Study on the Regulation Mechanism of Quality Deterioration Due to Chilling Stress and Dry Exposure during Anhydrous Storage and Transportation of Yesso Scallop Patinopecten yessoensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Raw Materials
2.2. Experimental Design and Methods
2.3. Analysis of the Test
2.3.1. Living Health Evaluation
2.3.2. H&E Staining and Histological Examination of Gill Tissues
2.3.3. Nucleotides and Associated Compounds
2.3.4. Proteomics
2.3.5. Metabolomics
2.4. Data Processing
3. Results
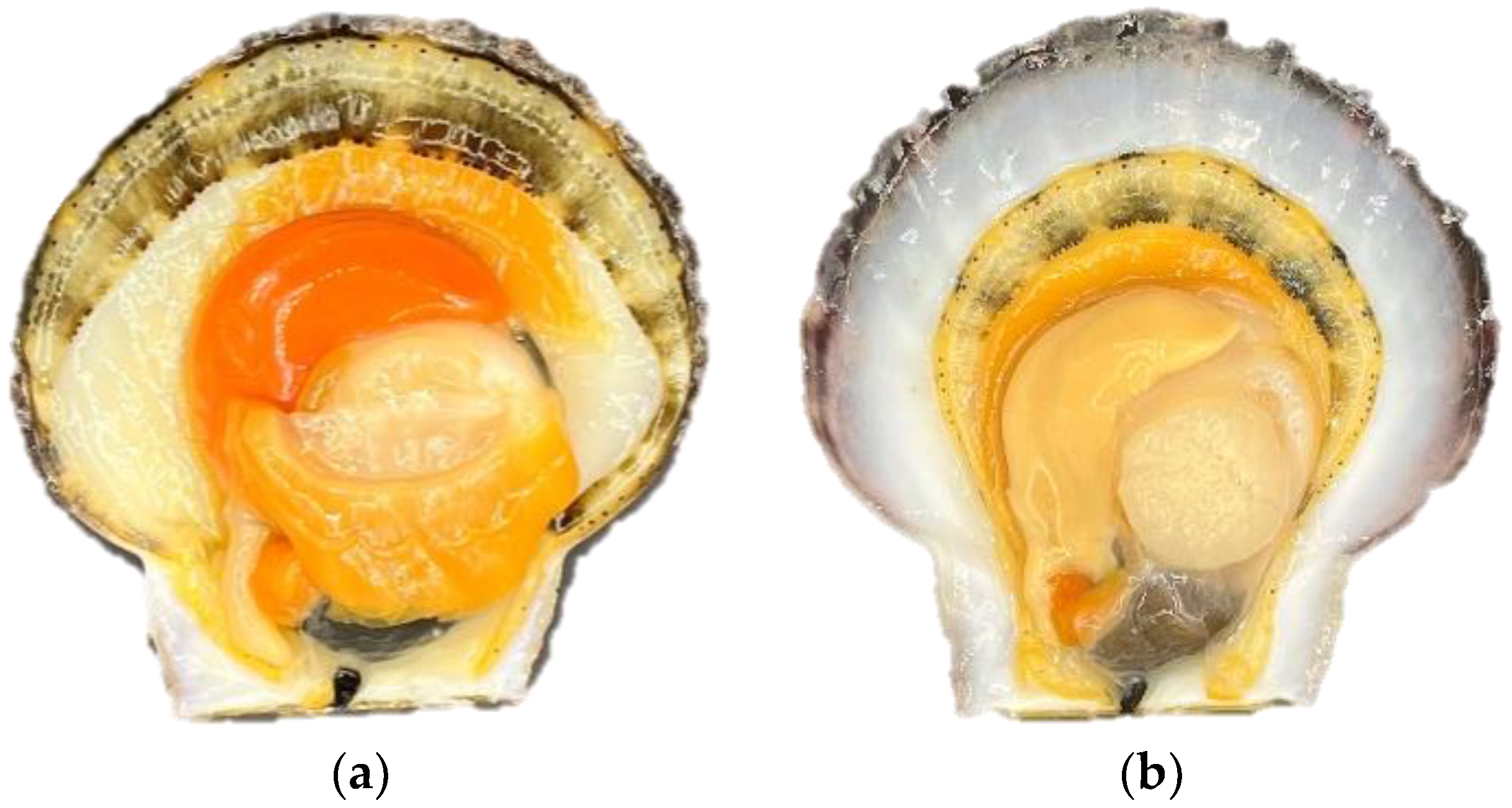
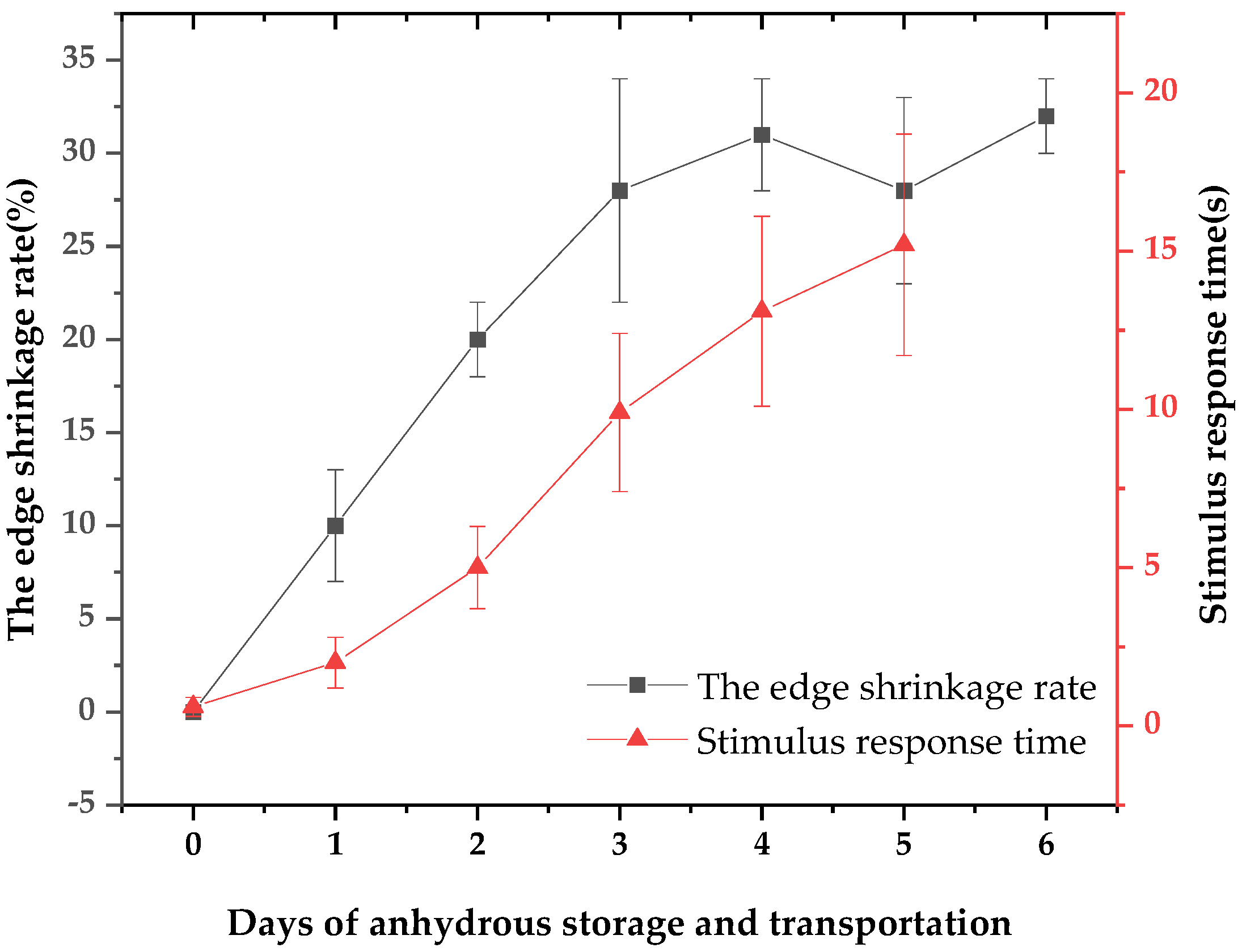
