Effect of Vanillin on the Anaesthesia of Crucian Carp: Effects on Physiological and Biochemical Indices, Pathology, and Volatile Aroma Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Determination of Effective Concentration of Vanillin for Anaesthesia of Crucian Carp
2.2.2. Determination of Behavioural Characteristics during the Recovery Phases of Anaesthesia, at the Optimal Vanillin Concentration
2.2.3. Effect of Vanillin Concentration on Blood and Serum Parameters of Crucian Carp
2.2.4. H&E Staining and Histological Examination of Liver and Gill Tissues
2.2.5. E-nose Analysis
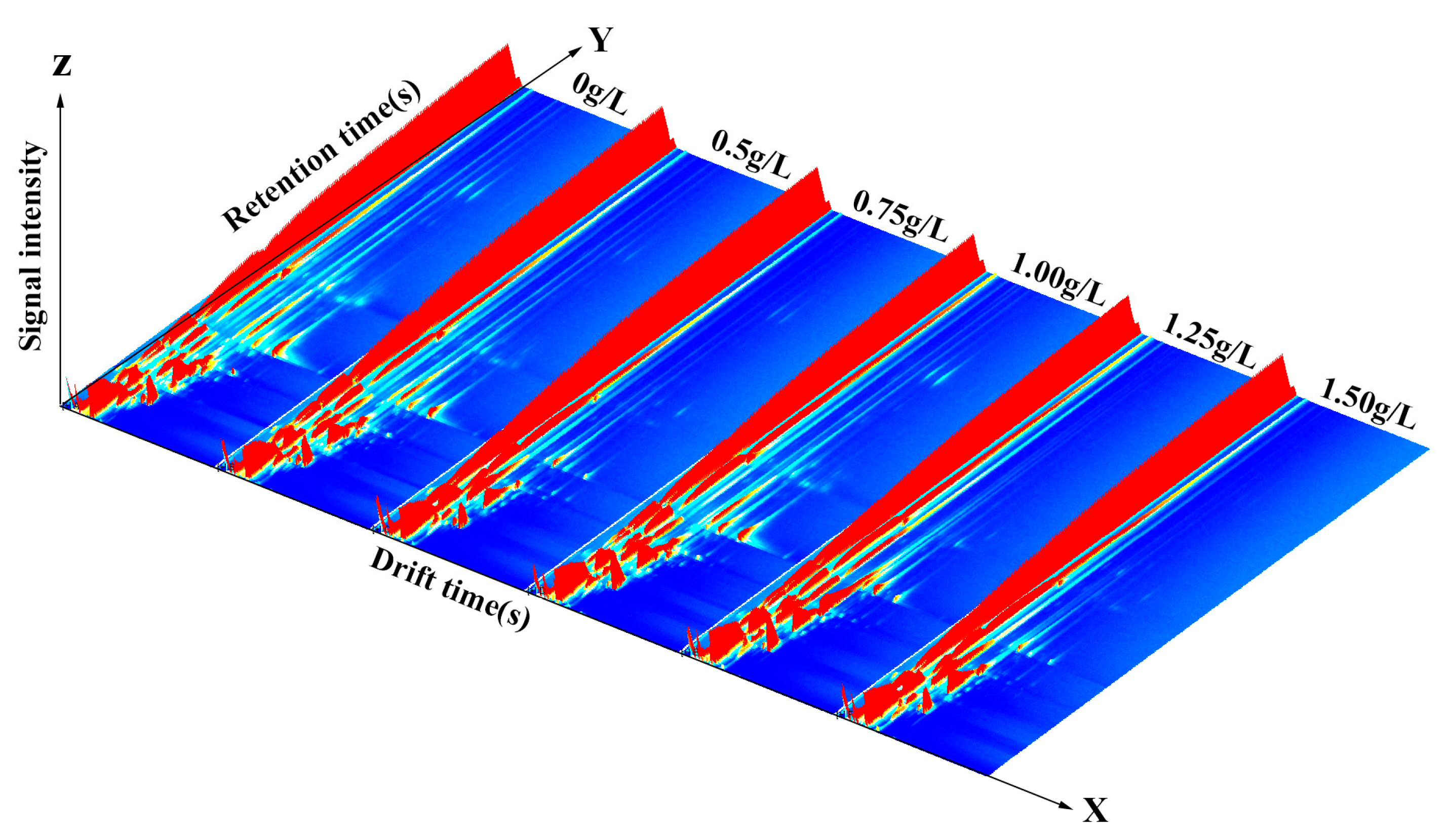
2.2.6. Gas Chromatography-Ion Mobility Spectrometry (GC-IMS) Analysis of Flavour Volatile Compounds
2.2.7. Statistical Analysis
3. Results
3.1. Determination of the Effective Vanillin Anaesthesia Concentration Range for Crucian Carp
Behavioural Characteristics of Crucian Carp during the Five Stages of Anaesthesia Onset and the Four Stages of Recovery
3.2. Effect of Vanillin Concentration on Blood and Serum Parameters of Crucian Carp
3.2.1. Effect of Vanillin Concentration on the Blood Composition Index of Crucian Carp
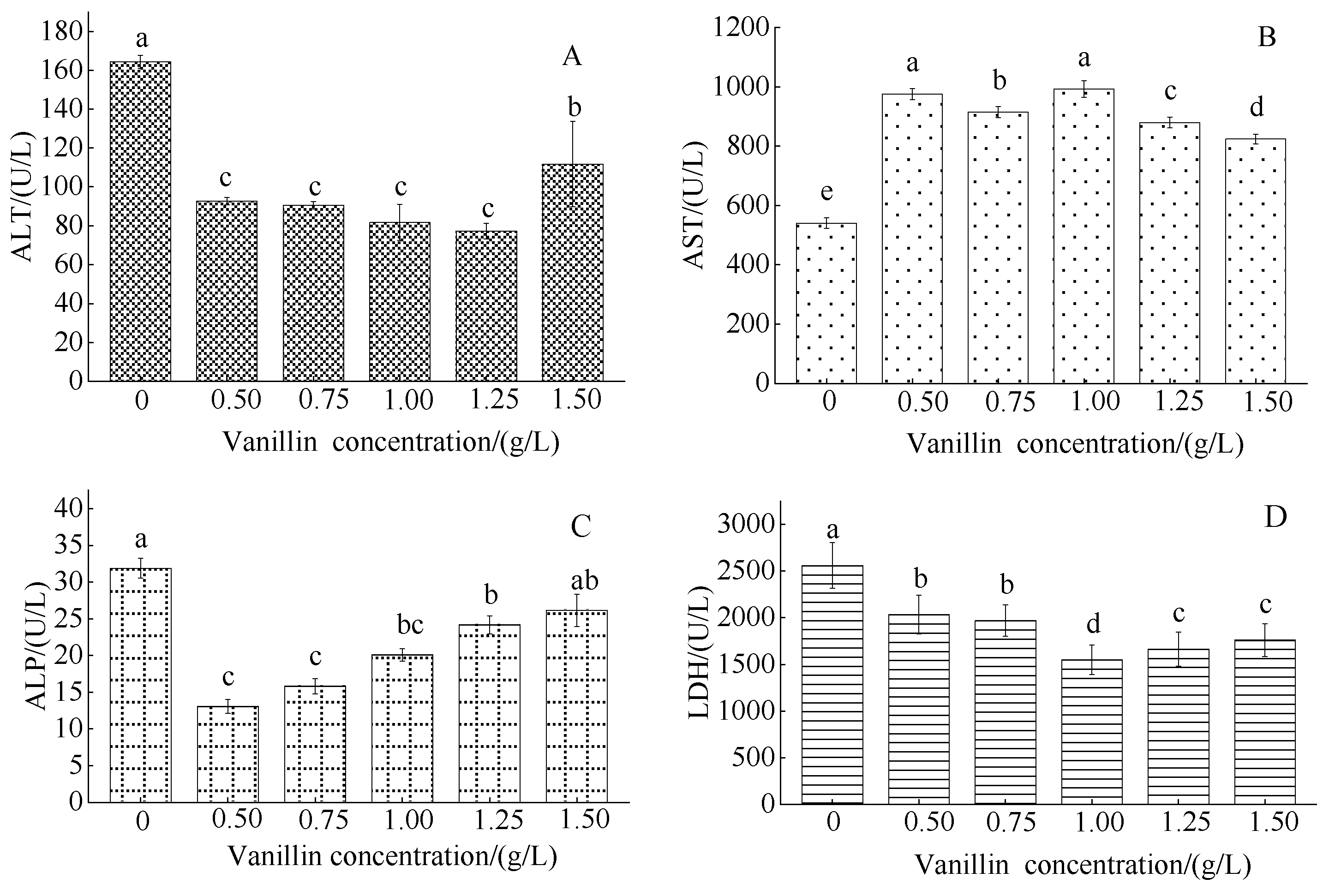
3.2.2. Effect of Vanillin Concentration on Blood Serum Enzymes in Crucian Carp
3.2.3. Effect of Vanillin Concentration on the Blood Serum Ion Concentrations of Crucian Carp
3.2.4. Effect of Vanillin Concentration on the Blood Serum Concentrations of Organic Components in Crucian Carp
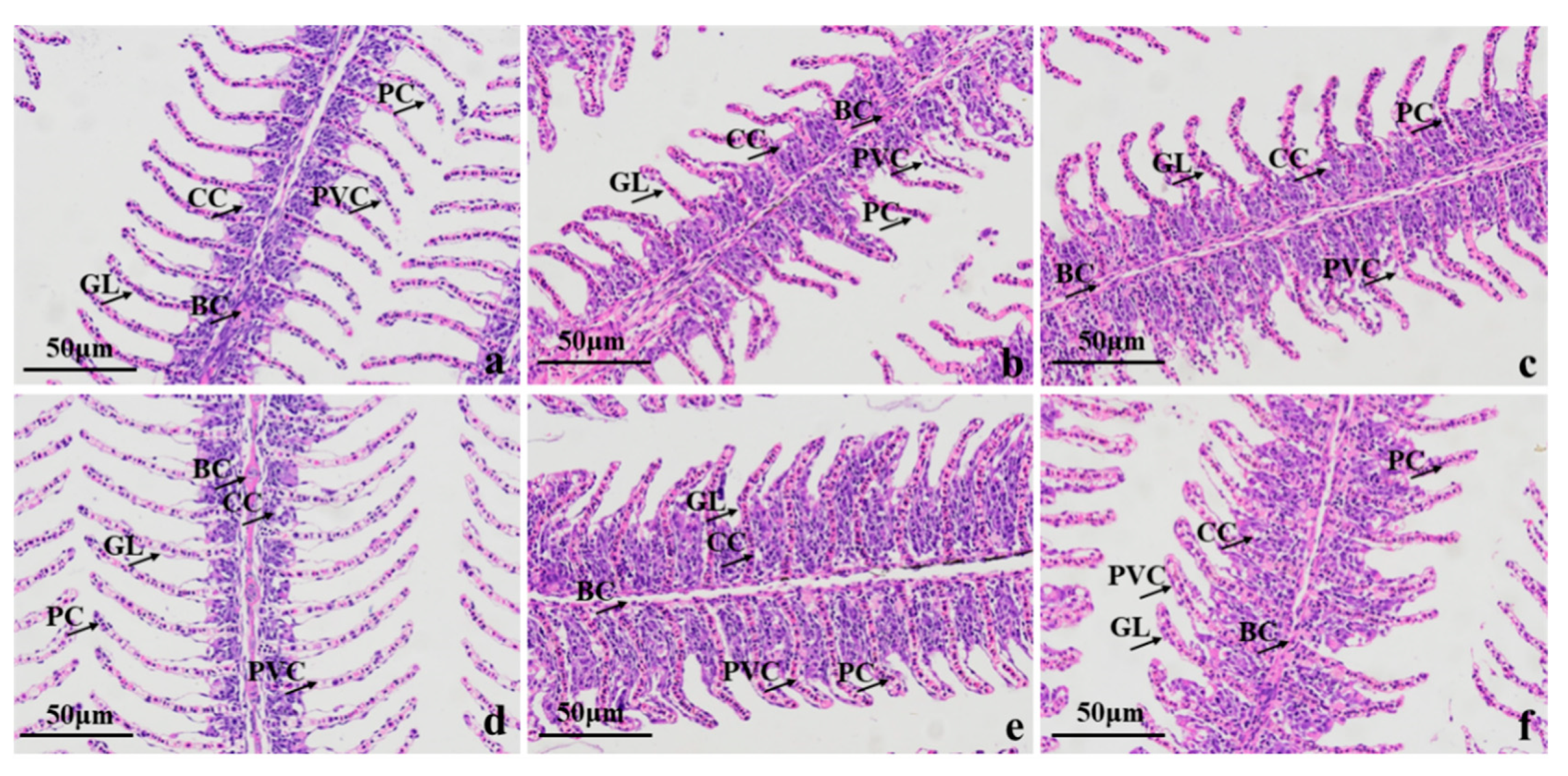
3.3. Histopathological Examination
3.4. Effect of Vanillin Concentration on the Volatile Flavour Compound Profile of Carp Muscle, Determined by E-Nose Analysis
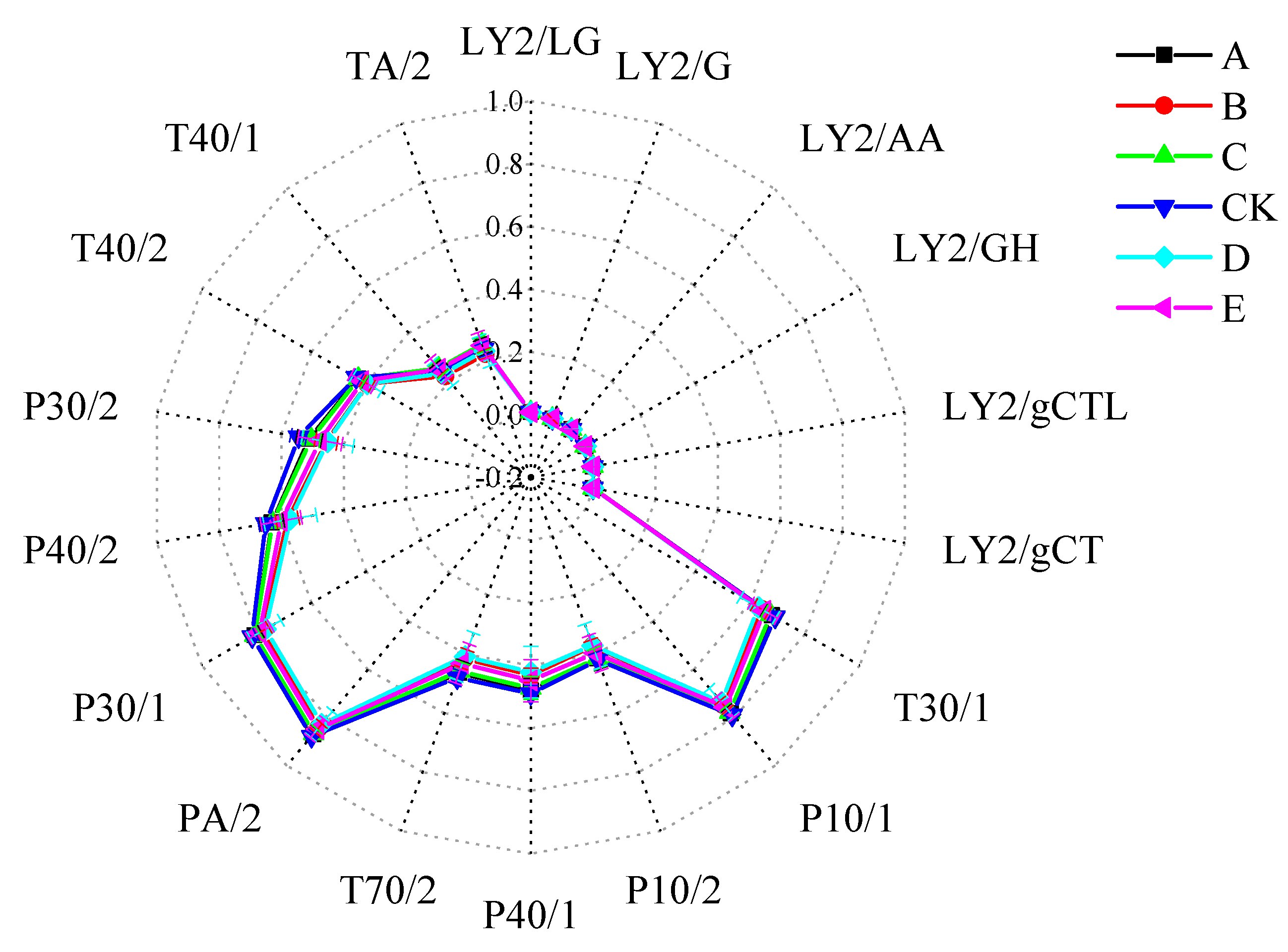
3.4.1. Radar Fingerprinting of the E-Nose Sensor Responses to Carp-Muscle Flavour Volatiles
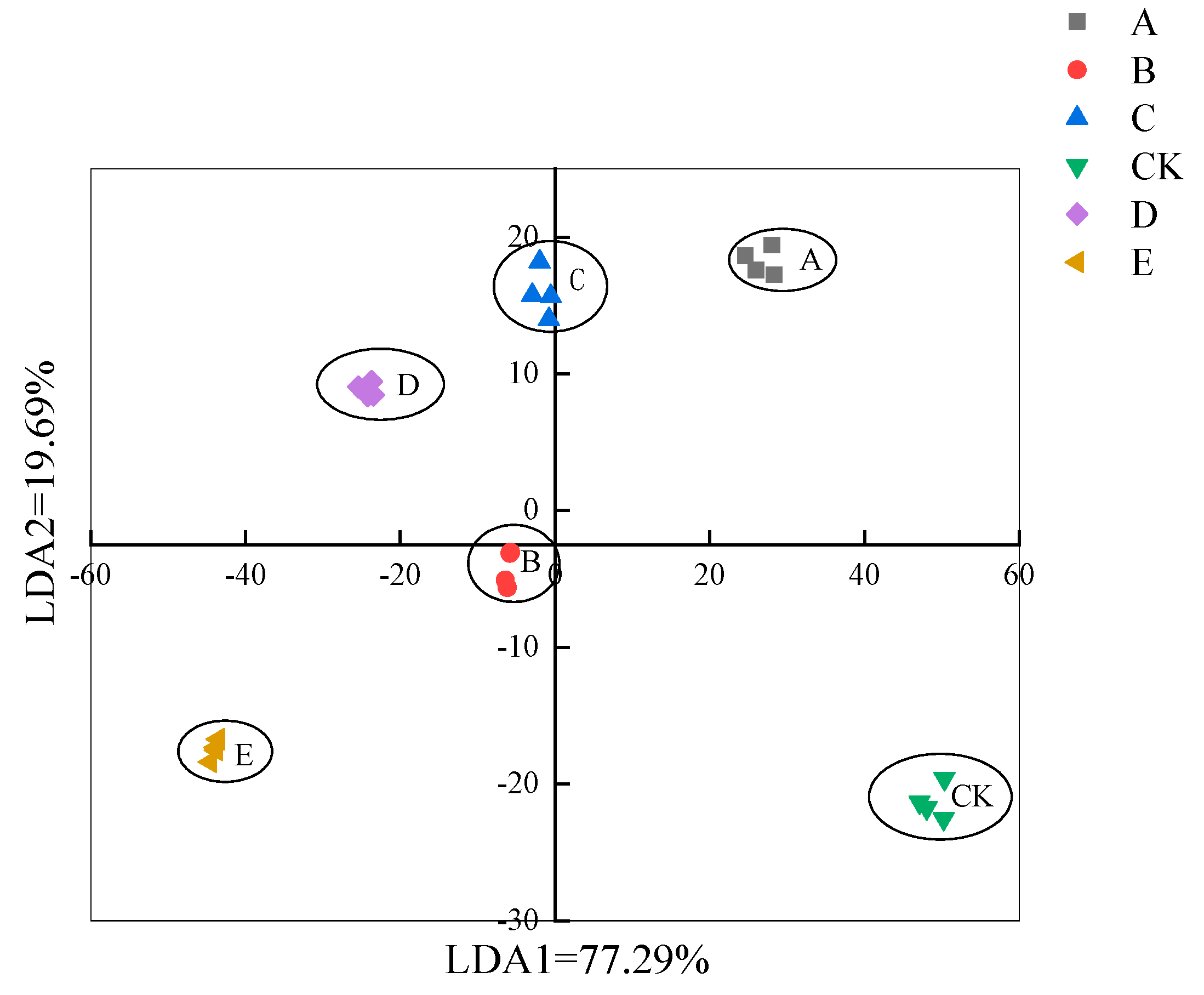
3.4.2. Linear Discriminant Analysis (LDA) of E-Nose Data
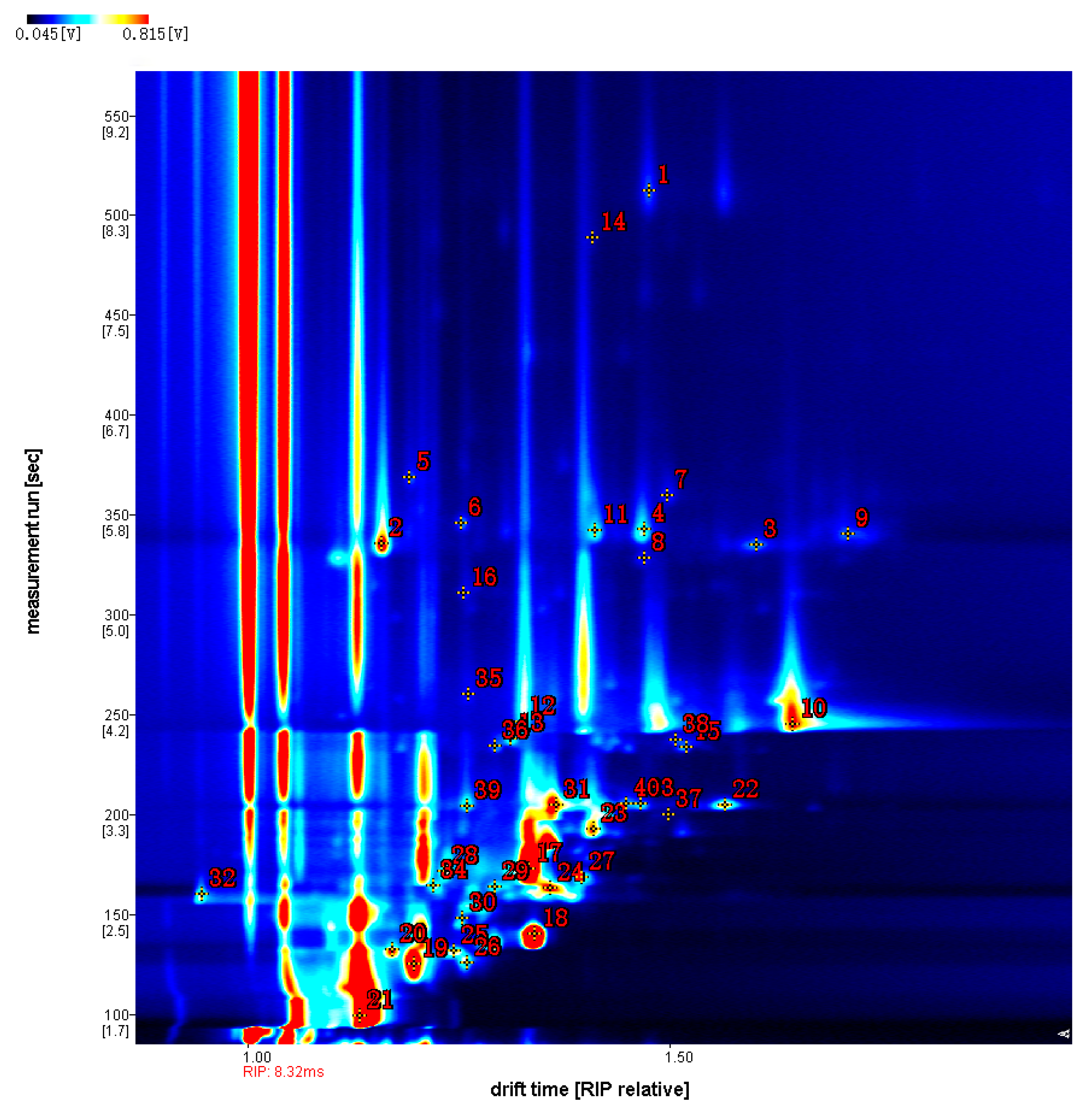
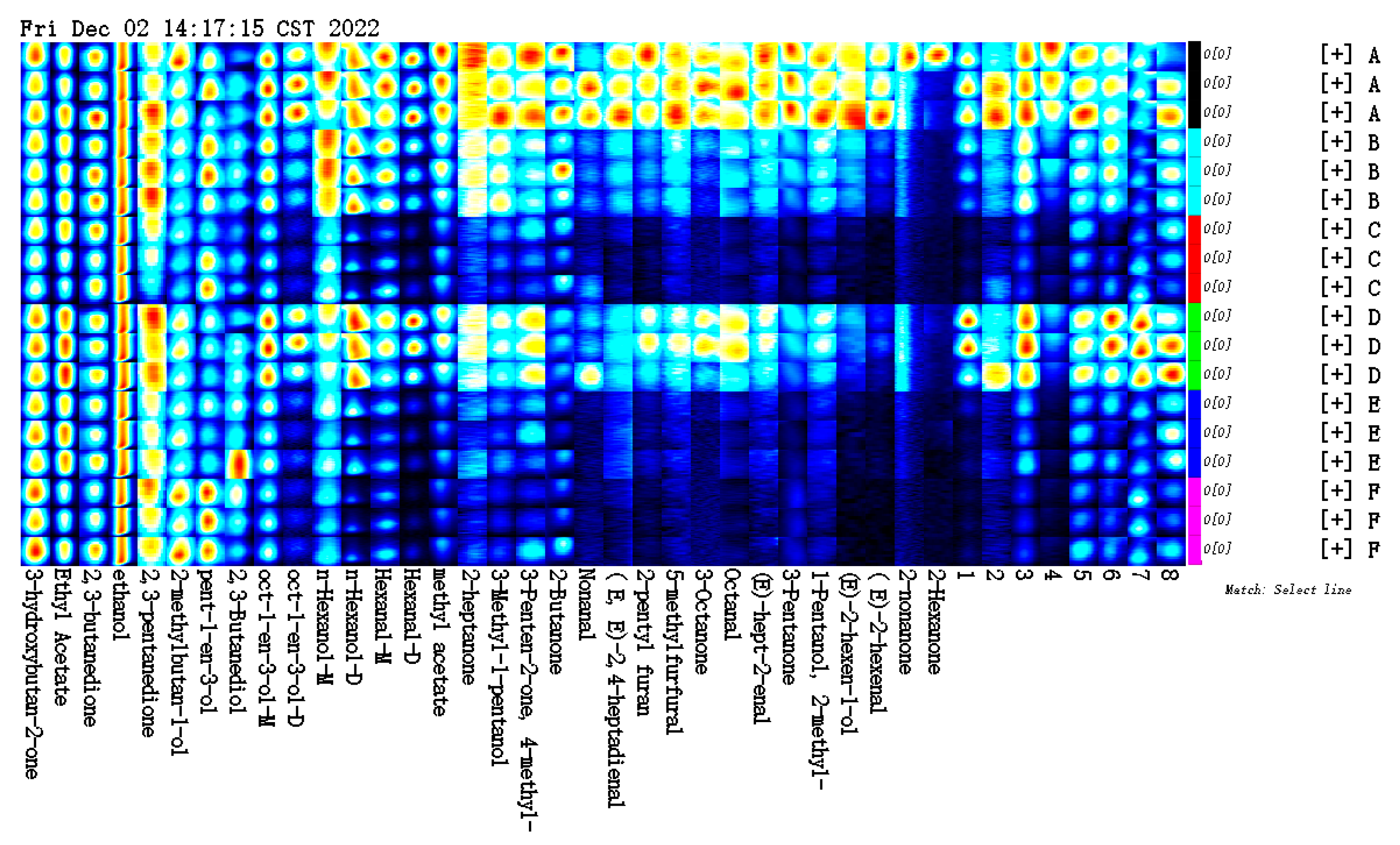
3.5. GC-IMS Analysis of Aroma Volatiles from Crucian Carp Muscle at Different Vanillin Treatment Concentrations
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bodur, T.; Leon, B.S.; Navarro, A.; Tort, L.; Afonso, J.M.; Montero, D. Effects of new plant based anesthetics Origanum Sp. and Eucalyptus Sp. oils on stress and welfare parameters in Dicentrarchus Labrax and their comparison with clove oil. Aquaculture 2018, 495, 402–408. [Google Scholar] [CrossRef]
- De Oliveira, C.P.B.; da Lemos, C.H.P.; Vidal, L.V.O. Anesthesia with eugenol in hybrid amazon catfish (Pseudoplatystoma Reticulatum × Leiarius Marmoratus) handling: Biochemical and haematological responses. Aquaculture 2019, 501, 255–259. [Google Scholar] [CrossRef]
