Effect of Treatment with Heated Scallop Shell Powder on the Inactivation of Naturally Existing Bacteria and Listeria monocytogenes Inoculated on Chicken Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. HSSP
2.2. HSSP Treatment of Chicken Thighs
2.2.1. Preparation of Samples and Inoculation with Pathogens
2.2.2. HSSP Treatment
2.3. Color Measurement
2.4. Statistical Analysis
3. Results and Discussion
3.1. Naturally Existing Bacteria
3.2. Inoculated Pathogenic Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFU | Colony forming unit |
| HSSP | heated scallop shell powder |
| L. monocytogenes | Listeria monocytogenes |
| ROS | reactive oxygen species |
References
- Tamaru, M.; Yabutani, T.; Motonaka, J. Multielement determination of trace metals in scallop samples. Bunseki Kagaku 2004, 55, 1435–1440. (In Japanese) [Google Scholar] [CrossRef]
- Ghimire, K.N.; Kai, H.; Inoue, K.; Ohto, K.; Kawakita, H.; Harada, H.; Morita, M. Heavy metal removal from contaminated scallop waste for feed and fertilizer application. Bioresour. Technol. 2008, 99, 2436–2441. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Sawai, J. Antimicrobial characteristics of heated scallop shell powder and its application. Biocontrol Sci. 2011, 16, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Shiga, H.; Kojima, H. Kinetic analysis of the bactericidal action of heated scallop-shell powder. Int. J. Food Microbiol. 2001, 71, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Fransisca, L.; Zhou, B.; Park, H.; Feng, H. The effect of calcinated calcium and chlorine treatments on Escherichia coli O157:H7 87-23 population reduction in radish sprouts. J. Food Sci. 2011, 76, M404–M412. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Mine, H.; Suhara, H.; Issiki, K. Effectiveness of calcium preparation on improvement for shelf-life period of food. Nippon Shokuhin Kagaku Kogaku Kaishi 1995, 42, 442–445. (In Japanese) [Google Scholar] [CrossRef]
- Yen, L.T.; Weng, C.H.; Than, N.A.T.; Tzeng, J.H.; Jacobson, A.R.; Iamsaard, K.; Dang, V.D.; Lin, Y. Mode of inactivation of Staphylococcus aureus and Escherichia coli by heated oyster-shell powder. Chem. Eng. J. 2022, 432, 134386. [Google Scholar] [CrossRef]
- Qu, C.L.; Lin, S.M.; Potiyaraj, P.; Meng, L.; Wu, C.S.; Yuan, L.; Luo, X.; Ge, F.F.; Tsou, C.H. Polymer packaging through the blending of biowaste oyster shell and low-density polyethylene: A sustainable approach for enhanced food preservation. Polymers 2023, 15, 3977. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, K.; Asada, T.; Yamamoto, K.; Wakabayashi, H.; Sasaki, M.; Sato, M.; Matsuda, J. Antibacterial activity of calcined shell calcium prepared from wild surf clam. J. Health Sci. 2000, 46, 98–103. [Google Scholar] [CrossRef]
- Li, M.; Yao, Z.T.; Chen, T.; Lou, Z.H.; Xia, M. The antibacterial activity and mechanism of mussel shell waste derived material. Powder Technol. 2014, 264, 577–582. [Google Scholar] [CrossRef]
