Theory and Practice; Naphthali Publishers Ltd.: Lagos, Nigeria; Pergamon Press: Oxford, UK, 2005; pp. 76–82. [Google Scholar]
- Olugbuyiro, J.A.; Oseh, J.A. Physico-chemical and sensory evaluation of market yoghurt in Nigeria. Pak. J. Nutr. 2011, 10, 914–918. [Google Scholar]
- Al-Kadamany, E.; Khattar, M.; Haddad, T.; Toufeili, I. Estimation of shelf life of concentrated yoghurt by monitoring selected microbiological and physiological changes during storage. LWT-Food Sci. Technol. 2003, 36, 407–414. [Google Scholar] [CrossRef]
- Okoye, J.I.; Animalu, I.L. Evaluation of physico-chemical and microbiological properties of stirred yoghurt stabilized with sweet potato (Ipomoea batatas) starch. Cont. J. Microbiol. 2009, 3, 27–30. [Google Scholar]
- Altemimi, A.; Watson, D.G.; Kinsel, M.; Lightfoot, D.A. Simultaneous extraction, optimization, and analysis of flavonoids and polyphenols from peach and pumpkin extracts using a TLC-densitometric method. Chem. Cent. J. 2015, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Andic, S.; Boran, G.; Tuncturk, Y. Effects of carboxyl methyl cellulose and edible cow gelatin on physico-chemical, textural and sensory properties of yoghurt. Int. J. Agric. Biol. 2013, 15, 245–251. [Google Scholar]
- Anwer, M.; Ahmad, S.; Sameen, A.; Ahmed, S. Effect of different heating temperatures on the rheological properties of lactic gel made from buffalo milk. J. Food Chem. Nutr. 2013, 1, 33–41. [Google Scholar]
- Kumar, P.; Mishra, H. Mango soy fortified set yoghurt: Effect of stabilizer addition on physicochemical, sensory and textural properties. Food Chem. 2004, 87, 501–507. [Google Scholar] [CrossRef]
- Seelee, W.; Tungjaroenchai, W.; Natvaratat, M. Development of low fat set type probiotic yoghurt from goat milk. Asian J. Food Agro-Ind. 2009, 2, 771–779. [Google Scholar]
- Hassan, F.A.; Helmy, W.A.; Enab, A.K. Utilization of some local polysaccharide in manufacture of yoghurt. Egypt. J. Dairy Sci. 2011, 2, 281–289. [Google Scholar]
- Kamruzzaman, M.N.; Rehman, M.M. Shelflife of different types of dahi at room and refrigeration temperature. Pak. J. Nutr. 2002, 1, 234–237. [Google Scholar]
- Isleten, M.; Karagul-Yuceer, Y. Effects of dried dairy ingredients on physical and sensory properties of nonfat yogurt. J. Dairy Sci. 2006, 89, 2865–2872. [Google Scholar] [CrossRef]
- Guven, M.; Yasar, K.; Karaca, O.B.; Hayaloglu, A.A. The effect of inulin as a fat replacer on the quality of set-type low-fat yoghurt manufacture. Int. J. Dairy Technol. 2005, 58, 180–184. [Google Scholar] [CrossRef]
- Hasan, M.; Huma, N.; Sameen, A.; Rafiq, S.; Gulzar, N. Use of meteroxylon sagu as a stabilizing agent in yoghurt. J. Food Chem. Nutr. 2014, 2, 19–26. [Google Scholar]
- Dave, R.L.; Dave, J.M.; Sannabhadti, S.S. Micorbiological quality of some market and household dahi samples. Asian J. Dairy Res. 1992, 10, 111–114. [Google Scholar]
- Ganesh, S. A Novel Yoghurt Product with Lactobacillus Acidophilus. Master’s Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2006. [Google Scholar]