- De Oliviera, C.P.B.; Lemosda Paixão Lemos, C.H.; e Silva, A.F.; De Souza, S.A.; Albinati, A.C.L.; Lima, A.O.; Copatti, C.E. Use of eugenol for the anesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 2019, 513, 734409. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Xu, S.L. The research status of the application of three fishery anesthetics was analyzed. China Food Saf. Mag. 2019, 9, 148–150. [Google Scholar]
- Aydın, B.; Barbas, L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J.; Effendi, I. Evaluation of the application of seaweed (Eucheuma cottonii) extract as fish anesthetic agent. Aquac. Int. 2021, 29, 1545–1560. [Google Scholar] [CrossRef]
- Santos, A.C.D.; Junior, G.B.; Zago, D.C.; Zeppenfeld, C.C.; Silva, D.T.D.; Heinzmann, B.M.; Baldisserotto, B.; Cunha, M.A.D. Anesthesia and anesthetic action mechanism of essential oils of Aloysia triphylla and Cymbopogon flexuosus in silver catfish (Rhamdia quelen). Vet. Anesth. Analg. 2017, 44, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Boaventura, T.P.; dos Santos, F.A.C.; Souza, A.D.S.; Batista, F.S.; Julio, G.S.C.; Luz, R.K. Thymol and linalool chemotypes of the essential oil of thymus vulgaris (thyme) as anesthetic for Colossoma macropomum: Physiology and feed consumption. Aquaculture 2022, 554, 738161. [Google Scholar] [CrossRef]
- Ananias, I.D.M.C.; de Melo, C.L.; Costa, D.C.; Ferreira, A.L.; Martins, E.D.F.F.; Takata, R.; Luz, R.K. Menthol as anesthetic for juvenile lophiosilurus alexandri: Induction and recovery time, ventilatory frequency, hematology and blood biochemistry. Aquaculture 2022, 546, 737373. [Google Scholar] [CrossRef]
- Han, Z.C.; Long, L.; Ding, S. Expression and characterization of carotenoid cleavage oxygenases from herbaspirillum seropedicae and rhodobacteraceae bacterium capable of biotransforming isoeugenol and 4-vinylguaiacol to vanillin. Front. Microbiol. 2019, 10, 1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.J. Development status of vanillin industry. Modern Food 2019, 7, 14–16. [Google Scholar]
- Wang, J.; Chen, P.S.; Meng, X.C. Effect of composite coating combined with UV treatment on the chilling quality of fresh-cut papaya. J. Agric. Eng. 2012, 2, 273–278. [Google Scholar]
- Mei, G.L.; Liu, W.Q.; Li, T.T.; Li, J.R.; Mou, W.L.; Guo, X.H. Characteristics of chitosan/vanillin/polyvinyl alcohol composite electrospun nanofiber film and its application in the preservation of turbot. Food Sci. 2021, 5, 221–227. [Google Scholar]
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Takahashi, Y.; Matsumoto, Y.; Okamoto, M.; Fujiwara, Y.; et al. Comprehensive behavioral study of the effects of vanillin inhalation in mice. Biomed. Pharmacother. 2019, 115, 108879. [Google Scholar] [CrossRef]
