Ultraviolet Irradiation Effect on Apple Juice Bioactive Compounds during Shelf Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
2.2. Juice Characterization Colorimetric Measurements
2.3. Colorimetric Measurements
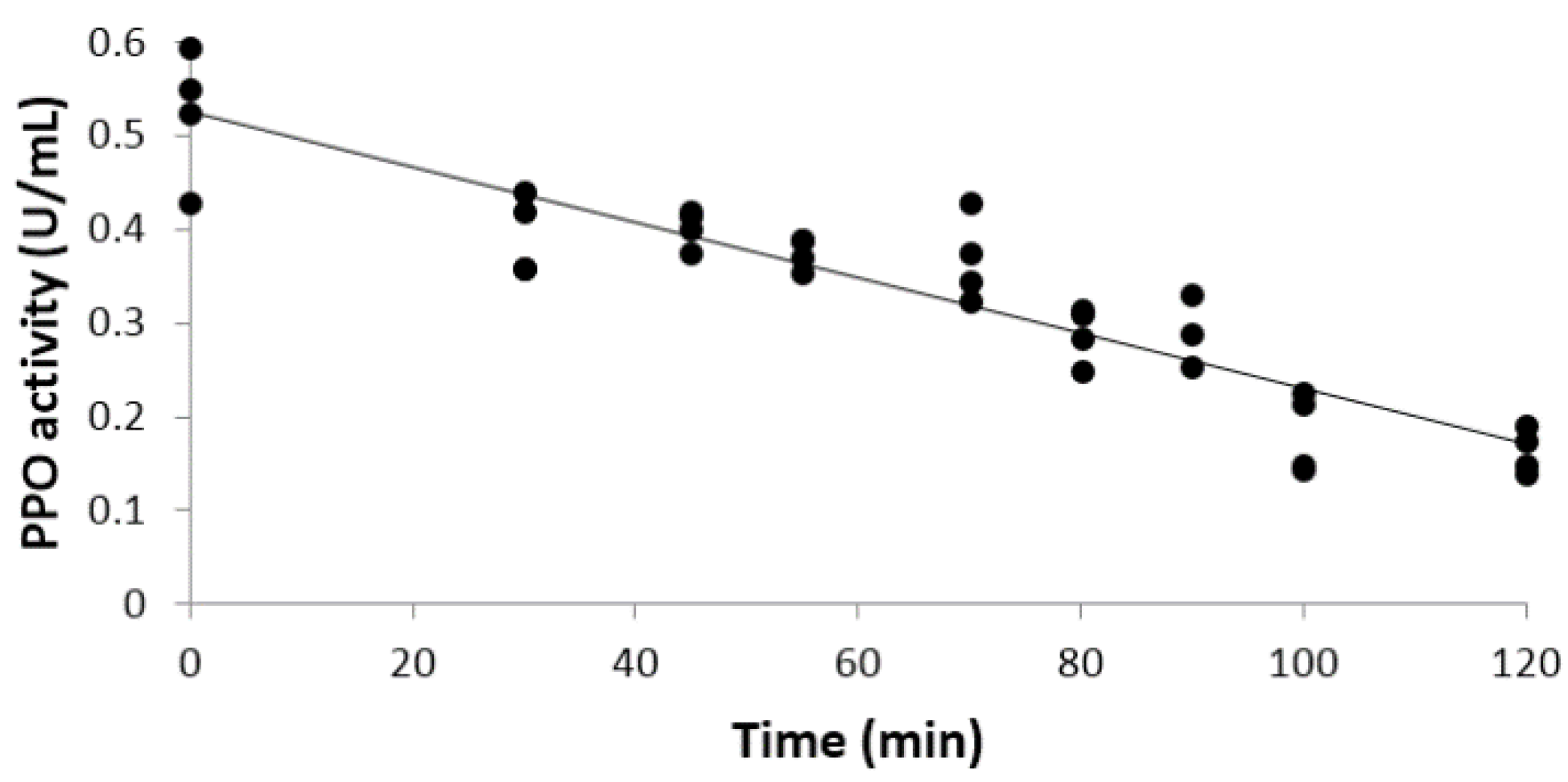
2.4. Polyphenol oxidase (PPO) Activity Determination
2.5. UV Treatment and Kinetic Data
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ravn-Haren, G.; Dragsted, L.; Buch-Andersen, T.; Jensen, E.; Jensen, R.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.; Koch, T.L.; Watzl, B.; Dietrich, H.; Will, F.; Bub, A. Moderate effects of apple juice consumption on obesity-related markers in obese men: Impact of diet–gene interaction on body fat content. Eur. J. Nutr. 2012, 51, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C. Cancer chemopreventive potential of apples, apple juice, and apple components. Plant Media 2008, 74, 1608–1624. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.; Huemmer, W.; Kempf, M.; Scheppach, W.; Erk, T.; Richling, E. Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. J. Agric. Food Chem. 2007, 55, 10605–10614. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, A.A.; Dekker, M.; Skrede, G.; Jongen, W.M.F. Activity and concentration of polyphenolic antioxidants in apple juice. 1. Effect of existing production methods. J. Agric. Food Chem. 2002, 50, 7211–7219. [Google Scholar] [CrossRef] [PubMed]
- Noci, F.; Riener, J.; Walkling-Ribeiro, M.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. J. Food Eng. 2008, 85, 141–146. [Google Scholar] [CrossRef]
- Donahue, D.W.; Canitez, N.; Bushway, A.A. UV inactivation of E. Coli o157:H7 in apple cider: Quality, Sensory and Shelf-Life Analysis. J. Food Process. Preserv. 2004, 28, 368–387. [Google Scholar] [CrossRef]
- Basaran, N.; Quintero-Ramos, A.; Moake, M.M.; Churey, J.J.; Worobo, R.W. Influence of apple cultivars on inactivation of different strains of Escherichia coli O157:H7 in apple cider by UV irradiation. App. Environ. Microbiol. 2004, 70, 6061–6065. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, I.; Palgan, I.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D.; Cronin, D.; Lyng, J. The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food Bioprocess Technol. 2012, 5, 680–686. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E. Evaluation of Traditional Thermal Method (HTST-UHT) and Alternative Methods (UV and HPP) on Clarified Apple Juice Pasteurization; Autonomous University of Chihuahua: Chihuahua, Mexico, 2013. [Google Scholar]