3.1. The Apparent Vitality of Patinopecten yessoensis Decreased during Anhydrous Storage and Transportation
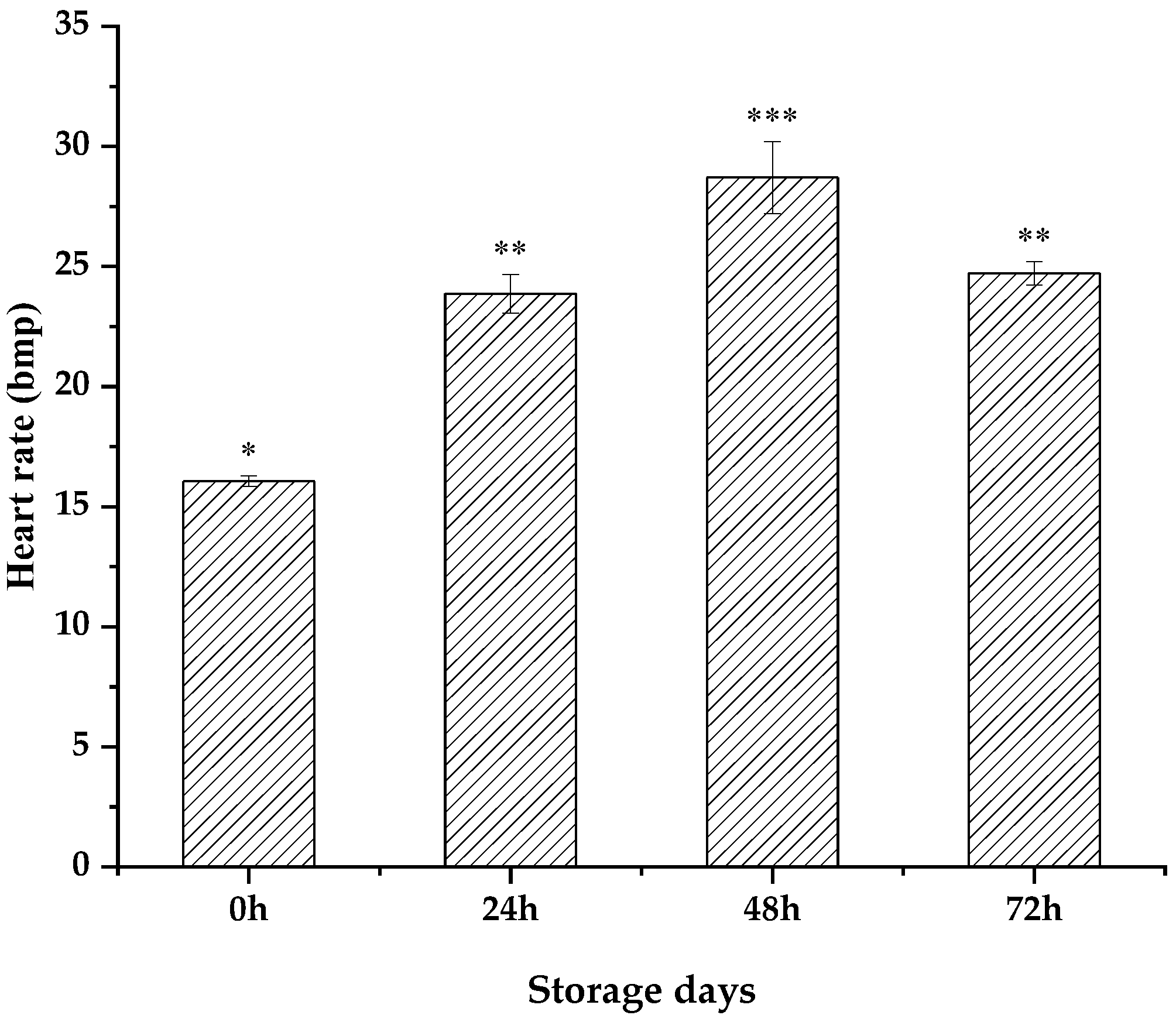
3.2. Patinopecten yessoensis Vitality Dropped during the