- Yan, X.; Liu, D.F.; Zhang, X.Y.; Liu, D.; Xu, S.Y.; Chen, G.X.; Huang, B.X.; Ren, W.Z.; Wang, W.; Fu, S.P.; et al. Vanillin protects dopaminergic neurons against inflammation-mediated cell death by inhibiting ERK1/2, P38 and the NF-κB signaling pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef]
- Orizano, P.E.; Char, C.; Sepulveda, F.; Ortiz, V.J. Heat sensitization of Escherichia coli by the natural antimicrobials vanillin and emulsified citral in blended carrot-orange juice. Food Microbiol. 2022, 107, 104058. [Google Scholar] [CrossRef]
- Lan, X.B.; Wang, Q.; Yang, J.M.; Ma, L.; Zhang, W.J.; Zheng, P.; Sun, T.; Niu, J.G.; Liu, N.; Yu, J.Q. Neuroprotective effect of vanillin on hypoxic-ischemic brain damage in neonatal rats. Biomed. Pharmacother. 2019, 118, 109196. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Liao, G.; Huang, Y.; Lv, X.; Liu, X.; Chen, W.; Zhang, L. Vanillin attenuates proinflammatory factors in a TMCAO mouse model via inhibition of TLR4/NF-KB signaling pathway. Neuroscience 2022, 491, 65–74. [Google Scholar] [CrossRef]
- Tripathi, A.S.; Awasthi, S.; Maurya, R.K.; Yasir, M.; Mohapatra, L.; Srivastav, V. Protective effect of vanillin on the management of cecal ligation and puncture induced sepsis rat model. Microb. Pathog. 2022, 165, 105493. [Google Scholar] [CrossRef]
- Marking, L.L.; Meyer, F.P. Are better anesthetics needed in fisheries? Fisheries 1985, 10, 2–5. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Ghelichpour, M.; Hoseini, S.M. Myrcene and linalool as new anesthetic and sedative agents in common carp, Cyprinus carpio-comparison with eugenol. Aquaculture 2016, 464, 165–170. [Google Scholar] [CrossRef]
- Chen, D.J.; Zhang, M.G.; Nie, X.B.; Jiang, P.H.; Zhang, Y.H.; Zhang, C.F.; Ren, F. Quality detection of turbot (Scophtalmus maximus) treated with electrostatic field using gas chromatography-ion mobility spectrometry. Food Sci. 2019, 24, 313–319. [Google Scholar]
- Jia, Z.; Chen, X.T.; Pan, N.; Cai, S.L.; Zhang, Y.; Liu, Z.Y. Changes of volatile flavor compounds in Takifugu bimaculatus during refrigeration storage. Food Sci. 2021, 20, 188–196. [Google Scholar]
- Li, H.D.; Xu, Q.S.; Liang, Y.Z. LibPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Cao, X.C.; Huang, X.L.; Sun, X.Y.; Lin, H.Z.; Shu, H.; Yang, Y.K.; Huang, Z. Anesthesia effects of eugenol on juvenile Siganus oramin. South China Fish. Sci. 2019, 3, 50–56. [Google Scholar]
- Zeng, X.; Dong, H.; Wu, J.; Wang, W.; Duan, Y.; Chen, J.; Zhang, J. Essential oil of Magnolia denudata is an effective anesthetic for spotted seabass (Lateolabrax maculatus): A test for its effect on blood biochemistry, physiology and gill morphology. Fish Physiol. Biochem. 2022, 48, 1349–1363. [Google Scholar] [CrossRef]
