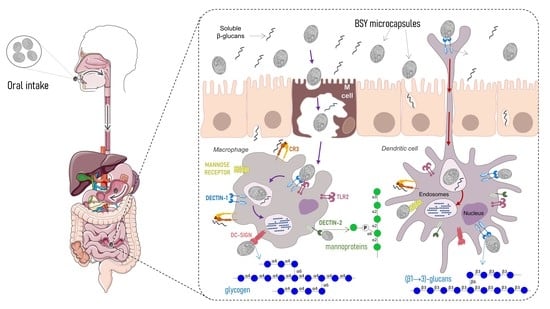
Feasibility of Brewer’s Spent Yeast Microcapsules as Targeted Oral Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brewer’s Spent Yeast Microcapsules Preparation
2.1.1. BSY Microcapsules Resultant from Alkaline Extractions
2.1.2. BSY Microcapsules Resultant from Subcritical Water Extractions (SWE)
2.2. In Vitro Digestion (IVD)
2.3. Chemical Composition Analysis of BSY Microcapsules before and after IVD
2.3.1. Protein Content
2.3.2. Sugar Analysis
2.3.3. Glycosidic Linkage Analysis
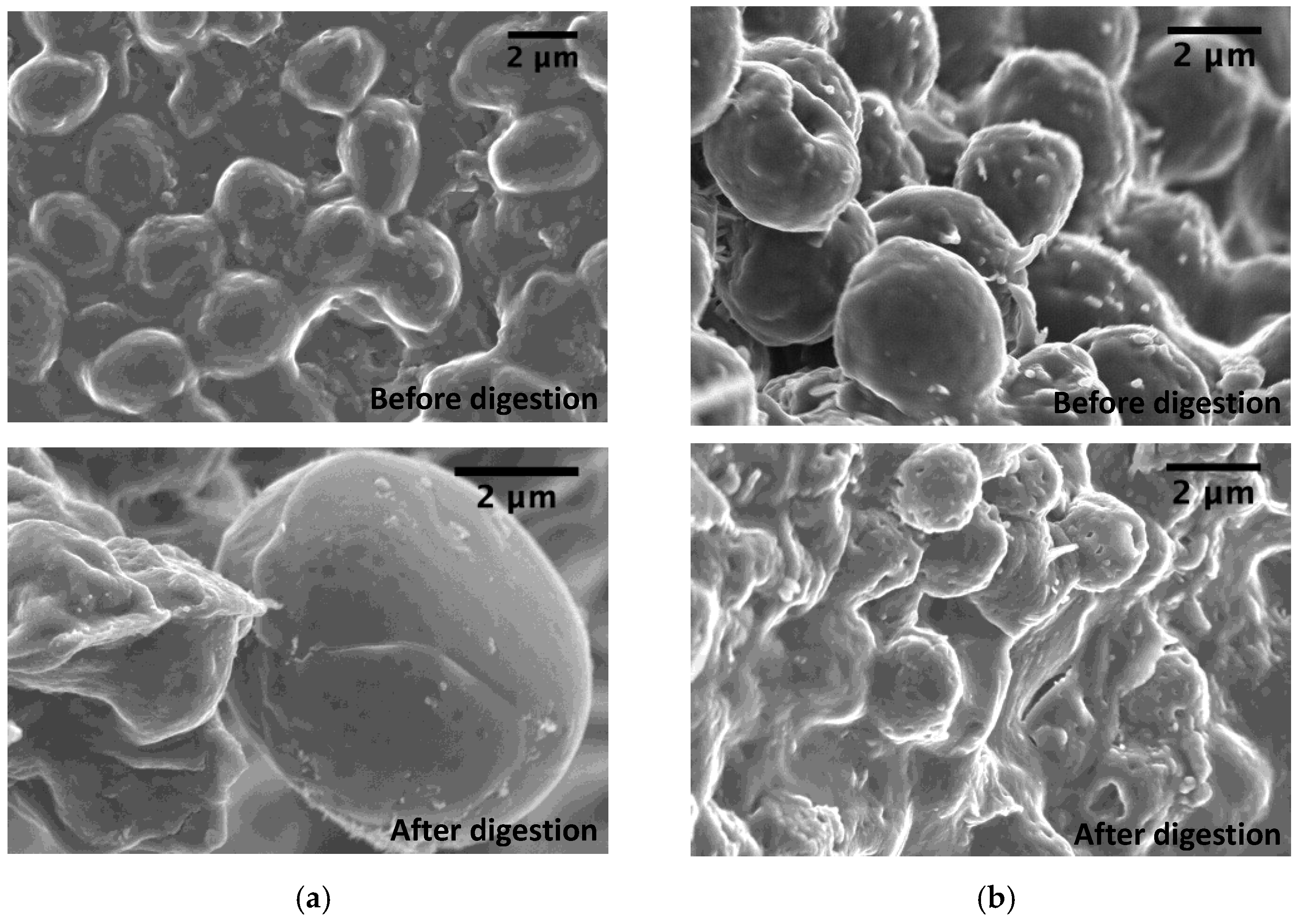
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Stimulation of Human Dectin-1 Receptor
2.6. Carbohydrate Microarray Construction and Analysis
2.6.1. Polysaccharides and Microarray Construction
2.6.2. Microarray Binding Analysis
3. Results and Discussion
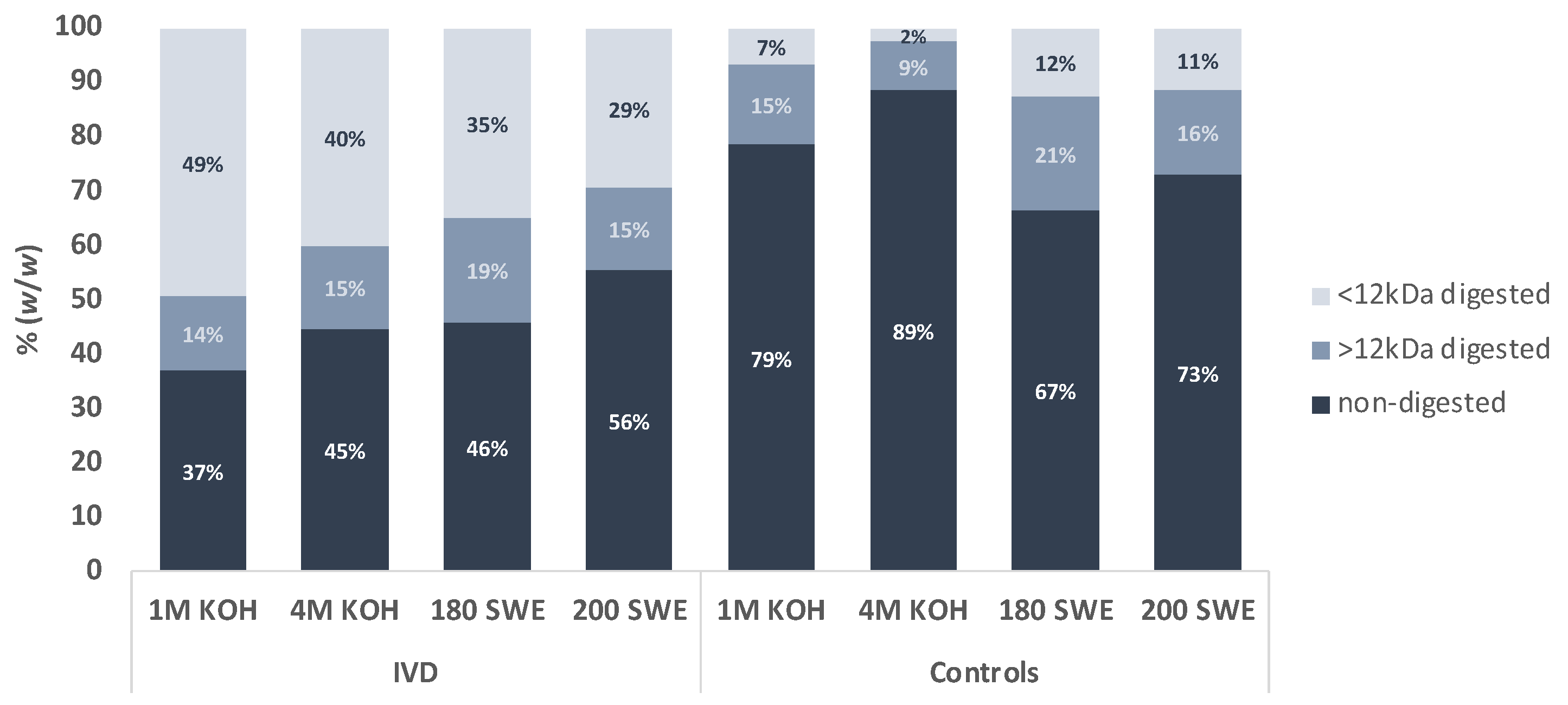
3.1. Resistance of Microcapsules to In Vitro Digestion (IVD)
3.2. Influence of In Vitro Digestion (IVD) on the Cell-Wall Polysaccharides Structure
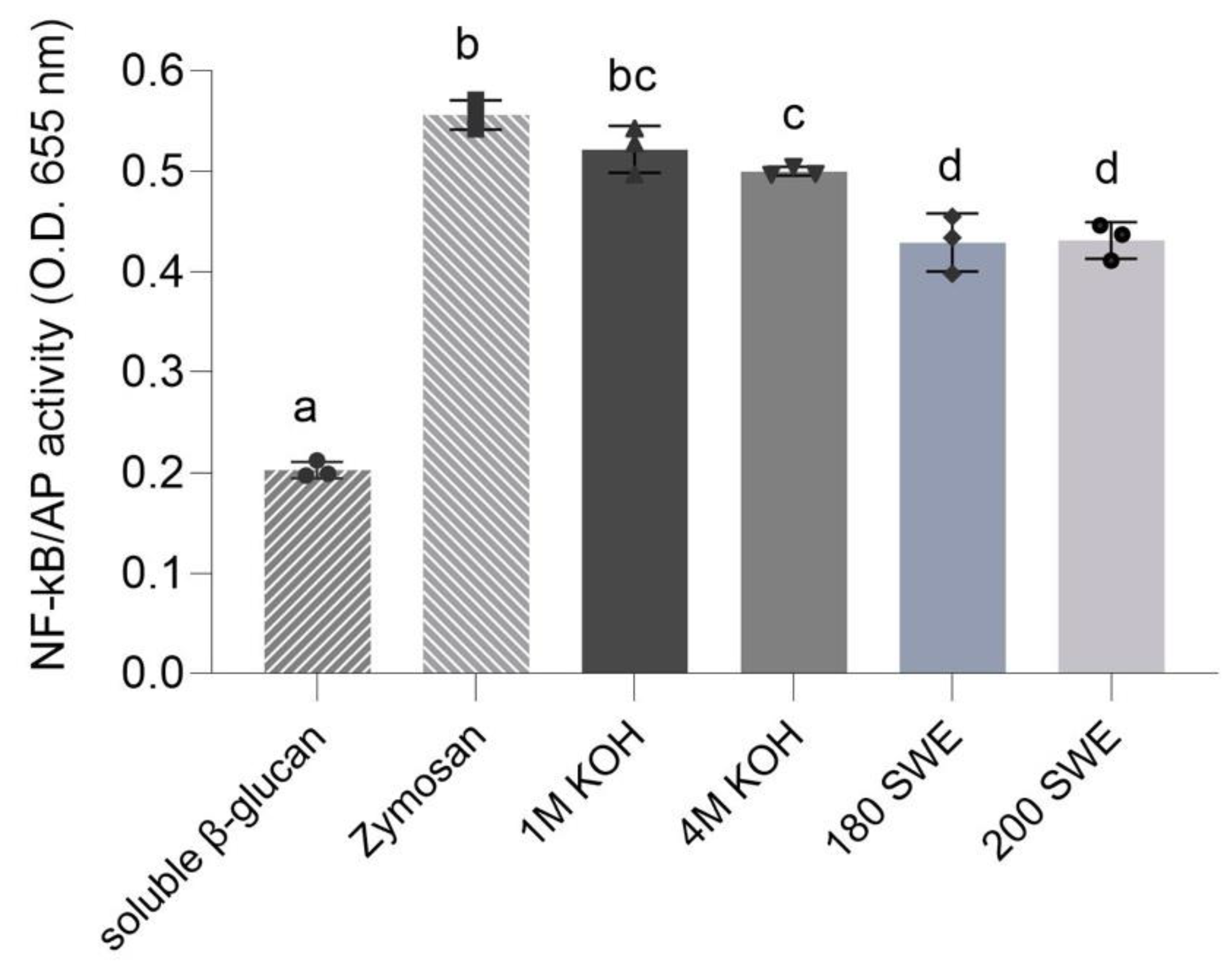
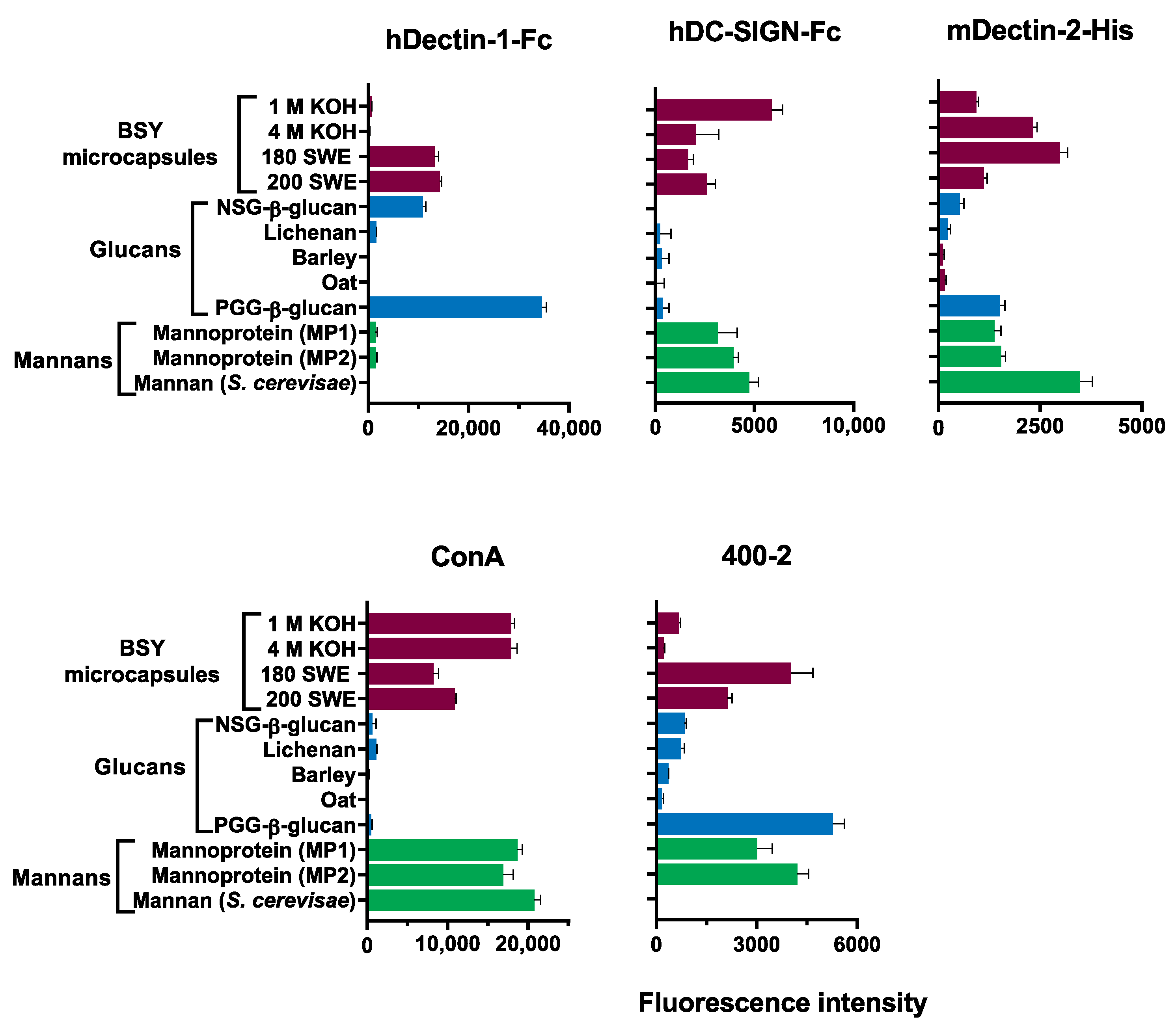
3.3. Recognition of Microcapsules by Dectin-1 Immune Receptor
3.4. Characterization of Digested Material from Microcapsules after In Vitro Digestion (IVD)
3.5. Recognition of Digested Material by Carbohydrate-Binding Proteins
3.6. Feasibility of BSY Microcapsules as Targeted Oral Carriers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent Brewer’s Yeast as a Source of High Added Value Molecules: A Systematic Review on Its Characteristics, Processing and Potential Applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Bastos, R.; Oliveira, P.G.; Gaspar, V.M.; Mano, J.F.; Coimbra, M.A.; Coelho, E. Brewer’s Yeast Polysaccharides—A Review of Their Exquisite Structural Features and Biomedical Applications. Carbohydr. Polym. 2022, 277, 118826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Wu, X.; Algharib, S.A.; Gong, F.; Hu, J.; Luo, W.; Zhou, M.; Pan, Y.; Yan, Y.; et al. Structure, Preparation, Modification, and Bioactivities of β-Glucan and Mannan from Yeast Cell Wall: A Review. Int. J. Biol. Macromol. 