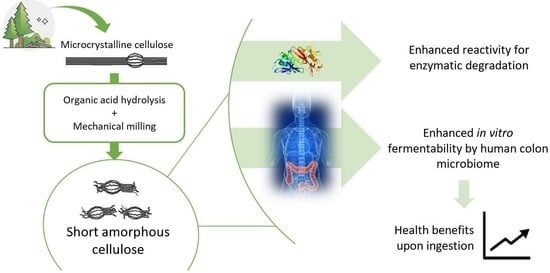
Decreasing the Crystallinity and Degree of Polymerization of Cellulose Increases Its Susceptibility to Enzymatic Hydrolysis and Fermentation by Colon Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Dietary Fiber Samples Starting from Microcrystalline Cellulose
2.3. Characterization of Dietary Fiber Samples
2.4. Enzymatic Digestibility Analysis
2.5. In Vitro Fermentation of Dietary Fiber Samples Using Human Fecal Inoculum
2.6. Short-Chain Fatty Acid Analysis
2.7. Microbial Analysis
2.8. Statistics
3. Results and Discussion
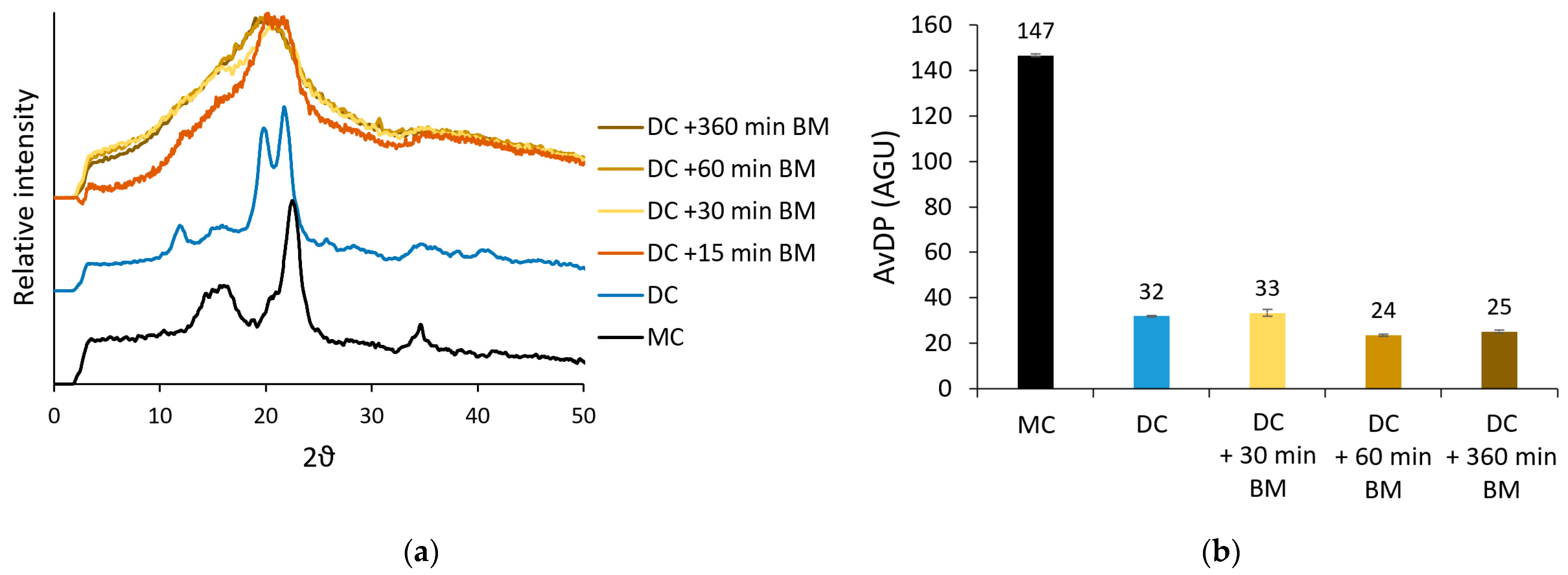
3.1. Production of Samples with Different DP and Crystallinity from Microcrystalline Cellulose
3.2. Influence of Cellulose Structural Properties on Enzymatic Accessibility
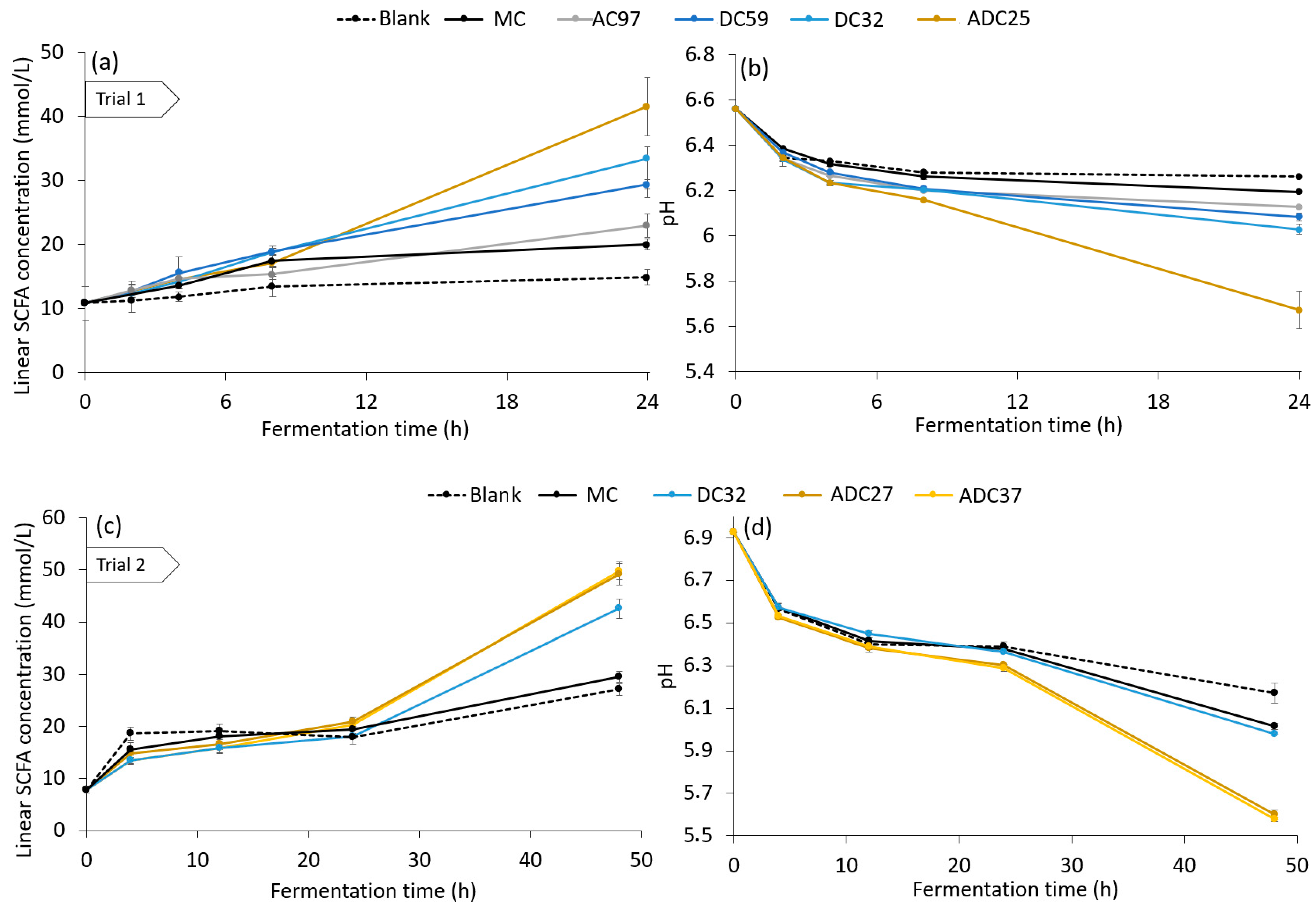
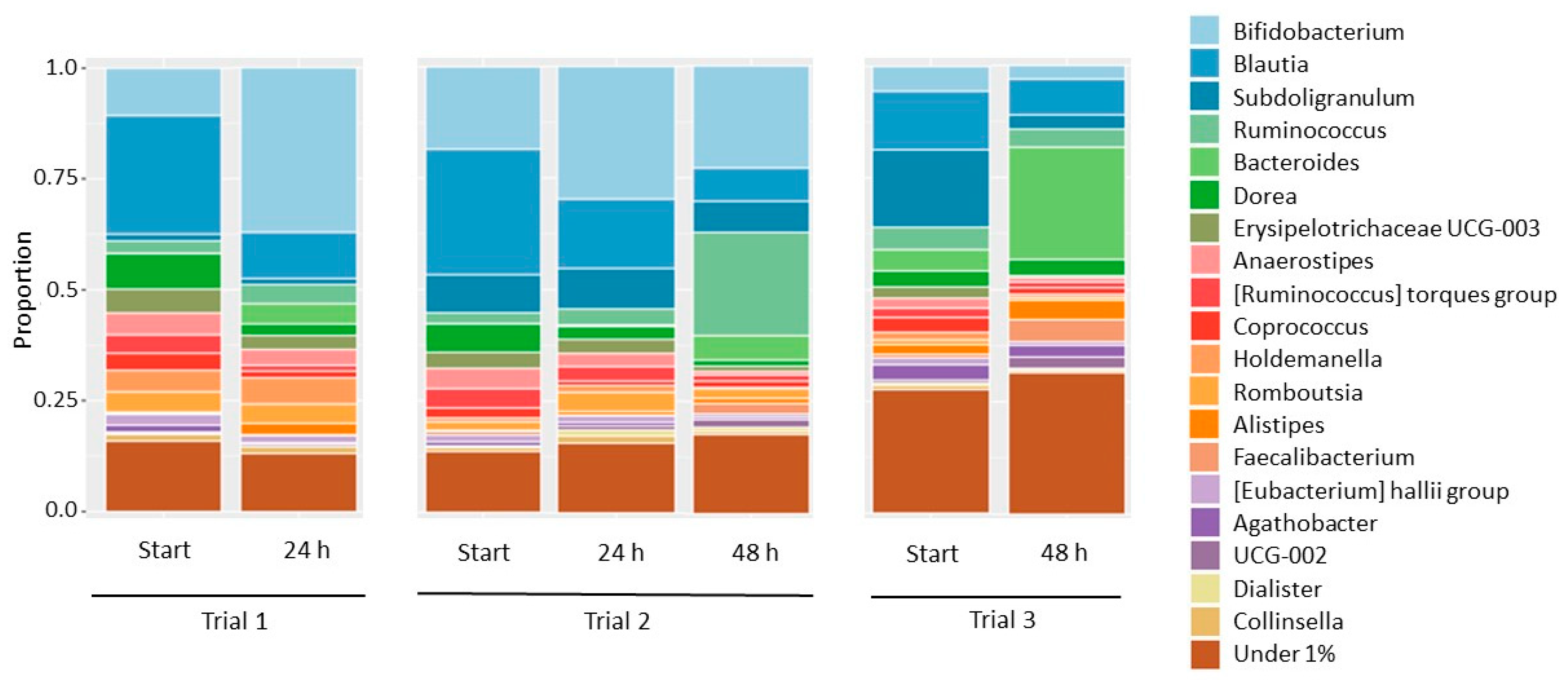
3.3. Effect of Enhanced Accessibility of Cellulose on Fermentation in the Human Colon
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ball Mill Time (min) | Ball Mill Speed (rpm) | Hydrolysis Time (h) | Hydrolysis Temperature (°C) | Ball Mill Time after Hydrolysis (min) | avDP (AGU) | Crystallinity Index | |
|---|---|---|---|---|---|---|---|
| MC | - | - | - | - | - | 168 ± 1 | 0.72 |
| AC124 | 260 | 400 | - | - | - | 124 ± 1 | <0.30 |
| AC97 | 360 | 500 | - | - | - | 97 ± 0 | <0.30 |
| DC | 360 | 500 | 16 | 120 | - | 32 ± 0 | 0.69 |
| DC28 | 260 | 400 | 16 | 130 | - | 28 ± 1 | 0.62 |
| DC42 | 260 | 400 | 16 | 110 | - | 42 ± 1 | 0.56 |
| DC85 | 260 | 400 | 2 | 110 | - | 85 ± 1 | 0.47 |
| DC104 | 260 | 400 | 2 | 90 | - | 104 ± 2 | 0.51 |
| DC32 | 360 | 500 | 16 | 120 | - | 32 ± 0 | 0.69 |
| DC59 | 360 | 500 | 3 | 100 | - | 59 ± 1 | 0.56 |
| ADC25 | 360 | 500 | 16 | 120 | 360 | 25 ± 1 | <0.30 |
| ADC27 | 360 | 500 | 16 | 120 | 60 | 27 ± 1 | <0.30 |
| ADC37 | 60 | 500 | 16 | 120 | 60 | 37 ± 2 | <0.30 |
