The Combined Effect of High Hydrostatic Pressure and Calcium Salts on the Stability, Solubility and Gel Formation of β-Lactoglobulin
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Solubility Experiments
2.3. HPLC to Determine Degree of Denaturation
2.4. Texture Analysis
3. Results and Discussion
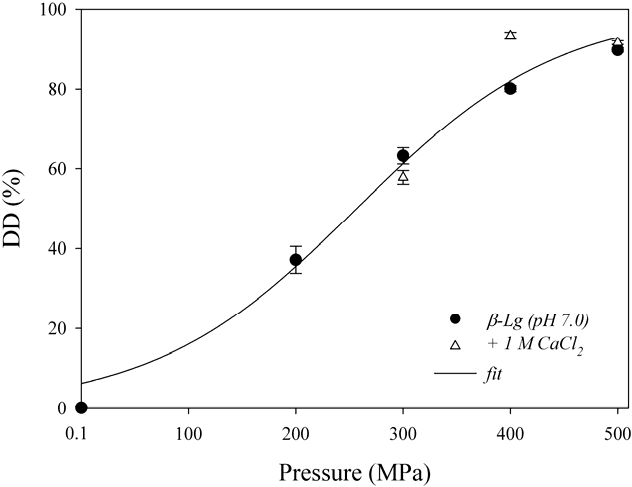


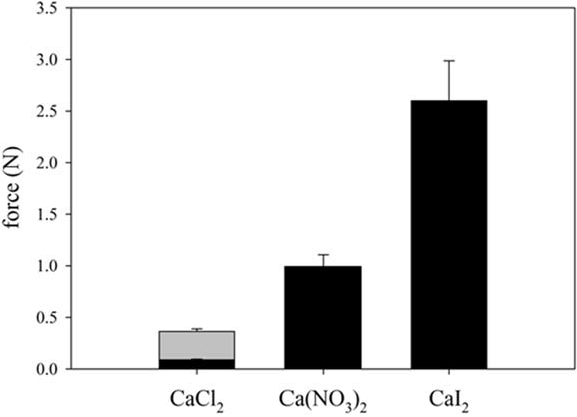
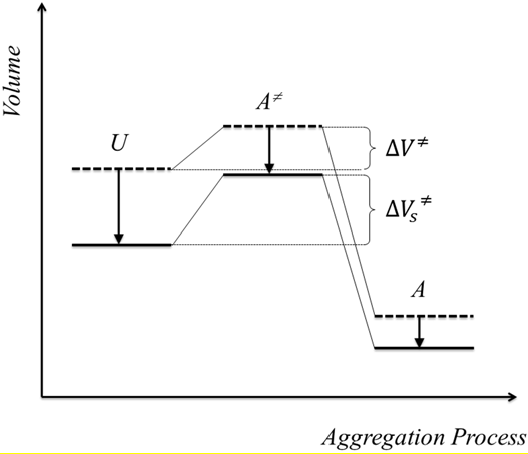
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheftel, J.C. Review: High-pressure, microbial inactivation and food preservation/Revision: Alta-presion, inactivacion microbiologica y conservacion de alimentos. Food Sci. Technol. Int. 1995, 1, 75–90. [Google Scholar] [CrossRef]
- Margosch, D.; Ehrmann, M.A.; Buckow, R.; Heinz, V.; Vogel, R.F.; Gänzle, M.G. High-pressure-mediated survival of Clostridium botulinum and Bacillus amyloliquefaciens endospores at high temperature. Appl. Environ. Microbiol. 2006, 72, 3476–3481. [Google Scholar] [CrossRef] [PubMed]
- Valente-Mesquita, V.L.; Botelho, M.M.; Ferreira, S.T. Pressure-Induced Subunit Dissociation and Unfolding of Dimeric β-Lactoglobulin. Biophys. J. 1998, 75, 471–476. [Google Scholar] [CrossRef]
- Gebhardt, R.; Toro-Sierra, J.; Kulozik, U. Pressure dissociation of β-lactoglobulin oligomers near their isoelectric point. Soft Matter 2012, 8, 11654–11660. [Google Scholar] [CrossRef]
- Stute, R.; Heilbronn; Klingler, R.W.; Boguslawski, S.; Eshtiaghi, M.N.; Knorr, D. Effects of High Pressures Treatment on Starches. Starch/Stärke 1996, 48, 399–408. [Google Scholar] [CrossRef]
- Gebhardt, R.; Hanfland, M.; Mezouar, M.; Riekel, C. High-pressure potato starch granule gelatinization: Synchrotron radiation micro-SAXS/WAXS using a diamond anvil cell. Biomacromolecules 2007, 8, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Aguirre, O.; Stuetz, W.; Grune, T.; Kessler, A.; Weiss, J.; Hinrichs, J. High pressure-assisted encapsulation of vitamin D 2 in reassembled casein micelles. High Press. Res. 2011, 31, 265–274. [Google Scholar] [CrossRef]
- Ueda, I.; Riegel, I.; Kulozik, U.; Gebhardt, R. Effect of hydrostatic pressure treatment on the structure–foaming relationships of β-lactoglobulin. High Press. Res. 2014, 34, 419–427. [Google Scholar] [CrossRef]
- Leberman, R.; Soper, A.K. Effect of high salt concentrations on water structure. Nature 1995, 378, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Parsegian, V.A. Hopes for Hofmeister. Nature 1995, 378, 335–336. [Google Scholar] [CrossRef]
- Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Gebhardt, R.; Kulozik, U. High pressure stability of protein complexes studied by static and dynamic light scattering. High Press. Res. 2011, 31, 243–252. [Google Scholar] [CrossRef]
- Gebhardt, R.; Takeda, N.; Kulozik, U.; Doster, W. Structure and stabilizing interactions of casein micelles probed by high-pressure light scattering and FTIR. J. Phys. Chem. B 2011, 115, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.-X.; Zhou, P.; Hu, B.-W.; Ji, D. An investigation into the effect of potassium ions on the folding of silk fibroin studied by generalized two-dimensional NMR-NMR correlation and Raman spectroscopy. FEBS J. 2008, 275, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R.; Vendrely, C.; Hanfland, M.; Riekel, C. Silk Fiber Formation after High-Pressure Treatment of Fibroin Solution in a Diamond Anvil Cell. Macromolecules 2008, 41, 9934–9936. [Google Scholar] [CrossRef]
