Research of Processing Technology of Longjing Tea with ‘Baiye 1’ Based on Non-Targeted Aroma Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Chemicals
2.3. Detection of Volatile Aroma Components
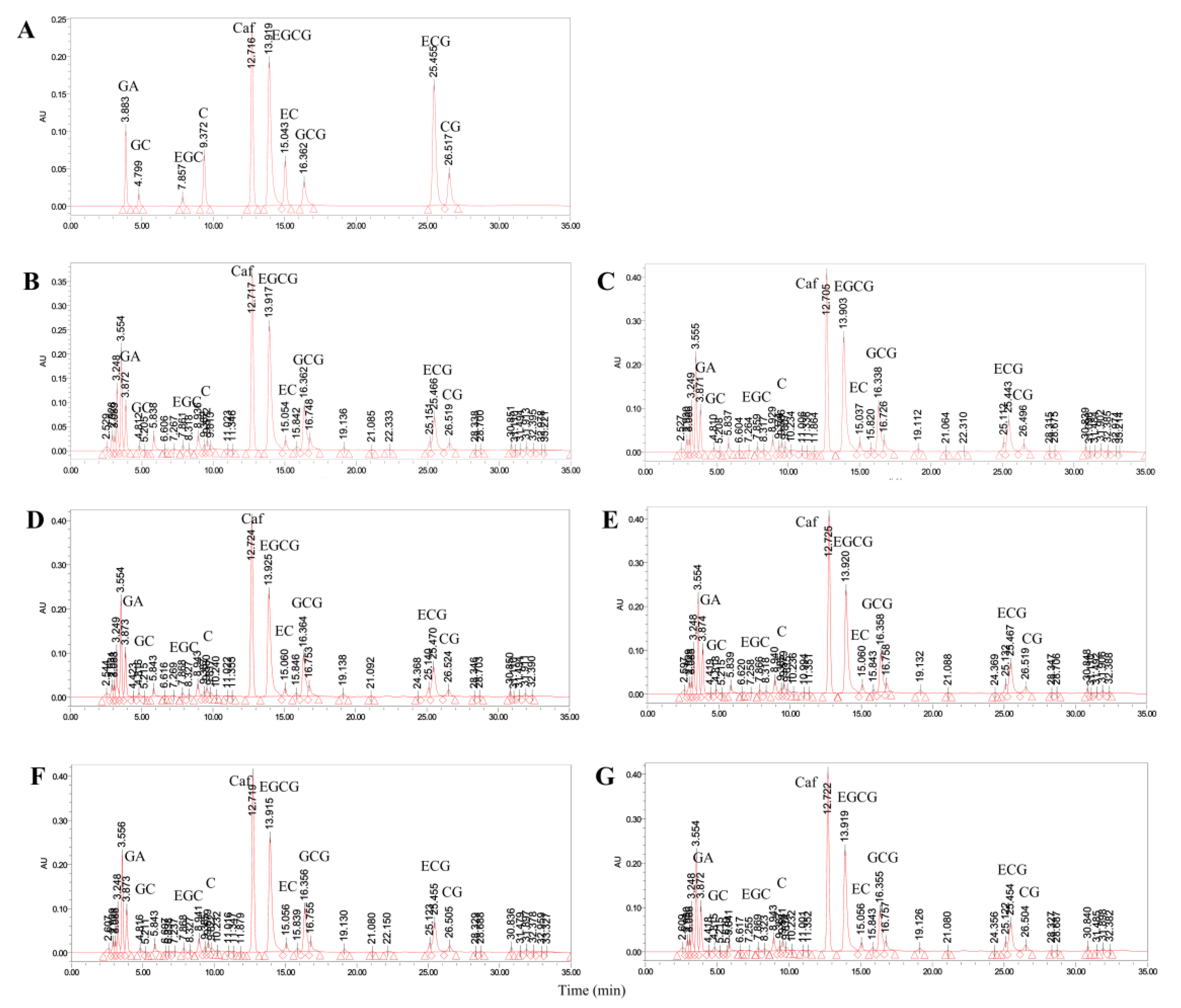
2.4. Qualitative and Quantitative Analyses of Aroma Components in Tea
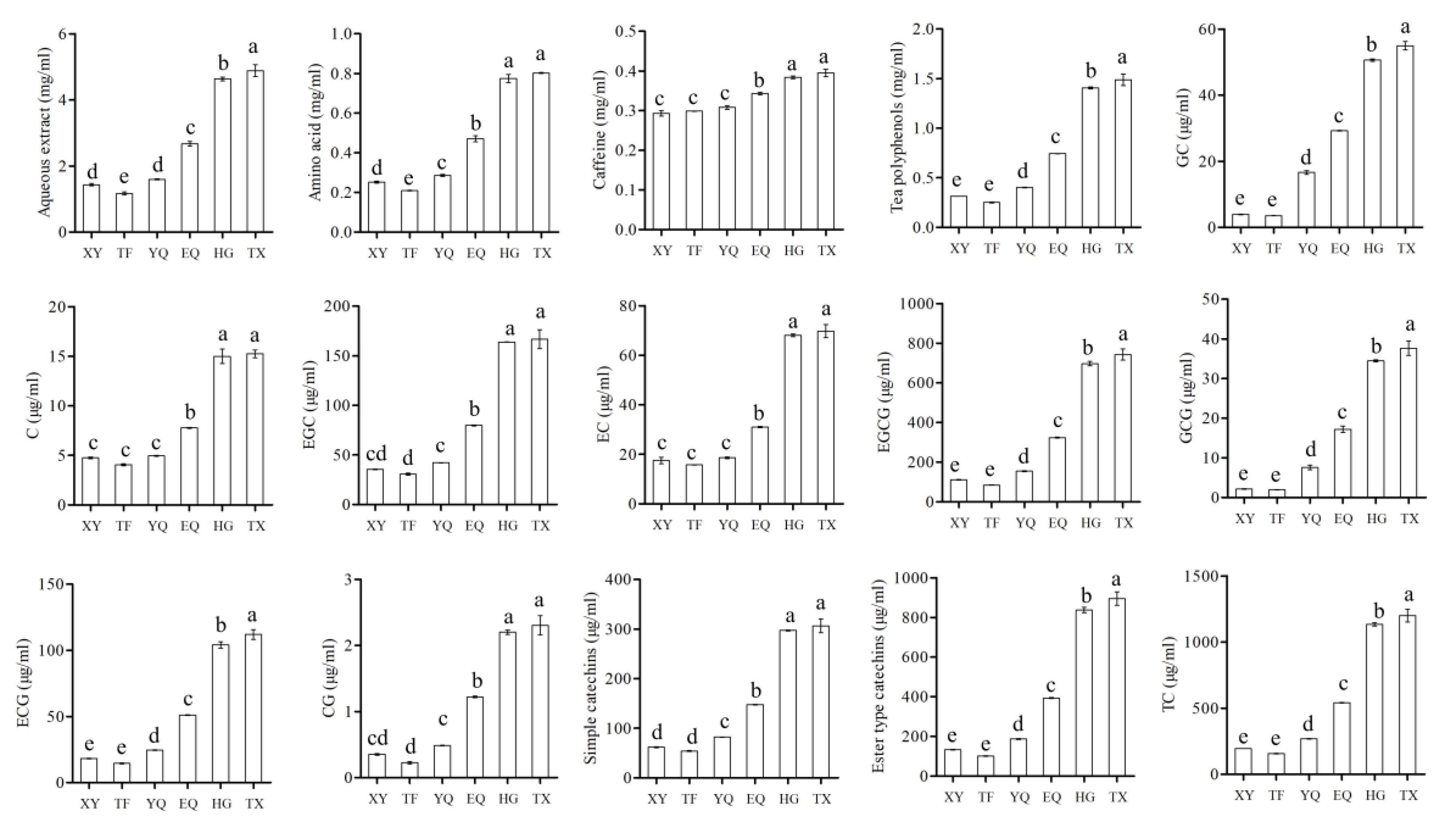
2.5. Determination of Chemical Constituents in Tea Leaves
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
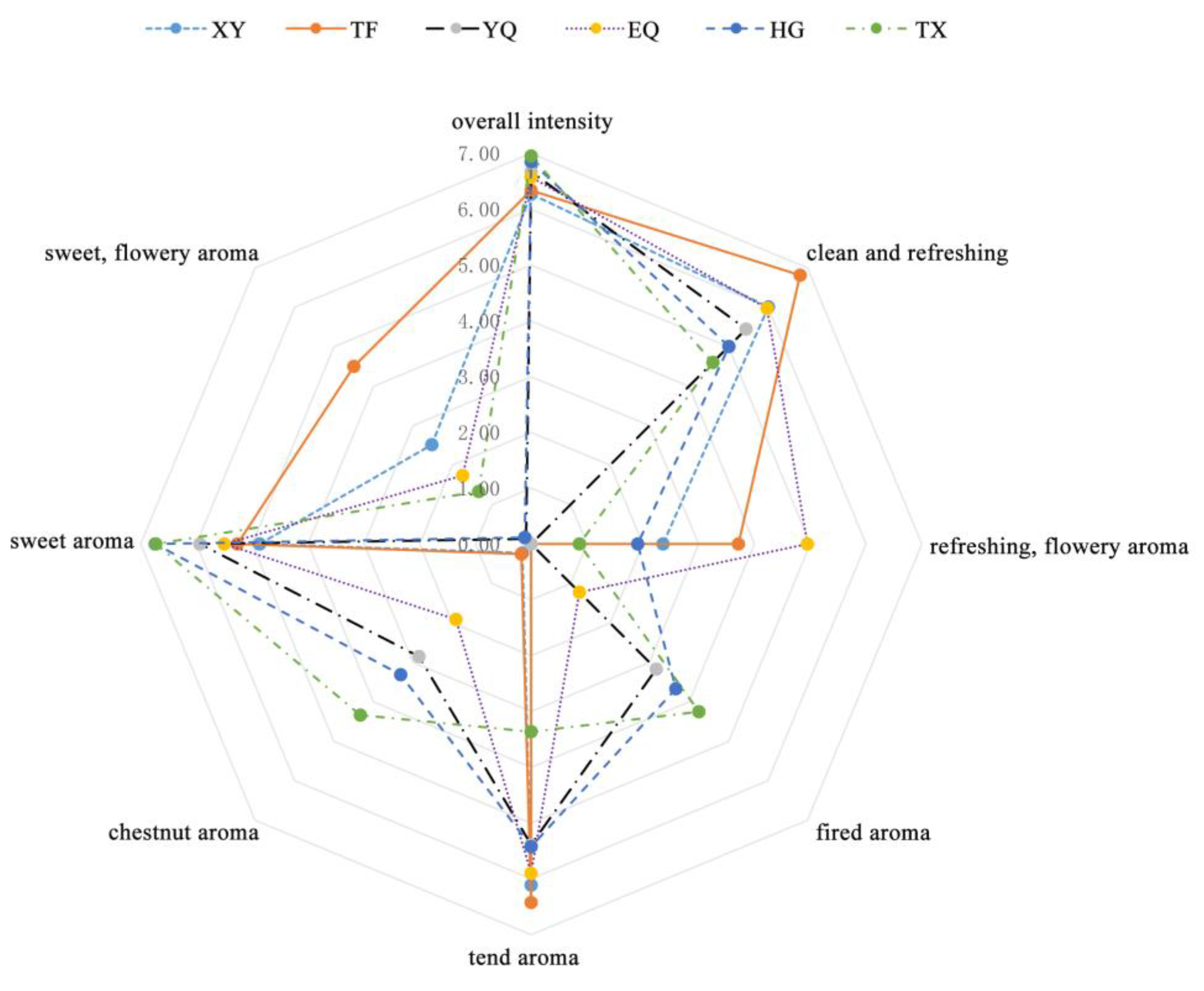
3.1. Aroma Quality Analysis
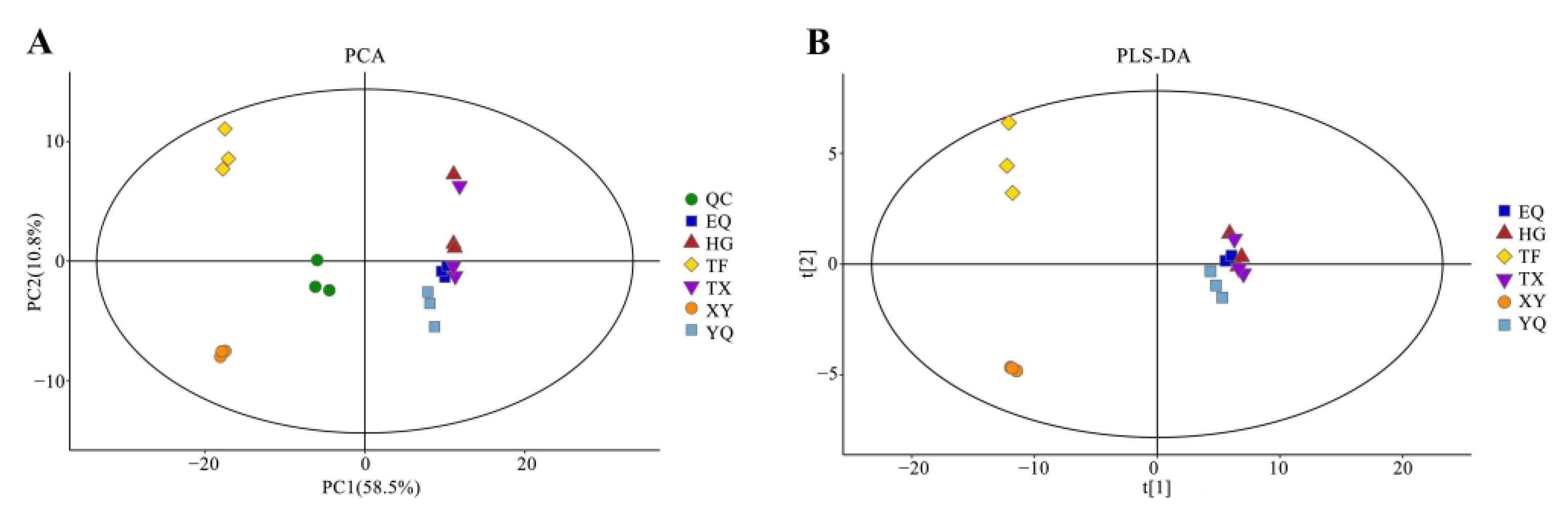
3.2. Multivariate Statistical Analysis of Aroma Metabolites during Tea Leaf Processing
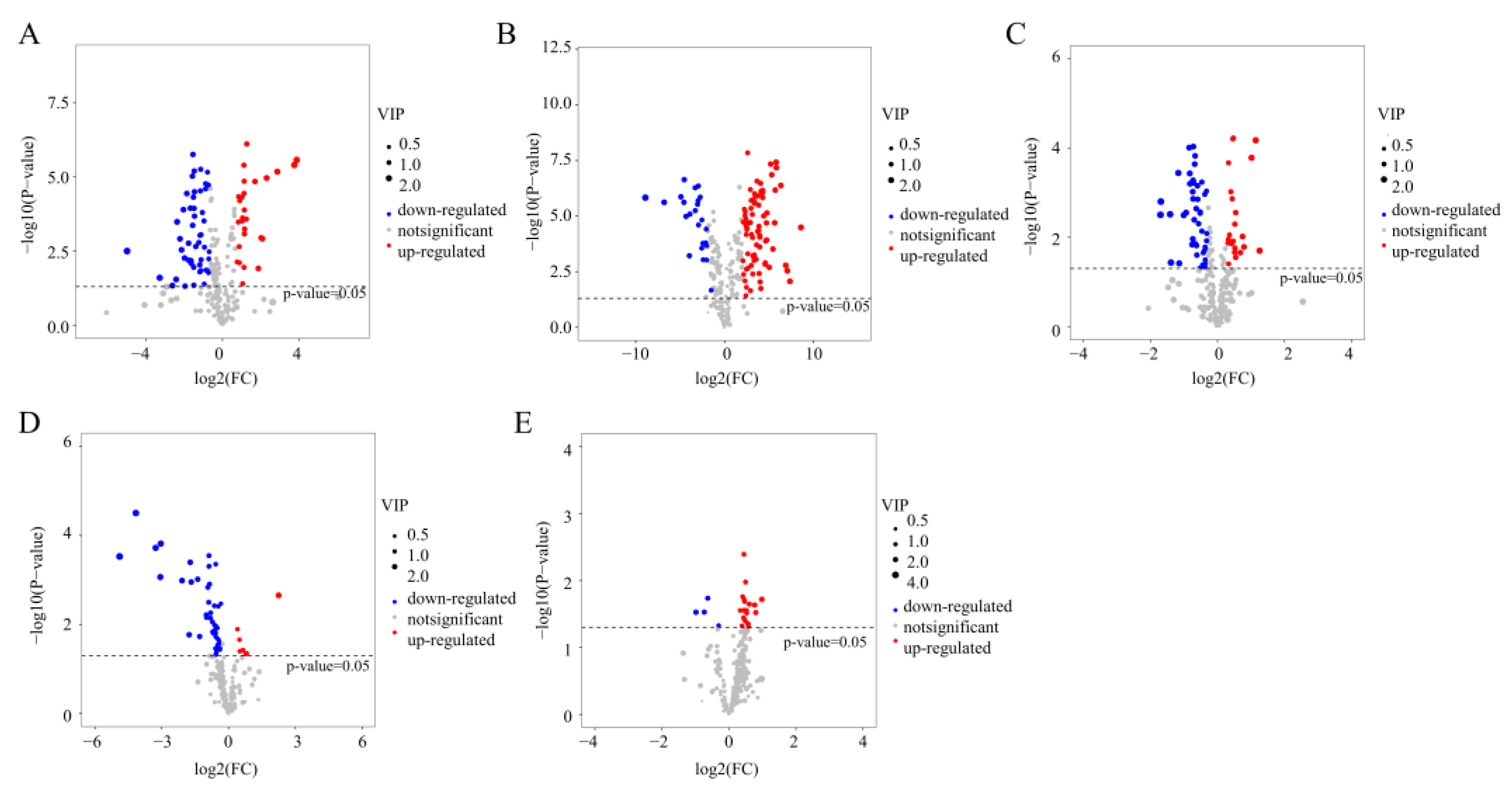
3.3. Metabolic Changes in Aroma Substances during Tea Processing
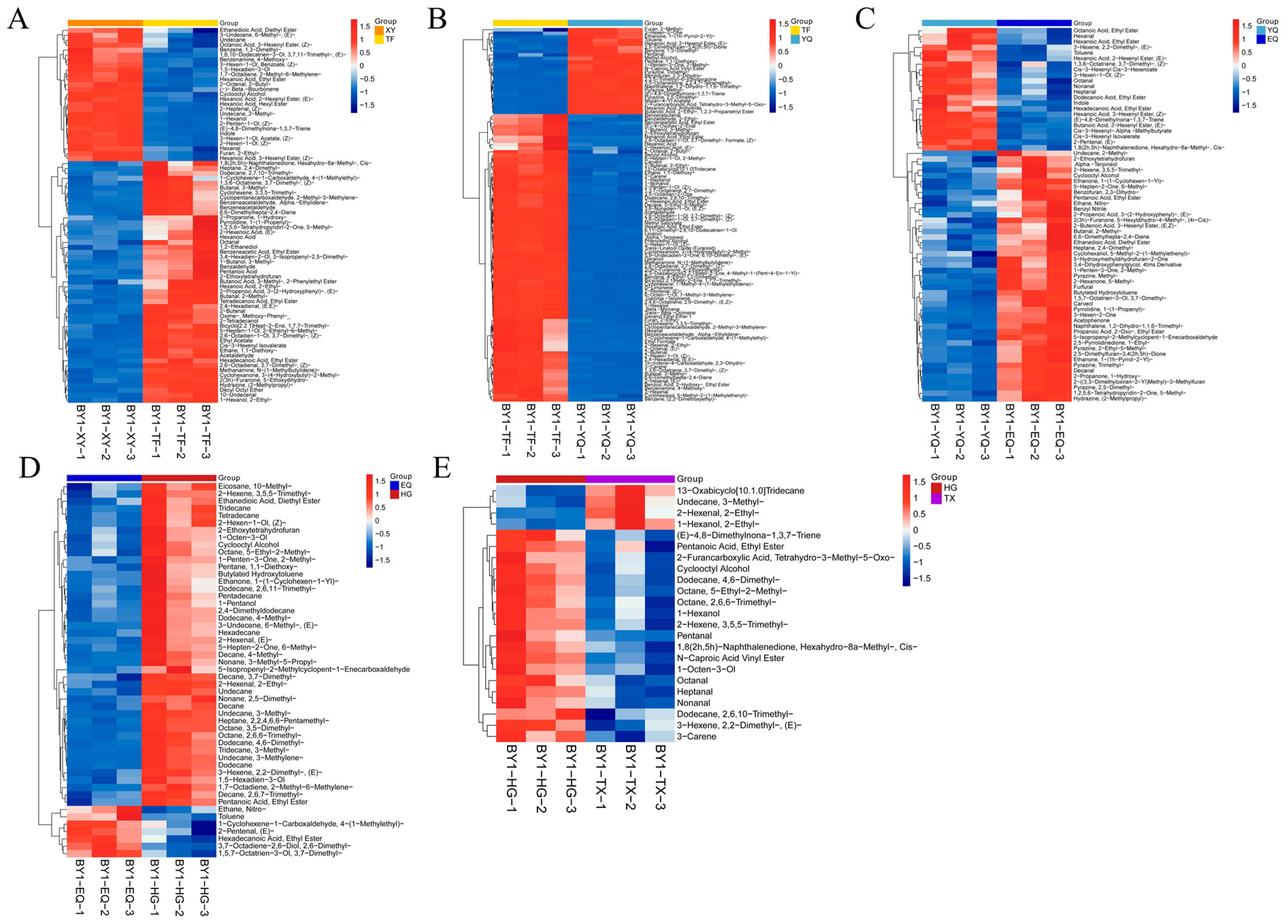
3.4. Metabolite Profiling Analysis
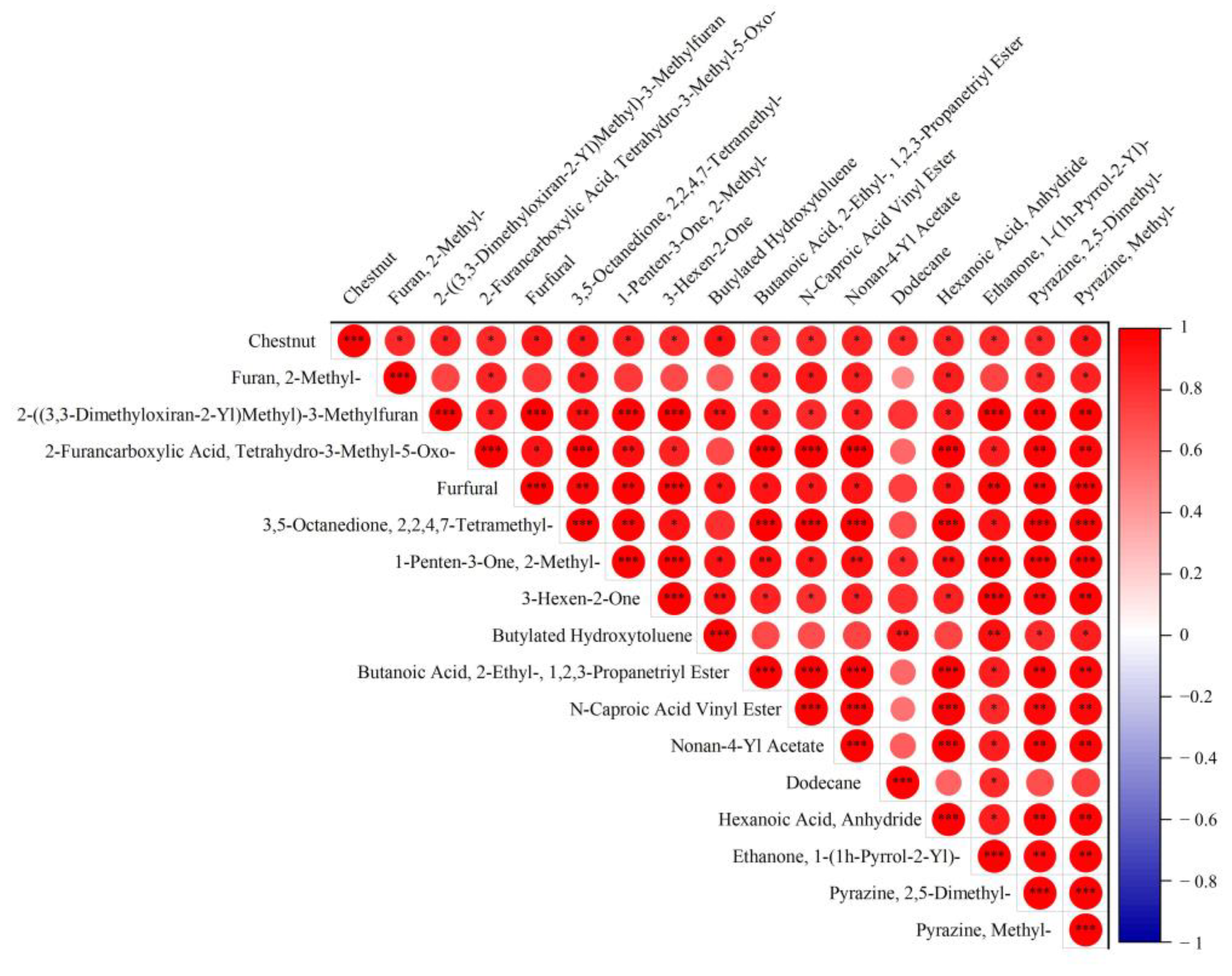
3.5. Characteristic Aroma Component
3.6. Changes in Flavor Substances during Tea Processing
3.7. Metabolic Changes in Taste Substances during Tea Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, P.; Kong, Y.S.; Liu, P.P.; Wang, J.J.; Zhu, Y.; Wang, G.M.; Sun, M.F.; Chen, Y.; Guo, G.Y.; Liu, Z.H. A critical review of key odorants in green tea: Identification and biochemical formation pathway. Trends Food Sci. Technol. 