Appendix B

Appendix C

References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Vincent, J.F.V. From Cellulose to Cell. J. Exp. Biol. 1999, 202, 3263–3268. [Google Scholar] [CrossRef]
- Dayib, M.; Larson, J.; Slavin, J. Dietary Fibers Reduce Obesity-Related Disorders: Mechanisms of Action. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Olivares, M.; Neyrinck, A.M.; Beaumont, M.; Kjølbæk, L.; Larsen, T.M.; Benítez-Páez, A.; Romaní-Pérez, M.; Garcia-Campayo, V.; Bosscher, D. Nutritional Interest of Dietary Fiber and Prebiotics in Obesity: Lessons from the MyNewGut Consortium. Clin. Nutr. 2020, 39, 414–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.J.; Ferguson, L.R. Dietary Fibre: Its Composition and Role in Protection against Colorectal Cancer. Mutat. Res. Mol. Mech. Mutagen. 1993, 290, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.; Giovannucci, E.; Michels, K.B.; Bergkvist, L.; Hansen, H.; Holmberg, L.; Wolk, A. Fruit, Vegetables, Dietary Fiber, and Risk of Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2001, 93, 525–533. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion on Dietary Reference Values for Carbohydrates and Dietary Fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H. Cellulose and the Human Gut. Gut 1984, 25, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassard, C.; Delmas, E.; Robert, C.; Bernalier-Donadille, A. The Cellulose-Degrading Microbial Community of the Human Gut Varies According to the Presence or Absence of Methanogens: Cellulolytic Microbiota and CH4 Production in the Human Gut. FEMS Microbiol. Ecol. 2010, 74, 205–213. [Google Scholar] [CrossRef]