Anhydrous Storage and Transportation Process
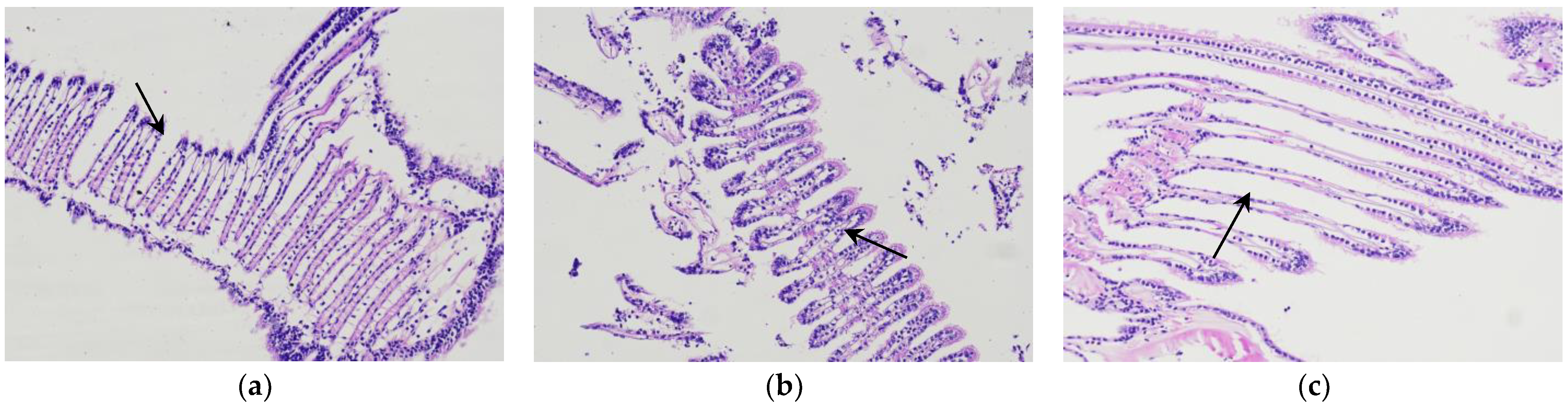
3.3. Changes in Microorganizational Structure of the Gills
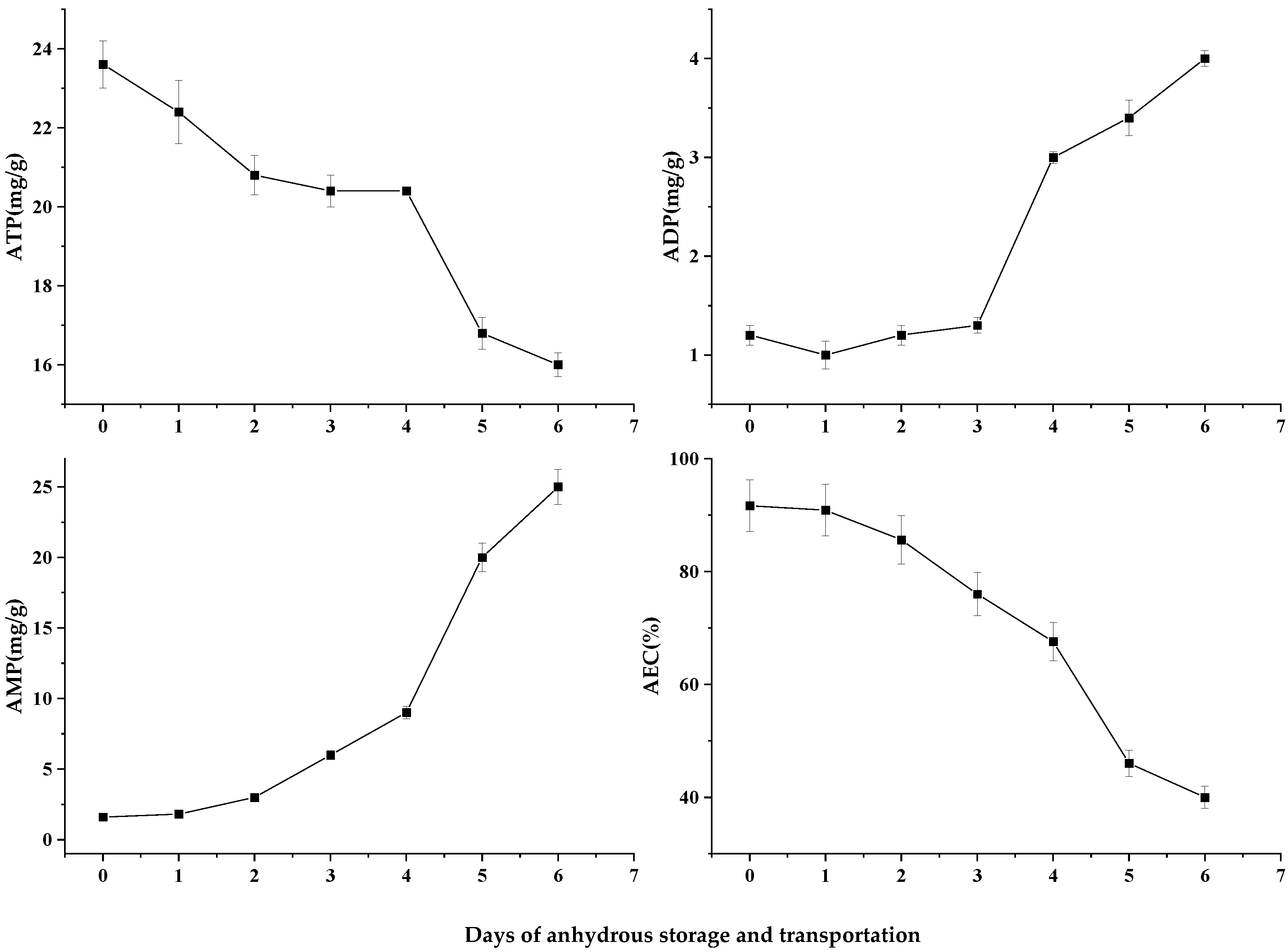
3.4. Nucleotide-Lineage Compounds