- Renard, D.; Lefebvre, J. Gelation of globular proteins: Effect of pH and ionic strength on the critical concentration for gel formation. A simple model and its application to β-lactoglobulin heat-induced gelation. Int. J. Biol. Macromol. 1992, 14, 287–291. [Google Scholar] [CrossRef]
- McSwiney, M.; Singh, H.; Campanella, O.H. Thermal aggregation and gelation of bovine β-lactoglobulin. Food Hydrocolloids 1994, 8, 441–453. [Google Scholar] [CrossRef]
- Spiegel, T. Whey protein aggregation under shear conditions-effects of lactose and heating temperature on aggregate size and structure. Int. J. Food Sci. Tech. 1999, 34, 523–531. [Google Scholar] [CrossRef]
- Verheul, M.; Sebastianus, P.F.M.R.; de Kruif, K.G. Kinetics of Heat-Induced Aggregation of β-Lactoglobulin. J. Agric. Food Chem. 1998, 46, 896–903. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 1998, 9, 143–151. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Anema, S.G.; Zhu, C.; Havea, P.; Coker, C. Water holding capacity and swelling of casein hydrogels. Food Hydrocolloids 2015, 44, 372–379. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Mechanism of protein salting in and salting out by divalent cation salts: Balance between hydration and salt binding. Biochemistry 1984, 23, 5912–5923. [Google Scholar] [CrossRef] [PubMed]
- Majhi, P.R.; Ganta, R.R.; Vanam, R.P.; Seyrek, E.; Giger, K.; Dubin, P.L. Electrostatically driven protein aggregation: Beta-lactoglobulin at low ionic strength. Langmuir ACS J. Surf. Colloids 2006, 22, 9150–9159. [Google Scholar] [CrossRef] [PubMed]
- Funtenberger, S.; Dumay, E.; Cheftel, J.C. High Pressure Promotes β-Lactoglobulin Aggregation through SH/S-S Interchange Reactions. J. Agric. Food Chem. 1997, 45, 912–921. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Dumay, E. Effects of high pressure on food biopolymers with special reference to β-lactoglobulin. In The Properties of Water in Foods ISOPOW 6; Reid, D., Ed.; Blackie Academic & Professional: New York, NY, USA, 1998; pp. 369–397. [Google Scholar]
- Toro-Sierra, J.; Tolkach, A.; Kulozik, U. Fractionation of α-Lactalbumin and β-Lactoglobulin from Whey Protein Isolate Using Selective Thermal Aggregation, an Optimized Membrane Separation Procedure and Resolubilization Techniques at Pilot Plant Scale. Food Bioprocess Technol. 2013, 6, 1032–1043. [Google Scholar] [CrossRef]
- Townend, R.; Winterbottom, R.J.; Timasheff, S.N. Molecular Interactions in β-Lactoglobulin. II. Ultracentrifugal and Electrophoretic Studies of the Association of β-Lactoglobulin below its Isoelectric Point 2. J. Am. Chem. Soc. 1960, 82, 3161–3168. [Google Scholar] [CrossRef]
- Botelho, M.M.; Valente-Mesquita, V.L.; Oliveira, Kesley M.G.; Polikarpov, I.; Ferreira, S.T. Pressure denaturation of β-lactoglobulin. Eur. J. Biochem. 2000, 267, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. High pressure effects on protein structure and function. Proteins 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Petit, J.; Herbig, A.-L.; Moreau, A.; Delaplace, G. Influence of calcium on β-lactoglobulin denaturation kinetics: Implications in unfolding and aggregation mechanisms. J. Dairy Sci. 2011, 94, 5794–5810. [Google Scholar] [CrossRef] [PubMed]