- Robert, C.; Bernalier-Donadille, A. The Cellulolytic Microflora of the Human Colon: Evidence of Microcrystalline Cellulose-Degrading Bacteria in Methane-Excreting Subjects. FEMS Microbiol. Ecol. 2003, 46, 81–89. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Gidley, M.J.; Williams, B.A. In Vitro Fermentation of Bacterial Cellulose Composites as Model Dietary Fibers. J. Agric. Food Chem. 2011, 59, 4025–4032. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Toh, Y.-R.; Yeh, A.-I. Enhancing Cellulose Functionalities by Size Reduction Using Media-Mill. Sci. Rep. 2018, 8, 11343. [Google Scholar] [CrossRef] [Green Version]
- Nsor-Atindana, J.; Zhou, Y.X.; Saqib, M.N.; Chen, M.; Douglas Goff, H.; Ma, J.; Zhong, F. Enhancing the Prebiotic Effect of Cellulose Biopolymer in the Gut by Physical Structuring via Particle Size Manipulation. Food Res. Int. 2020, 131, 108935. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; de Beeck, B.O.; Thielemans, K.; Ennaert, T.; Snelders, J.; Dusselier, M.; Courtin, C.M.; Sels, B.F. The Role of Pretreatment in the Catalytic Valorization of Cellulose. Mol. Catal. 2020, 487, 110883. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing Cellulose Degree of Polymerization and Its Relevancy to Cellulosic Ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Gomide, F.T.F.; da Silva, A.S.; da Silva Bon, E.P.; Alves, T.L.M. Modification of Microcrystalline Cellulose Structural Properties by Ball-Milling and Ionic Liquid Treatments and Their Correlation to Enzymatic Hydrolysis Rate and Yield. Cellulose 2019, 26, 7323–7335. [Google Scholar] [CrossRef]
- Glas, D.; Paesen, R.; Depuydt, D.; Binnemans, K.; Ameloot, M.; De Vos, D.E.; Ameloot, R. Cellulose Amorphization by Swelling in Ionic Liquid/Water Mixtures: A Combined Macroscopic and Second-Harmonic Microscopy Study. ChemSusChem 2015, 8, 82–86. [Google Scholar] [CrossRef]