3.5. Environmental Stress Induced Changes in the Proteome
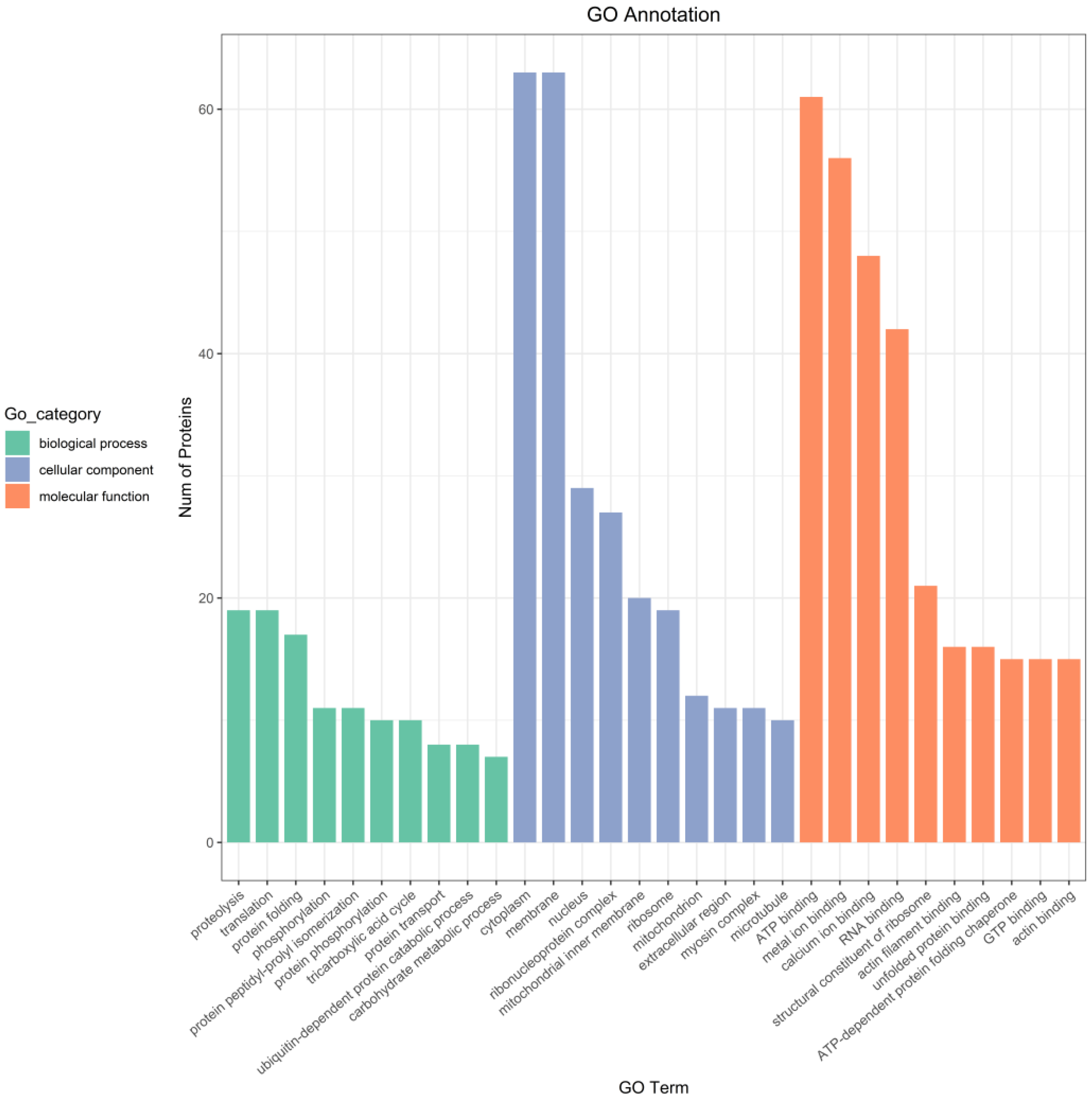
3.5.1. GO Annotation
3.5.2. COG Annotation
3.5.3. Analysis of Variance
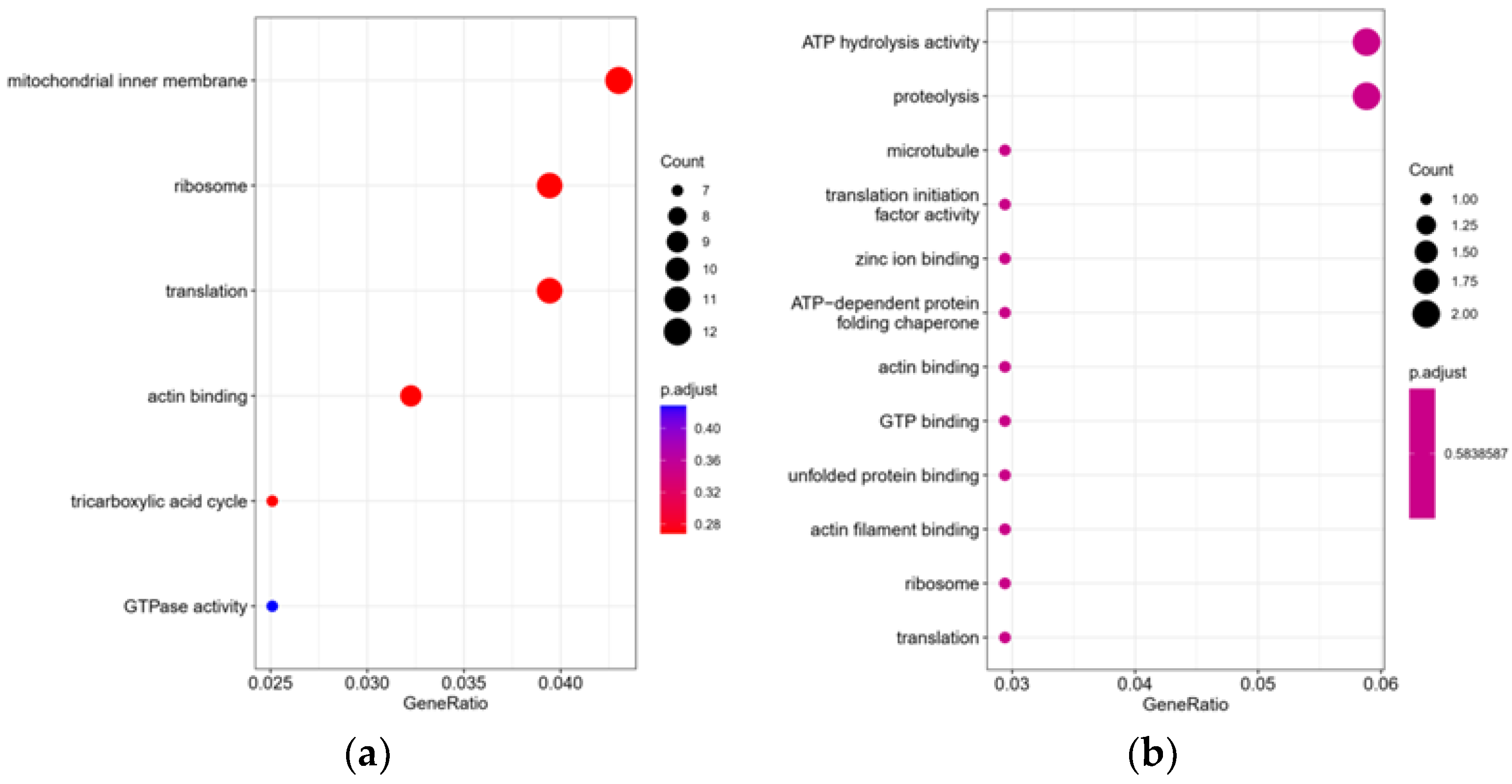