- Juarez-Enriquez, E.; Salmeron-Ochoa, I.; Gutierrez-Mendez, N.; Ramaswamy, H.S.; Ortega-Rivas, E. Shelf life studies on apple juice pasteurised by ultrahigh hydrostatic pressure. LWT-Food Sci. Technol. 2015, 62, 915–919. [Google Scholar] [CrossRef]
- Gómez, P.; Salvatori, D.; García-Loredo, A.; Alzamora, S. Pulsed light treatment of cut apple: Dose effect on color, structure, and microbiological stability. Food Bioprocess Technol. 2012, 5, 2311–2322. [Google Scholar] [CrossRef]
- Igual, M.; Contreras, C.; Camacho, M.M.; Martínez-Navarrete, N. Effect of thermal treatment and storage conditions on the physical and sensory properties of grapefruit juice. Food Bioprocess Technol. 2014, 7, 191–203. [Google Scholar] [CrossRef]
- Fernández-Vázquez, R.; Stinco, C.M.; Hernanz, D.; Heredia, F.J.; Vicario, I.M. Colour training and colour differences thresholds in orange juice. Food Qual. Preference 2013, 30, 320–327. [Google Scholar] [CrossRef]
- Cano, M.P.; Hernandez, A.; de Ancos, B. High pressure and temperature effects on enzyme inactivation in strawberry and orange products. J. Food Sci. 1997, 62, 85–88. [Google Scholar] [CrossRef]
- Gabriel, A.A.; Nakano, H. Inactivation of salmonella, E. Coli and listeria monocytogenes in phosphate-buffered saline and apple juice by ultraviolet and heat treatments. Food Control 2009, 20, 443–446. [Google Scholar] [CrossRef]
- Gachovska, T.K.; Kumar, S.; Thippareddi, H.; Subbiah, J.; Williams, F. Ultraviolet and pulsed electric field treatments have additive effect on inactivation of E. Coli in apple juice. J. Food Sci. 2008, 73, M412–M417. [Google Scholar] [CrossRef] [PubMed]
- Falguera, V.; Pagán, J.; Garza, S.; Garvín, A.; Ibarz, A. Inactivation of polyphenol oxidase by ultraviolet irradiation: Protective effect of melanins. J. Food Eng. 2012, 110, 305–309. [Google Scholar] [CrossRef]
- Falguera, V.; Pagán, J.; Ibarz, A. Effect of UV irradiation on enzymatic activities and physicochemical properties of apple juices from different varieties. LWT-Food Sci. Technol. 2011, 44, 115–119. [Google Scholar] [CrossRef]