- Meng, X.; Ragauskas, A.J. Recent Advances in Understanding the Role of Cellulose Accessibility in Enzymatic Hydrolysis of Lignocellulosic Substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Schüth, F. Acid Hydrolysis of Cellulose as the Entry Point into Biorefinery Schemes. ChemSusChem 2009, 2, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Sumari, S.; Roesyadi, A.; Sumarno, S. Effects of Ultrasound on the Morphology, Particle Size, Crystallinity, and Crystallite Size of Cellulose. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2013, 14, 229. [Google Scholar]
- Wu, Q.; Xu, J.; Zhu, S.; Kuang, Y.; Wang, B.; Gao, W. Crystalline Stability of Cellulose III Nanocrystals in the Hydrothermal Treatment and NaOH Solution. Carbohydr. Polym. 2020, 249, 116827. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Mais, U.; Esteghlalian, A.R.; Saddler, J.N.; Mansfield, S.D. Enhancing the Enzymatic Hydrolysis of Cellulosic Materials Using Simultaneous Ball Milling. In Biotechnology for Fuels and Chemicals: The Twenty–Third Symposium; Humana Press: Totowa, NJ, USA, 2002; pp. 815–832. [Google Scholar]
- Kuo, C.-H.; Lee, C.-K. Enhancement of Enzymatic Saccharification of Cellulose by Cellulose Dissolution Pretreatments. Carbohydr. Polym. 2009, 77, 41–46. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Y.; Lin, W.; Jin, Y.; Yong, Q.; Huang, C. Enhancing the Enzymatic Digestibility of Bamboo Residues by Biphasic Phenoxyethanol-Acid Pretreatment. Bioresour. Technol. 2021, 325, 124691. [Google Scholar] [CrossRef]
- Thielemans, K.; De Bondt, Y.; Van den Bosch, S.; Bautil, A.; Roye, C.; Deneyer, A.; Courtin, C.M.; Sels, B.F. Decreasing the Degree of Polymerization of Microcrystalline Cellulose by Mechanical Impact and Acid Hydrolysis. Carbohydr. Polym. 2022, 294, 119764. [Google Scholar] [CrossRef] [PubMed]
- AFNOR NF G06-037 norm. Essais Des Fibre–Cellulose–Détermination de l’indice de Viscosité Limite et Du Degré Du Polymérisation Moyen Viscosimétrique. Available online: https://m.boutique.afnor.org/Store/Preview/DisplayExtract?ProductID=8266&VersionID=6 (accessed on 20 January 2023).