- Huang, X.L.; Dai, C.; Yu, W.; Yang, J.; Yang, Y.K.; Li, T.; Lin, H.Z.; Huang, Z.; Sun, X.Y.; Shu, H. Anesthetic effect of eugenol on juvenile Trachinotus ovatus. J. Guangdong Ocean. Univ. 2020, 4, 124–131. [Google Scholar]
- Safuan, S.N.M.; Tomari, M.R.M.; Zakaria, W.N.W. White blood cell (WBC) counting analysis in blood smear images using various color segmentation methods. Measurement 2018, 116, 543–555. [Google Scholar] [CrossRef]
- Kumar, M.; Matoba, O.; Quan, X.; Rajput, S.K.; Morita, M.; Awatsuji, Y. Quantitative dynamic evolution of physiological parameters of RBC by highly stable digital holographic microscopy. Opt. Lasers Eng. 2022, 151, 106887. [Google Scholar] [CrossRef]
- Wang, K.; Bian, X.; Zheng, M.; Liu, P.; Lin, L.; Tan, X. Rapid determination of hemoglobin concentration by a novel ensemble extreme learning machine method combined with near-infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120138. [Google Scholar] [CrossRef]
- Liang, Z.Y.; An, L.N.; Dong, Z.J.; Miao, L.H.; Xu, P.; Xie, Z. The anesthetic effects of clove oil on tilapia (Oreochromis spp.) and its influence on haematological indices and hormone level. J. Shanghai Ocean. Univ. 2009, 5, 629–635. [Google Scholar]
- Amenyogbe, E.; Yang, E.; Xie, R.; Huang, J.; Chen, G. Influences of indigenous isolates Pantoea agglomerans RCS2 on growth, proximate analysis, haematological parameters, digestive enzyme activities, serum biochemical parameters, antioxidants activities, intestinal morphology, disease resistance, and molecular immune response in juvenile’s cobia fish (Rachycentron canadum). Aquaculture 2022, 551, 737942. [Google Scholar]
- Feng, C.J.; Wang, Y.L.; Zhang, Y.; Yang, H.L.; Wang, N.M.; Lyu, W.H.; Cao, D.C. Effects of different culture density on growth indices, serum biochemical indices and lipase activity of juvenile Huso dauricus. Heilongjiang Anim. Sci. Vet. Med. 2022, 6, 125–129. [Google Scholar]
- Zhong, Y.M. Clone and Expression Analysis of Alkaline Phosphatase Gene from S. Constricta under the Stress of Cu and Zn; Shanghai Ocean University: Shanghai, China, 2012. [Google Scholar]
- Yong, W.; Huang, Y.; Li, X.; Wu, X. Changes of AST, ALP, CK and LDH level in biological injury models caused by 18.4 mm rubber bullet. Int. J. Lab. Med. 2012, 33, 3. [Google Scholar]
- Li, Y.; Wang, Z.X.; Li, S.Y.; Gu, T.Y.; Lin, S.; Nie, X.B.; Huang, B.S.; Zhang, C.F. Effects of Buddleja lindleyana Fortune on anesthesia and serum biochemical indices in crucian carp (Carassius auratus). J. Dalian Ocean. Univ. 2020, 4, 491–495. [Google Scholar]
- Ding, Y.T.; Wang, Z.H.; Wang, L.L.; Shi, W.Z. Effect of MS-222 on survival of bream fish during anesthesia transportation. Fish. Sci. 2019, 3, 296–304. [Google Scholar]
- Zhang, Y.C.; Wen, H.S.; Li, L.M.; Feng, Q.C. Effect of acute temperature stress on serum cortisol and hematological physiology of gestated Sebastes schlegelii. J. Fish. China 2015, 12, 1872–1882. [Google Scholar]