2021, 173, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C.; Park, S. Advances in the Valorization of Spent Brewer’s Yeast. Innov. Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces Yeast Biomass: Characteristics and Potential Applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Reis, S.F.; Messias, S.; Bastos, R.; Martins, V.J.; Correia, V.G.; Pinheiro, B.A.; Silva, L.M.; Palma, A.S.; Coimbra, M.A.; Coelho, E. Structural Differences on Cell Wall Polysaccharides of Brewer’s Spent Saccharomyces and Microarray Binding Profiles with Immune Receptors. Carbohydr. Polym. 2023, 301, 120325. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of Brewers Spent Yeast Polysaccharides: A Structural Characterization Approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Pinto, M.; Pinto, R.J.B.; Freire, C.S.R.; Coimbra, M.A. Polysaccharide Characterization of Brewers Spent Yeast Insoluble Residue after Chlorite Oxidation Treatment. Trends Carbohydr. Res. 2015, 7, 33–40. [Google Scholar]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Modifications of Saccharomyces Pastorianus Cell Wall Polysaccharides with Brewing Process. Carbohydr. Polym. 2015, 124, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Magnani, M.; Calliari, C.M.; de Macedo, F.C.; Mori, M.P.; de Syllos Cólus, I.M.; Castro-Gomez, R.J.H. Optimized Methodology for Extraction of (1→3)(1→6)-β-d-Glucan from Saccharomyces Cerevisiae and in Vitro Evaluation of the Cytotoxicity and Genotoxicity of the Corresponding Carboxymethyl Derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, Q.; Cui, S.W.; Liu, H.-Z. A New Isolation Method of β-d-Glucans from Spent Yeast Saccharomyces Cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Freimund, S.; Sauter, M.; Käppeli, O.; Dutler, H. A New Non-Degrading Isolation Process for 1,3-β-d-Glucan of High Purity from Baker’s Yeast Saccharomyces Cerevisiae. Carbohydr. Polym. 2003, 54, 159–171. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, S.; Wu, T. Combination of Induced Autolysis and Sodium Hypochlorite Oxidation for the Production of Saccharomyces Cerevisiae (1→3)-β-D-Glucan. World J. Microbiol. Biotechnol. 