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Hu, T.; Zhao, X.; Liu, D. A Comparison of Several Organosolv Pretreatments for Improving the Enzymatic Hydrolysis of Wheat Straw: Substrate Digestibility, Fermentability and Structural Features. Appl. Energy 2015, 150, 224–232. [Google Scholar] [CrossRef]
- De Preter, V.; Falony, G.; Windey, K.; Hamer, H.M.; De Vuyst, L.; Verbeke, K. The Prebiotic, Oligofructose-Enriched Inulin Modulates the Faecal Metabolite Profile: An in Vitro Analysis. Mol. Nutr. Food Res. 2010, 54, 1791–1801. [Google Scholar] [CrossRef]
- Bautil, A.; Buyse, J.; Goos, P.; Bedford, M.R.; Courtin, C.M. Feed Endoxylanase Type and Dose Affect Arabinoxylan Hydrolysis and Fermentation in Ageing Broilers. Anim. Nutr. 2021, 7, 787–800. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Calvini, P. The Influence of Levelling-off Degree of Polymerisation on the Kinetics of Cellulose Degradation. Cellulose 2005, 12, 445–447. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Reiner, R.S. Detection and Quantitation of Cellulose II by Raman Spectroscopy. Cellulose 2021, 28, 9069–9079. [Google Scholar] [CrossRef]
- Ago, M.; Endo, T.; Hirotsu, T. Crystalline Transformation of Native Cellulose from Cellulose I to Cellulose II Polymorph by a Ball-Milling Method with a Specific Amount of Water. Cellulose 2004, 11, 163–167. [Google Scholar] [CrossRef]
- Kumar, R.; Wyman, C.E. Physical and Chemical Features of Pretreated Biomass That Influence Macro-/Micro-Accessibility and Biological Processing. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 281–310. [Google Scholar]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Inflammation-Modulating Effect of Butyrate in the Prevention of Colon Cancer by Dietary Fiber. Clin. Colorectal Cancer 2018, 17, e541–e544. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thielemans, K.; De Bondt, Y.; Comer, L.; Raes, J.; Everaert, N.; Sels, B.F.; Courtin, C.M. Decreasing the Crystallinity and Degree of Polymerization of Cellulose Increases Its Susceptibility to Enzymatic Hydrolysis and Fermentation by Colon Microbiota. Foods 2023, 12, 1100. https://doi.org/10.3390/foods12051100
Thielemans K, De Bondt Y, Comer L, Raes J, Everaert N, Sels BF, Courtin CM. Decreasing the Crystallinity and Degree of Polymerization of Cellulose Increases Its Susceptibility to Enzymatic Hydrolysis and Fermentation by Colon Microbiota. Foods. 2023; 12(5):1100. https://doi.org/10.3390/foods12051100
Chicago/Turabian StyleThielemans, Karel, Yamina De Bondt, Luke Comer, Jeroen Raes, Nadia Everaert, Bert F. Sels, and Christophe M. Courtin. 2023. "Decreasing the Crystallinity and Degree of Polymerization of Cellulose Increases Its Susceptibility to Enzymatic Hydrolysis and Fermentation by Colon Microbiota" Foods 12, no. 5: 1100. https://doi.org/10.3390/foods12051100