- Bijvelds, M.J.; Van, D.V.; Kolar, Z.I. Magnesium transport in freshwater teleosts. J. Exp. Biol. 1998, 201, 1981–1990. [Google Scholar] [CrossRef]
- Tang, B.G.; Yang, S.K.; Zhen, S.B.; Ni, F.R.; Zhou, X.X.; Xu, Y.R.; Xie, W.Y. Effects of MS-222 and benzocaine on electrolyte levels of Oreochromis niloticus and its correlation with content of erythrocyte. J. Guangdong Ocean. Univ. 2015, 6, 25–29. [Google Scholar]
- Wu, B.; Xie, J. Optimization of water temperature and salinity for live transportation of grouper. Food Sci. 2019, 16, 235–241. [Google Scholar]
- Toni, C.; Becker, A.G.; Simoes, L.N.; Pinheiro, C.G.; de Lima Silva, L.; Heinzmann, B.M.; Caron, B.O.; Baldisserotto, B. Fish anesthesia: Effects of the essential oils of Hesperozygis ringens and Lippia alba on the biochemistry and physiology of silver catfish (Rhamdia quelen). Fish Physiol. Biochem. 2014, 40, 701–714. [Google Scholar] [CrossRef]
- Bai, Z.; Shen, J.; Xu, W.Q.; Zhao, X.Y.; Guo, S.F.; Ma, T.T. Effects of electronarcosis on the behavior and blood biochemical indices of ♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus. Fish. Mod. 2022, 1, 89–96. [Google Scholar]
- Du, H.; Qin, X.M.; Fan, X.P.; Zhang, J.S.; Li, S.J. Synergistic effect of CO2 and low water temperature on the anesthesia and survival of Trachinotus ovatus. J. Guangdong Ocean. Univ. 2022, 01, 35–43. [Google Scholar]
- Cicek, S.; Ozogul, F. Effects of selenium nanoparticles on growth performance, hematological, serum biochemical parameters, and antioxidant status in fish. Anim. Feed. Sci. Technol. 2021, 281, 115099. [Google Scholar] [CrossRef]
- Zhu, T.; Ai, Q.; Mai, K. Feed intake, growth performance and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed defatted fish meal diets with graded levels of cholesterol. Aquaculture 2014, 428, 290–296. [Google Scholar] [CrossRef]
- Cooke, S.J.; Suski, C.D.; Ostrand, K.G.; Tufts, B.L.; Wahl, D.H. Behavioral and physiological assessment of low concentrations of clove oil anesthetic for handling and transporting largemouth bass (Micropterus salmoides). Aquaculture 2004, 239, 509–529. [Google Scholar] [CrossRef]
- Zheng, J.L.; Luo, Z.; Liu, C.X.; Chen, Q.L.; Tan, X.Y.; Zhu, Q.; Gong, Y. Differential effects of acute and chronic zinc (Zn) exposure on hepatic lipid deposition and metabolism in yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 2013, 132, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.j.; Liu, Q.Q.; Wen, J.F.; Li, J.E.; Li, H. The effects of acute low temperature stress on liver, muscle and gill tissues of juvenile Eleutheronema tetradactylum. Ecol. Sci. 2018, 5, 53–59. [Google Scholar]