- Agalya, P.; Suresh Kumar, G.; Srinivasan, R.; Prabu, K.M.; Karunakaran, G.; Cholan, S.; Kolesnikov, E.; Kim, M. Hydroxyapatite-based antibacterial bio-nanomaterials: An insight into the synthesis using mussel shell as a calcium source, physicochemical properties, and nanoindentation characteristics. Appl. Phys. A 2021, 127, 589. [Google Scholar] [CrossRef]
- Rusdaryanti, A.F.; Amalia, U.; Suharto, S. Antibacterial activity of CaO from blood cockle shells (Anadara granosa) calcination against Escherichia coli. Biodivers. J. Biol. Divers. 2020, 21, 2827–2831. [Google Scholar]
- Bae, D.H.; Yeon, J.H.; Park, S.Y.; Lee, D.H.; Ha, S.D. Bactericidal effects of CaO (scallop-shell powder) on foodborne pathogenic bacteria. Arch. Pharm. Res. 2006, 29, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Ro, E.Y.; Ko, Y.M.; Yoon, K.S. Survival of pathogenic enterohemorrhagic Escherichia coli (EHEC) and control with calcium oxide in frozen meat products. Food Microbiol. 2015, 49, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Shiga, H. Kinetic analysis of antifungal activity of heated scallop-shell powder against Trichophyton and possible application to the treatment of dermatophytosis. Biocontrol Sci. 2006, 11, 125–128. [Google Scholar] [CrossRef]
- Xing, R.; Qin, Y.; Guan, X.; Liu, S.; Yu, H.; Li, P. Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Sawai, J.; Miyoshi, H.; Kojima, H. Sporicidal kinetics of Bacillus subtilis spores by heated scallop shell powder. J. Food Prot. 2003, 66, 1482–1485. [Google Scholar] [CrossRef]
- Sawai, J.; Ohashi, S.; Miyoshi, H.; Shiga, H. Killing of Bacillus subtilis spores by heated scallop-shell powder containing calcium oxide as the main component. Bokin Bobai 2007, 35, 3–11. (In Japanese) [Google Scholar]
- Thammakarn, C.; Satoh, K.; Suguro, A.; Hakim, H.; Ruenphet, S.; Takehara, K. Inactivation of avian influenza virus, Newcastle disease virus and goose parvovirus using solution of nano-sized scallop shell powder. J. Vet. Med. Sci. 2014, 76, 1277–1280. [Google Scholar] [CrossRef]
- Akasaka, R.; Osawa, A.; Wada, R.; Sawai, J.; Nakagawa, Y. Antimicrobial activity and transparency of polyvinyl butyral paint containing heated scallop-shell powder. Coatings 2023, 13, 364. [Google Scholar] [CrossRef]
- Hatanaka, N.; Xu, B.; Yamashita, Y.; Kawakami, H.; Yasugi, M.; Yamasaki, S. ShellCoat, a calcinated calcium solution, effectively inactivates SARS-CoV-2. Biocontrol Sci. 2022, 27, 53–56. [Google Scholar] [CrossRef]
- Bodur, T.; Cagri-Mehmetoglu, A. Removal of Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 biofilms on stainless steel using scallop shell powder. Food Control 2012, 25, 1–9. [Google Scholar] [CrossRef]
- Sawai, J.; Nagasawa, K.; Kikuchi, M. Ability of heated scallop-shell powder to disinfect Staphylococcus aureus biofilm. Food Sci. Technol. Res. 2013, 19, 561–568. [Google Scholar] [CrossRef]
- Shimamura, N.; Irie, F.; Yamakawa, T.; Kikuchi, K.; Sawai, J. Heated scallop-shell powder treatment for killing and removal of Listeria sp. biofilm formed at low temperature. Biocontrol Sci. 2015, 20, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Kikuchi, M.; Sawai, J. Antimicrobial effects of heated scallop-shell powder against Salmonella biofilm. Bokin Bobai 2011, 39, 587–594. (In Japanese) [Google Scholar]