2003, 19, 947–952. [Google Scholar] [CrossRef]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust Stimulation of Humoral and Cellular Immune Responses Following Vaccination with Antigen-Loaded β-Glucan Particles. MBio 2010, 1, e00164-10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garello, F.; Arena, F.; Cutrin, J.C.; Esposito, G.; D’Angeli, L.; Cesano, F.; Filippi, M.; Figueiredo, S.; Terreno, E. Glucan Particles Loaded with a NIRF Agent for Imaging Monocytes/Macrophages Recruitment in a Mouse Model of Rheumatoid Arthritis. RSC Adv. 2015, 5, 34078–34087. [Google Scholar] [CrossRef] [Green Version]
- Ren, T.; Gou, J.; Sun, W.; Tao, X.; Tan, X.; Wang, P.; Zhang, Y.; He, H.; Yin, T.; Tang, X. Entrapping of Nanoparticles in Yeast Cell Wall Microparticles for Macrophage-Targeted Oral Delivery of Cabazitaxel. Mol. Pharm. 2018, 15, 2870–2882. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Duan, B.; Chen, H.; Xu, X. A Novel Strategy for Treating Inflammatory Bowel Disease by Targeting Delivery of Methotrexate through Glucan Particles. Adv. Healthc. Mater. 2020, 9, 1901805. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Yang, Y.; Liu, Z.; Wang, J.; Xu, Y.; Zhang, Y. Novel β-1,3-d-Glucan Porous Microcapsule Enveloped Folate-Functionalized Liposomes as a Trojan Horse for Facilitated Oral Tumor-Targeted Co-Delivery of Chemotherapeutic Drugs and Quantum Dots. J. Mater. Chem. B 2020, 8, 2307–2320. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Zhang, W.; Li, Y.; Liu, L.; Leng, T. Yeast Cell Wall Particle Mediated Nanotube-RNA Delivery System Loaded with MiR365 Antagomir for Post-Traumatic Osteoarthritis Therapy via Oral Route. Theranostics 2020, 10, 8479–8493. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef]
- Reis, S.F.; Abu-Ghannam, N. Antioxidant Capacity, Arabinoxylans Content and Invitro Glycaemic Index of Cereal-Based Snacks Incorporated with Brewer’s Spent Grain. LWT-Food Sci. Technol. 2014, 55, 269–277. [Google Scholar] [CrossRef]