- Melander, W.; Horváth, C. Salt effects on hydrophobic interactions in precipitation and chromatography of proteins: An interpretation of the lyotropic series. Arch. Biochem. Biophys. 1977, 183, 200–215. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N., III. Theory of protein solubility. In Methods in Enzymology: Diffraction Methods for Biological Macromolecules Part A; Wyckoff, H.W., Hirs, C.H.W., Timasheff, S.N., Eds.; Academic Press: Orlando, FL, USA, 1985; pp. 49–77. [Google Scholar]
- Shimada, K.; Cheftel, J.C. Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J. Agric. Food Chem. 1989, 37, 161–168. [Google Scholar] [CrossRef]
- Zittle, C.A.; DellaMonica, E.S.; Rudd, R.K.; Custer, J.H. The Binding of Calcium Ions by β-Lactoglobulin Both before and after Aggregation by Heating in the Presence of Calcium Ions. J. Am. Chem. Soc. 1957, 79, 4661–4666. [Google Scholar] [CrossRef]
- Simons, Jan-Willem F. A; Kosters, H.A.; Visschers, R.W.; de Jongh, Harmen H.J. Role of calcium as trigger in thermal β-lactoglobulin aggregation. Arch. Biochem. Biophys. 2002, 406, 143–152. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Abnormal solubility behavior of .beta.-lactoglobulin: Salting-in by glycine and sodium chloride. Biochemistry 1987, 26, 5147–5153. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Damodaran, S. Thermal gelation of globular proteins: Weight-average molecular weight dependence of gel strength. J. Agric. Food Chem. 1990, 38, 1157–1164. [Google Scholar] [CrossRef]
- Gross, M.; Jaenicke, R. Proteins under pressure. The influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur. J. Biochem. 1994, 221, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Foguel, D.; Silva, J.L. New insights into the mechanisms of protein misfolding and aggregation in amyloidogenic diseases derived from pressure studies. Biochemistry 2004, 43, 11361–11370. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, A.; Kulozik, U. Reaction kinetic pathway of reversible and irreversible thermal denaturation of β-lactoglobulin. Lait 2007, 87, 301–315. [Google Scholar] [CrossRef]
- Hirano, A.; Hamada, H.; Okubo, T.; Noguchi, T.; Higashibata, H.; Shiraki, K. Correlation Between Thermal Aggregation and Stability of Lysozyme with Salts Described by Molar Surface Tension Increment: An Exceptional Propensity of Ammonium Salts as Aggregation Suppressor. Protein J. 2007, 26, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Kelly, A.L.; Fox, P.F. Effects of high pressure on constituents and properties of milk. Int. Dairy J. 2002, 12, 561–572. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saalfeld, D.; Riegel, I.; Kulozik, U.; Gebhardt, R. The Combined Effect of High Hydrostatic Pressure and Calcium Salts on the Stability, Solubility and Gel Formation of β-Lactoglobulin. Foods 2015, 4, 229-239. https://doi.org/10.3390/foods4020229
Saalfeld D, Riegel I, Kulozik U, Gebhardt R. The Combined Effect of High Hydrostatic Pressure and Calcium Salts on the Stability, Solubility and Gel Formation of β-Lactoglobulin. Foods. 2015; 4(2):229-239. https://doi.org/10.3390/foods4020229
Chicago/Turabian StyleSaalfeld, Daniel, Ina Riegel, Ulrich Kulozik, and Ronald Gebhardt. 2015. "The Combined Effect of High Hydrostatic Pressure and Calcium Salts on the Stability, Solubility and Gel Formation of β-Lactoglobulin" Foods 4, no. 2: 229-239. https://doi.org/10.3390/foods4020229