| Yogurt Treatment | Extracted Potato Starch % (v/w) | Gelatin % (v/w) |
|---|---|---|
| YG | - | 0.6 |
| YC | 0 | - |
| Yp1 | 0.25 | - |
| Yp2 | 0.5 | - |
| Yp3 | 0.75 | - |
| Yp4 | 1 | - |
| Run | X1 (rpm) | X2 (Min) | Potato Starch Yield % |
|---|---|---|---|
| 1 | 3000 | 10 | 11 |
| 2 | 1000 | 15 | 8 |
| 3 | 2000 | 5 | 8 |
| 4 | 1000 | 10 | 7.5 |
| 5 | 3000 | 5 | 9.5 |
| 6 | 3000 | 15 | 16.5 |
| 7 | 2000 | 15 | 10 |
| 8 | 1000 | 5 | 7 |
| 9 | 1000 | 10 | 8 |
| 10 | 2000 | 5 | 7.5 |
| 11 | 2000 | 15 | 10 |
| 12 | 3000 | 10 | 13 |
| Source | Degree of Freedom | Sum of Square | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5 | 80.10 | 16.02 | 21.07 | 0.001 |
| X1 | 1 | 47.53 | 47.53 | 62.51 | 0.0002 |
| X2 | 1 | 19.53 | 19.53 | 25.68 | 0.0023 |
| X1X2 | 1 | 9 | 9 | 11.84 | 0.013 |
| X12 | 1 | 3.78 | 3.78 | 4.97 | 0.067 |
| X22 | 1 | 0.28 | 0.28 | 0.37 | 0.565 |
| Lack of fit | 2 | 2.31 | 1.16 | 2.06 | 0.2432 |
| Storage Period (Days) | Yogurt Treatments * | |||||
|---|---|---|---|---|---|---|
| YC | Yp1 | Yp2 | Yp3 | Yp4 | YG | |
| 1 | 0.75 | 0.75 | 0.74 | 0.49 | 0.53 | 0.55 |
| 5 | 1.22 | 0.98 | 0.95 | 0.42 | 0.44 | 0.41 |
| 10 | 1.75 | 1.78 | 1.75 | 0.51 | 0.52 | 0.48 |
| 15 | 1.95 | 1.89 | 1.88 | 0.61 | 0.59 | 0.53 |
| Means | 1.41 ± 0.54 a | 1.35 ± 0.56 a | 1.33 ± 0.56 a | 0.50 ± 0.07 b | 0.52 ± 0.06 b | 0.49 ± 0.06 b |
| Storage Period (Days) | Yogurt Treatments * | |||||
|---|---|---|---|---|---|---|
| YC | Yp1 | Yp2 | Yp3 | Yp4 | YG | |
| 1 | 4.74 | 4.75 | 4.71 | 4.75 | 4.74 | 4.74 |
| 5 | 4.16 | 4.21 | 4.25 | 4.73 | 4.75 | 4.72 |
| 10 | 3.89 | 4.03 | 4.11 | 4.69 | 4.72 | 4.70 |
| 15 | 3.64 | 3.67 | 3.75 | 4.61 | 4.63 | 4.59 |
| Means | 4.10 ± 0.47 a | 4.16 ± 0.45 a | 4.20 ± 0.39 a | 4.69 ± 0.06 b | 4.71 ± 0.05 b | 4.68 ± 0.06 b |
| Storage Period (Days) | Yogurt Treatments * | |||||
|---|---|---|---|---|---|---|
| YC | Yp1 | Yp2 | Yp3 | Yp4 | YG | |
| 1 | 6.15 | 5.98 | 6.08 | 2.21 | 2.19 | 2.18 |
| 5 | 4.85 | 4.91 | 4.81 | 2.15 | 2.01 | 2.07 |
| 10 | 3.92 | 3.65 | 3.83 | 1.87 | 1.86 | 1.91 |
| 15 | 2.88 | 2.77 | 2.81 | 1.79 | 1.77 | 1.75 |
| Means | 4.45 ± 1.39 a | 4.32 ± 1.40 a | 4.38 ± 1.39 a | 2.0 ± 0.20 b | 1.95 ± 0.18 b | 1.97 ± 0.18 b |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altemimi, A.B. Extraction and Optimization of Potato Starch and Its Application as a Stabilizer in Yogurt Manufacturing. Foods 2018, 7, 14. https://doi.org/10.3390/foods7020014
Altemimi AB. Extraction and Optimization of Potato Starch and Its Application as a Stabilizer in Yogurt Manufacturing. Foods. 2018; 7(2):14. https://doi.org/10.3390/foods7020014
Chicago/Turabian StyleAltemimi, Ammar B. 2018. "Extraction and Optimization of Potato Starch and Its Application as a Stabilizer in Yogurt Manufacturing" Foods 7, no. 2: 14. https://doi.org/10.3390/foods7020014
APA StyleAltemimi, A. B. (2018). Extraction and Optimization of Potato Starch and Its Application as a Stabilizer in Yogurt Manufacturing. Foods, 7(2), 14. https://doi.org/10.3390/foods7020014