- Fujihara, S.; Kasuga, A.; Aoyagi, Y.; Sugahara, T. Nitrogen-to-Protein Conversion Factors for Some Common Edible Mushrooms. J. Food Sci. 1995, 60, 1045–1047. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Delgadillo, I.; Waldron, K.W.; Selvendran, R.R. Isolation and Analysis of Cell Wall Polymers from Olive Pulp. In Plant Cell Wall Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 19–44. ISBN 978-3-642-60989-3. [Google Scholar]
- Ciucanu, I.; Kerek, F. A Simple and Rapid Method for the Permethylation of Carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Liu, Y.; McBride, R.; Stoll, M.; Palma, A.S.; Silva, L.; Agravat, S.; Aoki-Kinoshita, K.F.; Campbell, M.P.; Costello, C.E.; Dell, A.; et al. The Minimum Information Required for a Glycomics Experiment (MIRAGE) Project: Improving the Standards for Reporting Glycan Microarray-Based Data. Glycobiology 2017, 27, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Childs, R.A.; Palma, A.S.; Campanero-Rhodes, M.A.; Stoll, M.S.; Chai, W.; Feizi, T. Neoglycolipid-Based Oligosaccharide Microarray System: Preparation of NGLs and Their Noncovalent Immobilization on Nitrocellulose-Coated Glass Slides for Microarray Analyses. Methods Mol. Biol. 2012, 808, 117–136. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Simões, C.; Maciel, E.; Domingues, P.; Domingues, M.R.M.; Coimbra, M.A. Transglycosylation Reactions, a Main Mechanism of Phenolics Incorporation in Coffee Melanoidins: Inhibition by Maillard Reaction. Food Chem. 2017, 227, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.; Moreira, A.S.; Maria do Rosário, M.D.; Evtuguin, D.V.; Coelho, E.; Coimbra, M.A. Contribution of Non-Enzymatic Transglycosylation Reactions to the Honey Oligosaccharides Origin and Diversity. Pure Appl. Chem. 2019, 91, 1231–1242. [Google Scholar] [CrossRef]
- Nunes, F.M.; Lopes, E.S.; Moreira, A.S.P.; Simões, J.; Coimbra, M.A.; Domingues, R.M. Formation of Type 4 Resistant Starch and Maltodextrins from Amylose and Amylopectin upon Dry Heating: A Model Study. Carbohydr. Polym. 