- Tsukuda, H.; Akimoto, T.; Fukikoshi, N.; Wada, R.; Sawai, J. Antibiofilm effects of heated scallop shell powder on Campylobacter jejuni biofilms. Membranes 2021, 12, 43. [Google Scholar] [CrossRef]
- Jeong, M.S.; Park, J.S.; Song, S.H.; Jang, S.B. Characterization of antibacterial nanoparticles from the scallop Ptinopecten yessoensis. Biosci. Biotechnol. Biochem. 2007, 71, 2242–2247. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Fujimoto, R.; Kikuchi, M.; Sawai, J.; Yahata, S.; Satoh, S. Antibacterial characteristics of heated scallop-shell nano-particles. Biocontrol Sci. 2014, 19, 93–97. [Google Scholar] [CrossRef]
- Ishihara, M.; Hata, Y.; Hiruma, S.; Takayama, T.; Nakamura, S.; Sato, Y.; Ando, N.; Fukuda, K.; Murakami, K.; Yokoe, H. Safety of concentrated bioshell calcium oxide water application for surface and skin disinfections against pathogenic microbes. Molecules 2020, 25, 4502. [Google Scholar] [CrossRef]
- Takayama, T.; Ishihara, M.; Nakamura, S.; Sato, Y.; Hiruma, S.; Fukuda, K.; Murakami, K.; Yokoe, H. Bioshell calcium oxide (BiSCaO) ointment for the disinfection and healing of Pseudomonas aeruginosa-infected wounds in hairless rats. Int. J. Mol. Sci. 2020, 21, 4176. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Nimitkeatkai, H.; Choi, J.W.; Cheong, S.R. Calcinated calcium and mild heat treatment on storage quality and microbial populations of fresh-cut iceberg lettuce. Hortic. Environ. Biotechnol. 2011, 52, 408–412. [Google Scholar] [CrossRef]
- Mamun, A.A.; Simul, H.A.; Rahman, A.; Gazi, N.N.; Bari, L. Prevalence of foodborne pathogens and effectiveness of washing or cooking in reducing microbiological risk of contaminated red amaranth. Agric. Food Anal. Bacteriol. 2012, 2, 222–231. [Google Scholar]
- Nomoto, Y.; Sawada, S.; Abe, S.; Wakazawa, J.; Kikuchi, M.; Sawai, J. Sorbitol minimizes calcium carbonate scale generation while maintaining the disinfection effect of heated scallop-shell powder for fresh produce. Biocontrol Sci. 2018, 23, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Tsuruma, A.; Nomoto, Y.; Nishio, M.; Ishikawa, M.; Sawai, J. Efficacy of sorbitol-coated heated scallop-shell powder for the antimicrobial treatment of fresh vegetables. Food Control 2020, 110, 106972. [Google Scholar] [CrossRef]
- Chen, X.; Tango, C.N.; Daliri, E.B.M.; Oh, S.Y.; Oh, D.H. Disinfection efficacy of slightly acidic electrolyzed water combined with chemical treatments on fresh fruits at the industrial scale. Foods 2019, 8, 497. [Google Scholar] [CrossRef]
- Bodur, T.; Yaldirak, G.; Kola, O.; Çağri-mehmetoğlu, A. Inhibition of Listeria monocytogenes and Escherichia coli O157: H7 on frankfurters using scallop-shell powder. J. Food Saf. 2010, 30, 740–752. [Google Scholar] [CrossRef]
- Ahmed, S.; Akand, N.R.; Islam, M.T.; Mamun, A.A.; Bari, M.L. Effectiveness of scallop powder ice in reducing bacterial load on fresh whole fish and in the melted ice water. LWT Food Sci. Technol. 2015, 64, 270–274. [Google Scholar] [CrossRef]