2022, 129, 221–232. [Google Scholar] [CrossRef]
- Wang, J.Q.; Fu, Y.Q.; Chen, J.X.; Wang, F.; Feng, Z.H.; Yin, J.F.; Zeng, L.; Xu, Y.Q. Effects of baking treatment on the sensory quality and physicochemical properties of green tea with different processing methods. Food Chem. 2022, 380, 132217. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Q.; Ma, W.-J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC–MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, Y.; Lin, J.; Lv, H.; Zhou, W.; Zhou, X.; Qian, Y.; Zeng, X.; He, W.; Ye, Y. Comparison of volatile and nonvolatile metabolites in green tea under hot-air drying and four heat-conduction drying patterns using widely targeted metabolomics. Food Chem. X 2023, 19, 100767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qian, M.C.; Deng, Y.; Yuan, H.; Jiang, Y. Insight into aroma dynamic changes during the whole manufacturing process of chestnut-like aroma green tea by combining GC-E-Nose, GC-IMS, and GC × GC-TOFMS. Food Chem. 2022, 387, 132813. [Google Scholar] [CrossRef]
- Cui, H.; Mao, Y.; Zhao, Y.; Huang, H.; Yin, J.; Yu, J.; Zhang, J. Comparative metabolomics study of four kinds of Xihu Longjing tea based on machine fixing and manual fixing methods. Foods 2023, 12, 4486. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhai, X.; Guo, D.; Du, W.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C. Characterization of key odorants in xinyang maojian green tea and their changes during the manufacturing process. J. Agric. Food Chem. 2022, 70, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kurasawa, E.; Yamaguchi, Y.; Kubota, K.; Kobayashi, A. Analysis of glycosidically bound aroma precursors in tea leaves. 2. changes in glycoside contents and glycosidase activities in tea leaves during the black tea manufacturing process. J. Agric. Food Chem. 2001, 49, 1900–1903. [Google Scholar] [CrossRef]
- Guo, X.Y.; Song, C.; Ho, C.T.; Wan, X. Contribution of l-theanine to the formation of 2,5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018, 263, 18–28. [Google Scholar] [CrossRef]