- Azhuvalappil, Z.; Fan, X.; Geveke, D.J.; Zhang, H.Q. Thermal and nonthermal processing of apple cider: Storage quality under equivalent process conditions. J. Food Qual. 2010, 33, 612–631. [Google Scholar] [CrossRef]
- Tandon, K.; Worobo, R.W.; Churey, J.J.; Padilla-Zakour, O.I. Storage quality of pasteurized and UV treated apple cider. J. Food Process. Preserv. 2003, 27, 21–35. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V. Inactivation of saccharomyces cerevisiae and polyphenoloxidase in mango nectar treated with UV light. J. Food Prot. 2006, 69, 362–368. [Google Scholar] [PubMed]
- Miller, N.J.; Rice-Evans, C.A. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink. Food Chem. 1997, 60, 331–337. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Anantheswaran, R.C.; LaBorde, L.F. Ascorbic acid degradation in a model apple juice system and in apple juice during ultraviolet processing and storage. J. Food Sci. 2011, 76, H62–H71. [Google Scholar] [CrossRef] [PubMed]
- Clegg, K.M.; Morton, A.D. The phenolic compounds of blackcurrant juice and their protective effect on ascorbic acid. Int. J. Food Sci. Technol. 1968, 3, 277–284. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Martí, N.; Pérez-Vicente, A.; García-Viguera, C. Influence of storage temperature and ascorbic acid addition on pomegranate juice. J. Sci. Food Agric. 2002, 82, 217–221. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, G.H.; Lee, H.S. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int. 2002, 35, 753–759. [Google Scholar] [CrossRef]
| Property | Clarified Juice | UV Treatment |
|---|---|---|
| pH | 3.74 ± 0.01 a | 3.54 ± 0.03 b |
| Malic acid eq. (mg/mL) | 6.19 ± 0.04 a | 6.06 ± 0.04 a |
| °Brix | 17.97 ± 0.06 a | 18.05 ± 0.07 a |
| Ascorbic acid (ppm) | 119.22 ± 5.04 a | 121.16 ± 0.75 a |
| TEAC (mM) | 10.99 ± 0.16 a | 9.85 ± 0.96 c |
| Gallic acid eq. (mg/L) | 343.17 ± 1.02 a | 346.02 ± 5.04 a |
| Hue | 81.37 ± 0.59 a | 87.57 ± 0.51 b |
| Chroma | 3.29 ± 1.21 a | 5.10 ± 1.32 a |
| Days | Temp (°C) | pH | Acidity (mg/mL) | °Brix | Hue | Chroma | Ascorbic Acid (ppm) | Gallic Acid Eq. (mg/L) | TEAC (mM) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 °C | 3.54 ± 0.03 a | 6.056 ± 0.039 a | 18.15 ± 0.07 ab | 87.57 ± 0.52 a | 5.10 ± 1.32 a | 121.16 ± 0.75 a | 347.70 ± 4.58 a | 9.884 ± 0.079 a |