- Loyo, C.; Moreno-Serna, V.; Fuentes, J.; Amigo, N.; Sepúlveda, F.A.; Ortiz, J.A.; Rivas, L.M.; Ulloa, M.T.; Benavente, R.; Zapata, P.A. PLA/CaO nanocomposites with antimicrobial and photodegradation properties. Polym. Degrad. Stab. 2022, 197, 109865. [Google Scholar] [CrossRef]
- Cagri-Mehmetoglu, A. Inhibition of Listeria monocytogenes and Salmonella enteritidis on chicken wings using scallop-shell powder. Poult. Sci. 2011, 90, 2600–2605. [Google Scholar] [CrossRef]
- Sawai, J.; Kawada, E.; Kanou, F.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M. Detection of active oxygen generated from ceramic powders having antibacterial activity. J. Chem. Eng. Jpn. 1996, 29, 627–633. [Google Scholar] [CrossRef]
- Kubo, M.; Ohshima, Y.; Irie, F.; Kikuchi, M.; Sawai, J. Disinfection treatment of heated scallop-shell powder on biofilm of Escherichia coli ATCC 25922 surrogated for E. coli O157:H7. J. Biomater. Nanobiotechnol. 2013, 4, 40636. [Google Scholar] [CrossRef]
- Freeman, B.A.; Crapo, J.D. Biology of disease: Free radicals and tissue injury. Lab. Investig. 1982, 47, 412–426. [Google Scholar]
- Vodnar, D.C. Inhibition of Listeria monocytogenes ATCC 19115 on ham steak by tea bioactive compounds incorporated into chitosan-coated plastic films. Chem. Cent. J. 2012, 6, 74. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, B.; Cerf, O. Review–Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Mine, H.; Suhara, H.; Yamanaka, S.; Isshiki, K. Application of calcium preparation on meat processing. Nippon Shokuhin Kagaku Kogaku Kaishi 1995, 42, 268–272. (In Japanese) [Google Scholar] [CrossRef]
- Okai, M.; Aoki, H.; Ishida, M.; Urano, N. Antibiotic-resistance of fecal coliforms at the bottom of the Tama river, Tokyo. Biocontrol Sci. 2019, 24, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Ana, K.M.S.; Madriaga, J.; Espino, M.P. β-lactam antibiotics and antibiotic resistance in Asian lakes and rivers: An overview of contamination, sources and detection methods. Environ. Pollut. 2021, 275, 116624. [Google Scholar] [CrossRef]
- Sabri, N.; Schmitt, H.; Van der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2020, 8, 102245. [Google Scholar] [CrossRef]
- Basiry, D.; Entezari Heravi, N.; Uluseker, C.; Kaster, K.M.; Kommedal, R.; Pala-Ozkok, I. The effect of disinfectants and antiseptics on co-and cross-selection of resistance to antibiotics in aquatic environments and wastewater treatment plants. Front. Microbiol. 2022, 13, 1050558. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Aerobic Bacteria Population (log10 CFU/g) | |||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 7 | |
| No treatment (Control) | 4.6 ± 0.3 a,A | 5.6 ±1.4 a,AB | 6.5 ± 1.3 a,B | 6.7 ± 1.0 a,B |
| HSSP 1% | 3.9 ± 0.2 a,A | 3.5 ± 0.4 b,A | 6.1 ± 0.1 a,B | 5.6 ± 0.1 b,C |
| HSSP 5% | 3.8 ± 0.4 a,A | 4.2 ± 0.1 b,AB | 5.1 ±1.7 b,B | 3.8 ± 1.9 c,AC |
| Treatment | Coliform Population (log10 CFU/g) | |||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 7 | |