- Zhang, X.; Xu, Z.J.; Li, W.Y.; Yin, X.; Wang, Y.F.; Chen, S.A.; Ma, X.B. Effects of MS-222 on anesthesia, tissue structure and antioxidant enzyme activity of Larimichthys crocea. J. Dalian Ocean. Univ. 2022, 1–13. [Google Scholar] [CrossRef]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Groch, L.; Nepejchalova, L. Effects of clove oil anesthesia on common carp (Cyprinus Carpio L.). Vet. Med. 2005, 50, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Yigit, N.O.; Kocaayan, H. Efficiency of thyme (Origanum onites) and coriander (Coriandrum sativum) essential oils on anesthesia and histopathology of rainbow trout (Oncorhynchus mykiss). Aquaculture 2023, 562, 738813. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of electronic-nose technologies and VOC-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [Green Version]
- Wen, R.; Kong, B.; Yin, X.; Zhang, H.; Chen, Q. Characterisation of flavor profile of beef jerky inoculated with different autochthonous lactic acid bacteria using electronic nose and gas chromatography-ion mobility spectrometry. Meat Sci. 2022, 183, 108658. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Zheng, M.; Zhao, W.; Liu, Q.; Zeng, X.; Bai, W. Effect of marinating and frying on the flavor of braised pigeon. J. Food Process. Preserv. 2021, 45, 15219. [Google Scholar] [CrossRef]






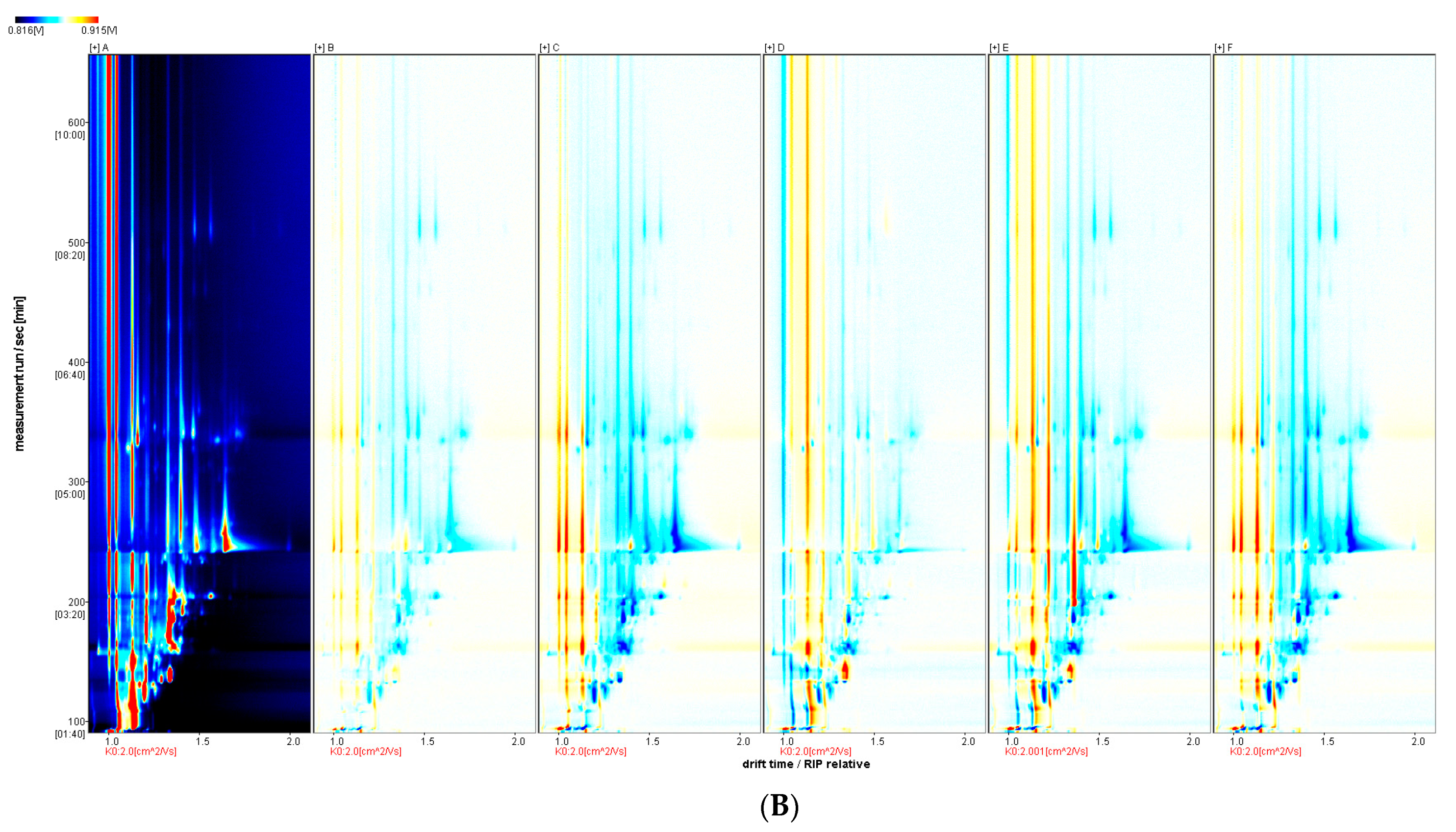
| Serial Number | Sensor Name | Sensor Response Characteristics |
|---|---|---|
| 1 | LY2/LG | chlorine, fluorine, nitrogen oxides, sulfides |
| 2 | LY2/G | ammonia, amine compounds, carbon oxides |
| 3 | LY2/AA | ethanol, acetone, ammonia |
| 4 | LY2/GH | ammonia, amine compounds |
| 5 | LY2/gCTL | hydrogen sulfide |
| 6 | LY2/gCT | propane, butane |
| 7 | T30/1 | polar compounds, hydrogen chloride |
| 8 | P10/1 | nonpolar; hydrocarbons, ammonia, chlorine |
| 9 | P10/2 | nonpolar; methane, ethane |
| 10 | P40/1 | fluorine, chlorine |
| 11 | T70/2 | toluene, xylene, carbon monoxide |
| 12 | PA/2 | ethanol, ammonia, amine compounds |
| 13 | P30/1 | hydrocarbons, ammonia, ethanol |
| 14 | P40/2 | chlorine, hydrogen sulfide, hydrogen fluoride |
| 15 | P30/2 | hydrogen sulfide, ketones |
| 16 | T40/2 | chlorine |
| 17 | T40/1 | fluorine |
| 18 | TA/2 | ethanol |
| Vanillin Concentration/(g/L) | Test the Number of Fish/Tail | Length/cm | Body Mass/g | Anaesthesia Time/min | Recovery Time/min | Recovery Rate/100% |
|---|---|---|---|---|---|---|
| 0.25 | 10 | 16.1 ± 0.35 | 271.7 ± 37.86 | 33.00 ± 8.54 | 2.58 ± 0.13 | 100 |
| 0.50 | 10 | 16.3 ± 0.95 | 273.3 ± 79.11 | 2.48 ± 0.03 | 2.13 ± 0.12 | 100 |
| 0.75 | 10 | 16.6 ± 0.96 | 291.6 ± 68.07 | 1.64 ± 0.35 | 2.21 ± 0.22 | 100 |
| 1.00 | 10 | 15.8 ± 0.41 | 263.4 ± 70.71 | 1.42 ± 0.52 | 2.46 ± 0.07 | 100 |
| 1.25 | 10 | 16.6 ± 0.80 | 288.3 ± 25.65 | 1.36 ± 0.13 | 2.48 ± 0.02 | 100 |