- Han, Z.X.; Rana, M.M.; Liu, G.F.; Gao, M.J.; Li, D.X.; Wu, F.G.; Li, X.B.; Wan, X.C.; Wei, S. Green tea flavour determinants and their changes over manufacturing processes. Food Chem. 2016, 212, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhou, X.; Mo, X.; Guo, Y.; Hu, Y.; Gong, X. Dynamic changes of aromatic substances during the formation process of aroma quality in roasted green tea. Guizhou Agric. Sci. 2017, 45, 122–126. [Google Scholar]
- Ni, D.; Chen, Y.; Hu, J. Effect of temperature on flavor during second drying of roasted tea. J. Huazhong Agric. Univ. 1996, 15, 94–99. [Google Scholar]
- Shao, C.Y.; Zhang, Y.; Lv, H.P.; Zhang, Z.F.; Zeng, J.M.; Peng, Q.H.; Zhu, Y.; Lin, Z. Aromatic profiles and enantiomeric distributions of chiral odorants in baked green teas with different picking tenderness. Food Chem. 2022, 388, 132969. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Yin, H.; Yang, Y.; Yao, Y.; Zhang, M.; Wang, J.; Jiang, Y.; Yuan, H. Discrimination of different characteristics of chestnut-like green tea based on gas chromatography-mass spectrometry and multivariate statistical data analysis. Food Sci. 2019, 40, 192–198. [Google Scholar]