2016, 141, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential High-Affinity Interaction of Dectin-1 with Natural or Synthetic Glucans Is Dependent upon Primary Structure and Is Influenced by Polymer Chain Length and Side-Chain Branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Palma, A.S.; Liu, Y.; Zhang, H.; Zhang, Y.; McCleary, B.V.; Yu, G.; Huang, Q.; Guidolin, L.S.; Ciocchini, A.E.; Torosantucci, A.; et al. Unravelling Glucan Recognition Systems by Glycome Microarrays Using the Designer Approach and Mass Spectrometry. Mol. Cell. Proteomics 2015, 14, 974–988. [Google Scholar] [CrossRef] [Green Version]
- Vendele, I.; Willment, J.A.; Silva, L.M.; Palma, A.S.; Chai, W.; Liu, Y.; Feizi, T.; Spyrou, M.; Stappers, M.H.T.; Brown, G.D.; et al. Mannan Detecting C-Type Lectin Receptor Probes Recognise Immune Epitopes with Diverse Chemical, Spatial and Phylogenetic Heterogeneity in Fungal Cell Walls. PLoS Pathog. 2020, 16, e1007927. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kwon, Y.; Hwang, J.; Choi, Y.; Kim, K.; Koo, H.-J.; Seo, Y.; Jeon, H.; Choi, J. Synthesis and Functionalization of β-Glucan Particles for the Effective Delivery of Doxorubicin Molecules. ACS Omega 2019, 4, 668–674. [Google Scholar] [CrossRef]
- Gao, C.; Stavenhagen, K.; Eckmair, B.; McKitrick, T.R.; Mehta, A.Y.; Matsumoto, Y.; McQuillan, A.M.; Hanes, M.S.; Eris, D.; Baker, K.J.; et al. Differential Recognition of Oligomannose Isomers by Glycan-Binding Proteins Involved in Innate and Adaptive Immunity. Sci Adv. 2021, 7, eabf6834. [Google Scholar] [CrossRef] [PubMed]
- Geissner, A.; Reinhardt, A.; Rademacher, C.; Johannssen, T.; Monteiro, J.; Lepenies, B.; Thepaut, M.; Fieschi, F.; Mrazkova, J.; Wimmerova, M.; et al. Microbe-Focused Glycan Array Screening Platform. Proc. Natl. Acad. Sci. USA 2019, 116, 1958–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meikle, P.J.; Bonig, I.; Hoogenraad, N.J.; Clarke, A.E.; Stone, B.A. The Location of (1→3)-β-glucans in the Walls of Pollen Tubes of Nicotiana Alata using a (1→3)-β-Glucan-Specific Monoclonal Antibody. Planta 1991, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.S.; Feizi, T.; Zhang, Y.; Stoll, M.S.; Lawson, A.M.; Díaz-Rodríguez, E.; Campanero-Rhodes, M.A.; Costa, J.; Gordon, S.; Brown, G.D.; et