| 6 | 4 °C | 3.50 ± 0.03 a,b | 6.213 ± 0.077 a | 18.25 ± 0.07 a | 87.59 ± 0.41 a | 4.18 ± 0.64 a,b | 108.72 ± 0.83 b | 334.35 ± 1.11 b | 8.031 ± 0.144 b |
| 12 | 4 °C | 3.48 ± 0.02 b | 6.168 ± 0.005 a | 17.25 ± 0.07 c | 88.01 ± 0.52 a | 3.32 ± 0.37 a,b | 105.60 ± 0.62 b,c | 332.35 ± 1.59 b | 7.445 ± 0.571 c |
| 18 | 4 °C | 3.46 ± 0.02 b | 6.663 ± 0.131 b | 17.85 ± 0.21 a,b | 87.55 ± 0.95 a | 3.98 ± 0.14 a,b | 102.25 ± 5.48 c | 318.06 ± 1.97 b | 7.154 ± 0.250 c |
| 24 | 4 °C | 3.44 ± 0.02 b | 7.084 ± 0.140 c | 17.70 ± 0.14 bc | 85.74 ± 1.41 a | 3.11 ± 0.13 b | 74.42 ± 2.32 d | 337.86 ± 5.06 c | 6.780 ± 0.121 c |
| 1 | 20 °C | 3.54 ± 0.03 a | 6.056 ± 0.039 a | 18.05 ± 0.07 a | 87.57 ± 0.52 a | 5.10 ± 1.32 a | 121.16 ± 0.75 a | 347.70 ± 4.58 a | 9.884 ± 0.079 a |
| 6 | 20 °C | 3.50 ± 0.03 a | 6.213 ± 0.077 a | 17.95 ± 0.07 a | 86.72 ± 0.45 a | 3.68 ± 0.52 a,b | 109.27 ± 0.64 b | 330.21 ± 1.11 b | 8.031 ± 0.144 b |
| 12 | 20 °C | 3.51 ± 0.03 a | 7.017 ± 0.205 b | 17.70 ± 0.14 a | 88.02 ± 0.85 a | 2.91 ± 0.37 b | 106.14 ± 0.93 c | 310.09 ± 11.92 c | 7.751 ± 0.187 b |
| 18 | 20 °C | 3.53 ± 0.02 a | 6.927 ± 0.353 b | 17.60 ± 1.14 a | 82.27 ± 2.80 b | 2.81 ± 0.34 b | 104.44 ± 0.09 d | 315.13 ± 3.31 b,c | 7.980 ± 0.081 b |
| 24 | 20 °C | 3.54 ± 0.01 a | 7.375 ± 0.201 b | 17.65 ± 0.21 a | 86.40 ± 1.32 a | 2.59 ± 0.14 b | 96.07 ± 1.93 e | 330.57 ± 1.54 b | 6.876 ± 0.029 c |
| Parameter | p |
|---|---|
| pH | 0.928 |
| Malic acid eq. | 0.978 |
| °Brix | 0.123 |
| Ascorbic acid | <0.001 |
| Gallic acid eq. | 0.882 |
| Antioxidant capacity | 0.953 |
| Chroma | 0.714 |
| Hue | 0.362 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juarez-Enriquez, E.; Salmerón, I.; Gutierrez-Mendez, N.; Ortega-Rivas, E. Ultraviolet Irradiation Effect on Apple Juice Bioactive Compounds during Shelf Storage. Foods 2016, 5, 10. https://doi.org/10.3390/foods5010010
Juarez-Enriquez E, Salmerón I, Gutierrez-Mendez N, Ortega-Rivas E. Ultraviolet Irradiation Effect on Apple Juice Bioactive Compounds during Shelf Storage. Foods. 2016; 5(1):10. https://doi.org/10.3390/foods5010010
Chicago/Turabian StyleJuarez-Enriquez, Edmundo, Ivan Salmerón, Nestor Gutierrez-Mendez, and Enrique Ortega-Rivas. 2016. "Ultraviolet Irradiation Effect on Apple Juice Bioactive Compounds during Shelf Storage" Foods 5, no. 1: 10. https://doi.org/10.3390/foods5010010