- Feng, Z.; Li, Y.; Zhang, P.; Wang, J.; Xu, Y.; Feng, Y.; Zhai, X.; Yang, X.; Wan, X.; Yin, J. Formation and isomerization of (Z)-methyl epijasmonate, the key contributor of the orchid-like aroma, during tea processing. Food Res. Int. 2023, 172, 113186. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Sawai, Y.; Yamaguchi, Y. Analysis of key odorants in roasted green tea. Tea Res. J. 2008, 2008, 10543. [Google Scholar] [CrossRef]
- Wang, B.; Shu, N.; Lu, A.; Liao, X.; Yan, J.; Xie, G.; Tong, H. Aroma formation of green tea effected by different pan-fire temperature. Food Ferment. Ind. 2020, 46, 197–203. [Google Scholar]
- Zhang, M.; Yin, H.; Deng, Y.; Yao, Y.; Jiang, Y.; Hua, J.; Yuan, H.; Yang, Y. Analysis of key odorants responsible for different chestnut-like aromas of green teas based on headspace solid-phase microextraction coupled with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry and odor activity value. Food Sci. 2020, 41, 244–252. [Google Scholar]
- An, H.; Ou, X.; Zhang, Y.; Li, S.; Xiong, Y.; Li, Q.; Huang, J.; Liu, Z. Study on the key volatile compounds and aroma quality of jasmine tea with different scenting technology. Food Chem. 2022, 385, 132718. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, J.; Ao, C.; Huang, H.; Zheng, X.; Zhao, Y.; Shi, D.; Yu, J. Difference quality characteristics of Xihu Longjing tea with different processing technology. Sci. Technol. Food Ind. 2021, 42, 268–273. [Google Scholar]
- GB/T 23776-2009; Tea-Determination of Free Amino Acids Content. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Ye, T.; Mengxin, W.; Jinhe, W.; Baoyu, H. Correlation of low temperature with soluble sugar and amino acid content in fresh tea leaves. J. Tea Sci. 2015, 35, 567–573. [Google Scholar]
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Botelho, B.G.; Reis, N.; Oliveira, L.S.; Sena, M.M. Development and analytical validation of a screening method for simultaneous detection of five adulterants in raw milk using mid-infrared spectroscopy and PLS-DA. Food Chem. 2015, 181, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Wang, J.; Yuan, H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, Y.; Yin, H.; Yang, Y.; Deng, Y.; Dong, C.; Li, J.; Hua, J.; Wang, J. Chestnut-like aroma of green tea: Research progress of formation and technology. Chin. Agric. Sci. Bull. 2020, 36, 129–137. [Google Scholar]
- Cui, J.; Katsuno, T.; Totsuka, K.; Ohnishi, T.; Takemoto, H.; Mase, N.; Toda, M.; Narumi, T.; Sato, K.; Matsuo, T.; et al. Characteristic fluctuations in glycosidically bound volatiles during tea processing and identification of their unstable derivatives. J. Agric. Food Chem. 2016, 64, 1151–1157. [Google Scholar] [CrossRef]
- Hattori, S.; Takagaki, H.; Fujimori, T. A Comparison of the volatile compounds in several green teas. Food Sci. Technol. Int. Tokyo 2005, 11, 82–86. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent advances in volatiles of teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Zhang, Y.; Shi, J.; Lin, Z.; Lü, H. Analysis of volatile composition and key aroma compounds of green teas with fresh scent flavor. Food Sci. 2019, 40, 219–228. [Google Scholar]
- Ye, Y. Effects of Withering on the Main Physical and Chemical Properties of Manufactured Tea Leaves; Southwest University: Chongqing, China, 2018. [Google Scholar]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compos. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Huang, J.; Ding, Y.; Xu, Y.; Lei, P.; Wu, Q. Effects of fresh tea leaves spreading on the quality of green tea. Chin. Tea Process. 2013, 14–18. [Google Scholar] [CrossRef]
- Yu, X. Effects of Different Withering Methods on Components Metabolism Related to Color, Aroma and Taste Quality in Green Tea; Huazhong Agricultural University: Wuhan, China, 2020. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, R.; Ao, C.; Huang, H.; Shi, D.; Mao, Y.; Zheng, X.; Zhao, Y. Research of Processing Technology of Longjing Tea with ‘Baiye 1’ Based on Non-Targeted Aroma Metabolomics. Foods 2024, 13, 1338. https://doi.org/10.3390/foods13091338
Teng R, Ao C, Huang H, Shi D, Mao Y, Zheng X, Zhao Y. Research of Processing Technology of Longjing Tea with ‘Baiye 1’ Based on Non-Targeted Aroma Metabolomics. Foods. 2024; 13(9):1338. https://doi.org/10.3390/foods13091338
Chicago/Turabian StyleTeng, Ruimin, Cun Ao, Haitao Huang, Daliang Shi, Yuxiao Mao, Xuxia Zheng, and Yun Zhao. 2024. "Research of Processing Technology of Longjing Tea with ‘Baiye 1’ Based on Non-Targeted Aroma Metabolomics" Foods 13, no. 9: 1338. https://doi.org/10.3390/foods13091338