al. Ligands for the β-Glucan Receptor, Dectin-1, Assigned Using “Designer” Microarrays of Oligosaccharide Probes (Neoglycolipids) Generated from Glucan Polysaccharides*. J. Biol. Chem. 2006, 281, 5771–5779. [Google Scholar] [CrossRef] [Green Version]
- Pejchal, R.; Doores, K.J.; Walker, L.M.; Khayat, R.; Huang, P.S.; Wang, S.K.; Stanfield, R.L.; Julien, J.P.; Ramos, A.; Crispin, M.; et al. A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield. Science 2011, 334, 1097–1103. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.M.; Correia, V.G.; Moreira, A.S.P.; Domingues, M.R.M.; Ferreira, R.M.; Figueiredo, C.; Azevedo, N.F.; Marcos-Pinto, R.; Carneiro, F.; Magalhães, A.; et al. Helicobacter Pylori Lipopolysaccharide Structural Domains and Their Recognition by Immune Proteins Revealed with Carbohydrate Microarrays. Carbohydr. Polym. 2021, 253, 117350. [Google Scholar] [CrossRef]
| BSY Microcapsules | Protein (Total %) | Carbohydrates (Total %) | Man mol % | Glc mol % | |
|---|---|---|---|---|---|
| 1 M KOH | Before IVD | 31 | 58 | 9 | 91 |
| After IVD | 15 | 79 | 6 | 94 | |
| Control | 24 | 57 | 7 | 93 | |
| 4 M KOH | Before IVD | 32 | 61 | 14 | 86 |
| After IVD | 13 | 79 | 13 | 87 | |
| Control | 30 | 46 | 9 | 91 | |
| 180 SWE | Before IVD | 32 | 41 | 23 | 77 |
| After IVD | 26 | 44 | 4 | 96 | |
| Control | 46 | 28 | 5 | 95 | |
| 200 SWE | Before IVD | 32 | 13 | 40 | 60 |
| After IVD | 56 | 42 | 30 | 70 | |
| Control | 60 | 41 | 46 | 54 | |
| Linkage | 1 M KOH | 4 M KOH | ||||
|---|---|---|---|---|---|---|
| Before IVD | After IVD | Control | Before IVD | After IVD | Control | |
| t-Man | 3.7 | 6.2 | 3.0 | 8.9 | 11.2 | 7.0 |
| 2-Man | 0.7 | 1.9 | 1.1 | 0.7 | 3.6 | 2.7 |
| 3-Man | 0.3 | ------ | 0.2 | 0.3 | 0.8 | 0.8 |
| 6-Man | ------ | ------ | 0.1 | 0.2 | ------ | 0.4 |
| 2,3-Man | 0.1 | 3.0 | 0.3 | 0.7 | 1.6 | 0.9 |
| 2,6-Man | ------ | 0.5 | 1.2 | 2.0 | 0.6 | 1.3 |
| 2,3,4,6-Man | 0.2 | 0.6 | 0.1 | 0.7 | 0.7 | 0.2 |
| Total Man | 5.1 | 12.2 | 6.0 | 18.0 | 18.5 | 13.2 |