| 1.50 | 10 | 17.8 ± 0.72 | 310.1 ± 13.22 | 1.24 ± 0.09 | 4.59 ± 0.27 | 100 |
| Stages | Behavioural Characteristics | Minute |
|---|---|---|
| A0 stress period | A stress response occurs, swimming is accelerated, and operculum opening and closing are accelerated | 0.40 ± 0.06 |
| A1 sedation period | The response to external stimuli is weakened, the ability to swim is weakened, the body is slightly out of balance, and the breathing rate is further increased | 0.75 ± 0.12 |
| A2 Mild anaesthesia phase | The body rolls on its side, the ability to respond to external stimuli continues to weaken, swimming slowly, and the rate of operculum opening and closing decreases | 1.09 ± 0.15 |
| A3 anaesthesia period | The body of the fish is out of balance, ventral face up, stationary, and the operculum opening is reduced but continuous | 2.60 ± 0.13 |
| A4 deep anaesthesia period | The body of the fish is stationary, and the operculum opens and closes extremely slowly and irregularly | 4.45 ± 0.42 |
| R1 recovery stage 1 | The ventral side of the fish is stationary and breathing begins to slowly resume continuously | 1.44 ± 0.12 |
| R2 recovery stage 2 | The fish can swim slowly laterally, but the sense of direction is not clear, and the frequency of operculum opening is close to that before anaesthesia | 1.91 ± 0.10 |
| R3 recovery stage 3 | The fish body was completely restored to its preanaesthesia state, and the operculum and upper and lower jaw opening frequency returned to normal | 3.61 ± 0.18 |
| R4 recovery stage 4 | The operculum opens and closes normally, fully returns to normal swimming, and responds rapidly to stimuli | 4.78 ± 0.27 |
| Category | Characteristic Peak Number | Name of the Compound | CAS# | Retention Index | Retention Time/min | Migration Time/min | Description of the Incense |
|---|---|---|---|---|---|---|---|
| Aldehydes | 1 | Nonanal | C124196 | 1107.9 | 512.244 | 1.47608 | grease, cucumber and sweet orange flavours |
| 5 | (E,E)-2,4-heptadienal | C4313035 | 1012.9 | 368.968 | 1.19232 | aroma of grass and chicken | |
| 8 | 5-methylfurfural | C620020 | 974.9 | 328.377 | 1.47055 | cocoa, almonds | |
| 11 | Octanal | C124130 | 989.8 | 342.276 | 1.41258 | fat, soap | |
| 15 | (E)-2-hexenal | C6728263 | 844.7 | 233.629 | 1.5202 | fruity, green and vegetable | |
| 16 | (E)-hept-2-enal | C18829555 | 955.1 | 310.888 | 1.25698 | aroma of grass and oil | |
| 22 | Hexanal-D | C66251 | 788.4 | 204.748 | 1.56613 | grassy flavour | |
| 39 | Hexanal-M | C66251 | 787.5 | 204.354 | 1.26096 | grassy flavour | |
| Alcohols | 2 | 1-octen-3-ol- M | C3391864 | 982.7 | 335.632 | 1.15976 | mushroom, lavender, rose and hay aromas |
| 3 | 1-octen-3-ol-D | C3391864 | 982.2 | 335.107 | 1.60295 | mushroom, lavender, rose and hay aromas | |