| No treatment (Control) | 3.9 ± 0.8 a,A | 5.0 ± 1.7 a,B | 5.4 ± 1.6 a,B | 5.4 ± 1.1 a,B |
| HSSP 1% | 3.5 ± 0.4 ab,A | 3.5 ± 0.2 b,A | 4.3 ± 0.1 b,B | 4.8 ± 0.1 a,B |
| HSSP 5% | 2.9 ± 0.3 b,A | 3.2 ± 1.0 b,AB | 3.9 ± 0.4 b,B | 3.6 ± 1.1 b,B |
| Treatment | Storage Time | |||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 7 | |
| No treatment (Control) | 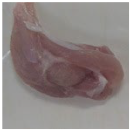 | 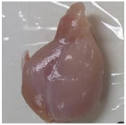 | 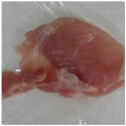 |  |
| HSSP 1% | 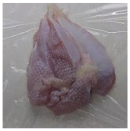 | 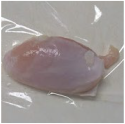 | 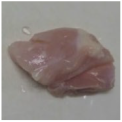 | 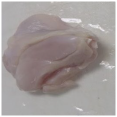 |
| HSSP 5% |  |  |  |  |
| Hunter Color Values | Treatment | Storage Time | |||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 7 | ||
| L* | No treatment (Control) | 44.8 ± 5.2 a,A | 47.8 ± 4.3 a,AC | 51.6 ± 4.4 a,BC | 52.7 ± 5.2 a,BC |
| HSSP 1% | 61.6 ± 9.4 b,A | 57.8 ± 5.9 b,A | 51.8 ± 1.6 a,B | 59.9 ± 5.6 b,A | |
| HSSP 5% | 61.6 ± 9.4 b,A | 57.8 ± 5.9 b,A | 51.8 ± 1.6 a,B | 59.9 ± 5.6 b,A | |
| a* | No treatment (Control) | 2.9 ± 2.3 a,A | 4.8 ± 1.0 a,AB | 8.7 ± 0.3 a,D | 5.2 ± 1.9 a,CB |
| HSSP 1% | 3.4 ± 1.4 a,A | 1.6 ± 0.8 b,A | 3.4 ± 2.5 b,A | 2.2 ± 2.9 b,A | |
| HSSP 5% | 0.9 ± 1.0 b,A | 0.9 ± 0.6 b,A | 1.7 ± 0.5 c,A | 2.6 ± 1.9 b,A | |
| b* | No treatment (Control) | 5.1 ± 2.8 a,ABC | 2.8 ± 2.2 ab,B | 4.9 ± 4.3 a,ABC | 7.8 ± 2.6 a,C |
| HSSP 1% | 4.7 ± 3.0 a,AC | −0.2 ± 3.8 b,B | 2.6 ± 2.8 a,ABC | 5.5 ± 2.1 a,C | |
| HSSP 5% | 9.1 ± 5.1b,A | 4.2 ± 2.7 ac,A | 8.5 ± 1.1 b,A | 7.9 ± 5.1 a,A | |
| Bacteria | Treatment | Bacterial Population (log10 CFU/g) | |||
|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 5 | Day 7 | ||
| Aerobic bacteria | No treatment (Control) | 5.6 ± 0.2 a,A | 7.2 ± 0.8 a,B | 8.9 ± 0.4 a,C | 9.2 ± 0.2 a,C |
| HSSP 5% | 3.6 ± 1.5 b,A | 4.2 ± 2.1 b,A | 4.4 ± 1.3 b,A | 3.5 ± 1.1 b,A | |
| Listeria | No treatment (Control) | 5.5 ± 0.3 a,A | 7.0 ± 0.8 a,B | 7.1 ± 1.4 c,B | 9.0 ± 0.3 a,C |
| HSSP 5% | 3.7 ± 1.4 b,A | 3.9 ± 1.6 b,A | 3.3 ± 0.9 b,A | 2.8 ± 0.2 b,A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omura, K.; Kaibara, E.; Yamaguchi, S.; Aoyagi, H.; Nishio, M.; Tomita, K.; Sawai, J. Effect of Treatment with Heated Scallop Shell Powder on the Inactivation of Naturally Existing Bacteria and Listeria monocytogenes Inoculated on Chicken Meat. Foods 2024, 13, 370. https://doi.org/10.3390/foods13030370
Omura K, Kaibara E, Yamaguchi S, Aoyagi H, Nishio M, Tomita K, Sawai J. Effect of Treatment with Heated Scallop Shell Powder on the Inactivation of Naturally Existing Bacteria and Listeria monocytogenes Inoculated on Chicken Meat. Foods. 2024; 13(3):370. https://doi.org/10.3390/foods13030370
Chicago/Turabian StyleOmura, Kiuta, Emi Kaibara, Sae Yamaguchi, Hana Aoyagi, Mari Nishio, Kazuhisa Tomita, and Jun Sawai. 2024. "Effect of Treatment with Heated Scallop Shell Powder on the Inactivation of Naturally Existing Bacteria and Listeria monocytogenes Inoculated on Chicken Meat" Foods 13, no. 3: 370. https://doi.org/10.3390/foods13030370