| t-Glc | 11.7 | 14.2 | 11.1 | 9.2 | 12.4 | 10.5 |
| 3-Glc | 18.5 | 57.1 | 15.0 | 20.0 | 55.6 | 22.6 |
| 4-Glc | 57.6 | 1.6 | 60.3 | 40.7 | 2.1 | 45.0 |
| 6-Glc | 1.4 | 5.8 | 2.8 | 3.8 | 4.9 | 3.5 |
| 3,6-Glc | 1.3 | 3.3 | 1.1 | 1.3 | 2.8 | 1.8 |
| 4,6-Glc | 3.3 | 0.1 | 2.6 | 3.8 | 0.1 | 2.0 |
| 2,3,4,6-Glc | 1.0 | 4.8 | 0.9 | 2.5 | 2.9 | 1.2 |
| Total Glc | 94.8 | 86.8 | 93.7 | 81.5 | 80.7 | 86.7 |
| Linkage | 180 SWE | 200 SWE | ||||
|---|---|---|---|---|---|---|
| Before IVD | After IVD | Control | Before IVD | After IVD | Control | |
| t-Man | 9.9 | 1.7 | 2.9 | 15.3 | 5.0 | 6.9 |
| 2-Man | 6.4 | 0.6 | ------ | 7.7 | 1.7 | ------ |
| 3-Man | 1.6 | ------ | ------ | 1.3 | ------ | ------ |
| 6-Man | 0.8 | ------ | ------ | 1.5 | ------ | ------ |
| 2,3-Man | 0.2 | 0.3 | 0.9 | ------ | ------ | ------ |
| 2,6-Man | 6.3 | 0.1 | 0.4 | 9.6 | 0.7 | 0.6 |
| 2,3,4,6-Man | 0.2 | 0.3 | 0.7 | 1.4 | 4.5 | 2.3 |
| Total Man | 25.5 | 2.9 | 5.0 | 36.9 | 11.9 | 9.8 |
| t-Glc | 8.7 | 10.5 | 13.8 | 8.7 | 4.7 | 5.4 |
| 3-Glc | 41.0 | 77.4 | 61.6 | 17.7 | 27.0 | 22.5 |
| 4-Glc | 13.9 | 1.5 | 4.7 | 21.6 | 12.5 | 24.8 |
| 6-Glc | 5.8 | 1.6 | 5.6 | 4.0 | 1.2 | ------ |
| 3,6-Glc | 3.6 | 2.0 | 3.6 | 1.2 | 1.4 | 1.8 |
| 4,6-Glc | 0.2 | tr | 0.3 | 1.6 | 0.7 | 1.4 |
| 2,3,4,6-Glc | 0.7 | 3.4 | 4.3 | 5.9 | 34.8 | 29.5 |
| Total Glc | 73.9 | 96.4 | 93.9 | 60.6 | 82.3 | 85.4 |
| BSY Microcapsules | Protein (Total %) | Carbohydrates (Total %) | Man mol % | Glc mol % | |
|---|---|---|---|---|---|
| 1 M KOH | Digested > 12 kDa | 39 | 35 | 42 | 58 |
| Control | 36 | 52 | 30 | 70 | |
| 4 M KOH | Digested > 12 kDa | 43 | 44 | 67 | 33 |
| Control | 20 | 32 | 36 | 64 | |
| 180 SWE | Digested > 12 kDa | 43 | 33 | 68 | 32 |
| Control | 33 | 49 | 56 | 44 | |
| 200 SWE | Digested > 12 kDa | 52 | 23 | 54 | 46 |
| Control | 49 | 28 | 46 | 54 | |
| Linkage | 1 M KOH | 4 M KOH | ||
|---|---|---|---|---|
| Digested > 12 kDa | Control | Digested > 12 kDa | Control | |
| t- Man | 22.7 | 13.4 | 34.3 | 15.4 |
| 2-Man | 4.8 | 4.0 | 7.3 | 4.0 |
| 3-Man | 1.5 | 1.4 | 2.9 | 1.4 |
| 6-Man | ------ | ------ | ------ | ------ |