| 10 | n-Hexanol-D | C111273 | 865.2 | 245.133 | 1.64596 | herbal flavour | |
| 12 | n-Hexanol-M | C111273 | 867 | 246.147 | 1.32588 | herbal flavour | |
| 13 | 3-Methyl-1-pentanol | C589355 | 852.3 | 237.822 | 1.31307 | fermented taste | |
| 21 | ethanol | C64175 | 421.1 | 99.501 | 1.13351 | alcohol | |
| 28 | 2-methylbutan-1-ol | C137326 | 714.7 | 171.67 | 1.23403 | aromatic with wine and ether | |
| 31 | 2,3-Butanediol | C513859 | 788.4 | 204.748 | 1.36608 | fermented taste | |
| 32 | pent-1-en-3-ol | C616251 | 684.2 | 159.959 | 0.94764 | fruity aroma | |
| 36 | 1-Pentanol,2-methyl | C105306 | 845.8 | 234.228 | 1.29357 | fermented taste | |
| 38 | (E)-2-hexen-1-ol | C928950 | 851.7 | 237.511 | 1.50757 | grassy, fruity | |
| Ketones | 9 | 3-Octanone | C106683 | 987.9 | 340.428 | 1.71197 | fruity aroma |
| 14 | 2-nonanone | C821556 | 1094.4 | 488.937 | 1.40882 | fruity, sweet and green notes | |
| 17 | 3-hydroxybutan-2-one | C513860 | 717.3 | 172.744 | 1.33455 | aromatic smell | |
| 20 | 2,3-butanedione | C431038 | 580.2 | 132.579 | 1.17293 | Fermented aroma, sweet aroma | |
| 24 | 3-Pentanone | C96220 | 693.3 | 163.079 | 1.36017 | sweet scent | |
| 25 | 2-Butanone | C78933 | 576.6 | 131.72 | 1.24487 | aromatic smell | |
| 34 | 2,3-pentanedione | C600146 | 697 | 164.522 | 1.22074 | Caramel aroma, diluted with a creamy smell | |
| 35 | 2-heptanone | C110430 | 890.4 | 260.017 | 1.26204 | fruity aroma | |
| 37 | 2-Hexanone | C591786 | 779 | 200.268 | 1.50009 | spicy smell | |
| 40 | 3-Penten-2-one, 4-methyl | C141797 | 790.2 | 205.63 | 1.45009 | sweet scent | |
| Esters | 18 | ethyl acetate | C141786 | 610.7 | 140.096 | 1.34145 | pineapple flavour |
| 19 | methyl acetate | C79209 | 548.8 | 125.276 | 1.19757 | pineapple flavour | |
| Furans | 6 | 2-pentyl furan | C3777693 | 993.6 | 345.924 | 1.25402 | fruity, grassy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Tang, J.; Huang, B.; Zhang, C.; Jiang, P.; Chen, D. Effect of Vanillin on the Anaesthesia of Crucian Carp: Effects on Physiological and Biochemical Indices, Pathology, and Volatile Aroma Components. Foods 2023, 12, 1614. https://doi.org/10.3390/foods12081614
Jiang L, Tang J, Huang B, Zhang C, Jiang P, Chen D. Effect of Vanillin on the Anaesthesia of Crucian Carp: Effects on Physiological and Biochemical Indices, Pathology, and Volatile Aroma Components. Foods. 2023; 12(8):1614. https://doi.org/10.3390/foods12081614
Chicago/Turabian StyleJiang, Lexia, Jiaming Tang, Baosheng Huang, Changfeng Zhang, Peihong Jiang, and Dongjie Chen. 2023. "Effect of Vanillin on the Anaesthesia of Crucian Carp: Effects on Physiological and Biochemical Indices, Pathology, and Volatile Aroma Components" Foods 12, no. 8: 1614. https://doi.org/10.3390/foods12081614