| 2,3-Man | ------ | ------ | ------ | ------ |
| 2,6-Man | 1.6 | 2.1 | 0.8 | 1.5 |
| 2,3,4,6-Man | 0.7 | 0.1 | 1.0 | 0.2 |
| Total Man | 31.3 | 21.0 | 46.2 | 22.4 |
| t-Glc | 25.9 | 8.5 | 17.8 | 8.3 |
| 3-Glc | 0.2 | 0.2 | 0.3 | 0.9 |
| 4-Glc | 29.1 | 62.6 | 22.4 | 60.4 |
| 6-Glc | 2.3 | 2.3 | 2.6 | 4.0 |
| 3,6-Glc | 1.3 | 0.5 | 1.1 | 0.5 |
| 4,6-Glc | 7.1 | 3.9 | 6.0 | 2.2 |
| 2,3,4,6-Glc | 2.6 | 1.1 | 3.2 | 1.4 |
| Total Glc | 68.5 | 79.0 | 53.4 | 77.6 |
| Linkage | 180 SWE | 200 SWE | ||
|---|---|---|---|---|
| Digested > 12 kDa | Control | Digested > 12 kDa | Control | |
| t-Man | 44.4 | 29.3 | 32.1 | 54.7 |
| 2-Man | 10.9 | 7.0 | 10.4 | 4.7 |
| 3-Man | 3.4 | 1.7 | 1.9 | 2.2 |
| 6-Man | 0.5 | ------ | 0.4 | ------ |
| 2,3-Man | ------ | ------ | ------ | ------ |
| 2,6-Man | 1.1 | 1.4 | 1.4 | 1.8 |
| 2,3,4,6-Man | 1.2 | 1.5 | 1.0 | 1.8 |
| Total Man | 61.5 | 40.9 | 47.1 | 65.1 |
| t-Glc | 6.1 | 10.6 | 8.0 | 16 |
| 3-Glc | 15.9 | 13.2 | 26.7 | 14 |
| 4-Glc | 5.3 | 22.1 | 7.0 | 4.9 |
| 6-Glc | 2.0 | 5.9 | 2.0 | ------ |
| 3,6-Glc | 0.9 | 0.9 | 1.3 | ------ |
| 4,6-Glc | 1.0 | 2.7 | 1.1 | ------ |
| 2,3,4,6-Glc | 6.9 | 4.5 | 6.1 | 1.8 |
| Total Glc | 38.1 | 59.8 | 52.3 | 36.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, S.F.; Martins, V.J.; Bastos, R.; Lima, T.; Correia, V.G.; Pinheiro, B.A.; Silva, L.M.; Palma, A.S.; Ferreira, P.; Vilanova, M.; et al. Feasibility of Brewer’s Spent Yeast Microcapsules as Targeted Oral Carriers. Foods 2023, 12, 246. https://doi.org/10.3390/foods12020246
Reis SF, Martins VJ, Bastos R, Lima T, Correia VG, Pinheiro BA, Silva LM, Palma AS, Ferreira P, Vilanova M, et al. Feasibility of Brewer’s Spent Yeast Microcapsules as Targeted Oral Carriers. Foods. 2023; 12(2):246. https://doi.org/10.3390/foods12020246
Chicago/Turabian StyleReis, Sofia F., Vitor J. Martins, Rita Bastos, Tânia Lima, Viviana G. Correia, Benedita A. Pinheiro, Lisete M. Silva, Angelina S. Palma, Paula Ferreira, Manuel Vilanova, and et al. 2023. "Feasibility of Brewer’s Spent Yeast Microcapsules as Targeted Oral Carriers" Foods 12, no. 2: 246. https://doi.org/10.3390/foods12020246