3.5.4. GO Annotation and Enrichment Analysis of Significantly Differential Proteins
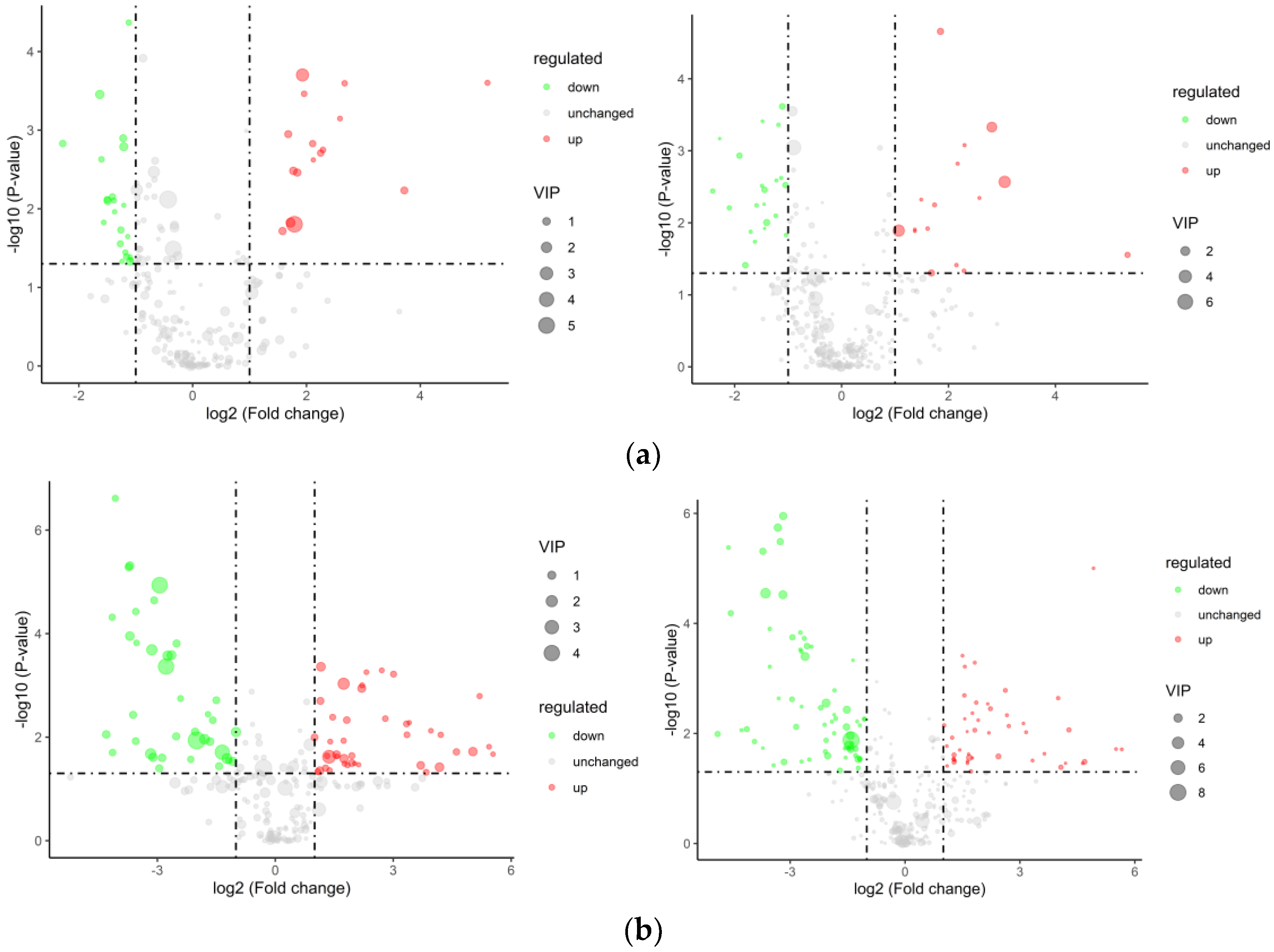
3.6. Environmental Stress Induced Changes in the Metabolome
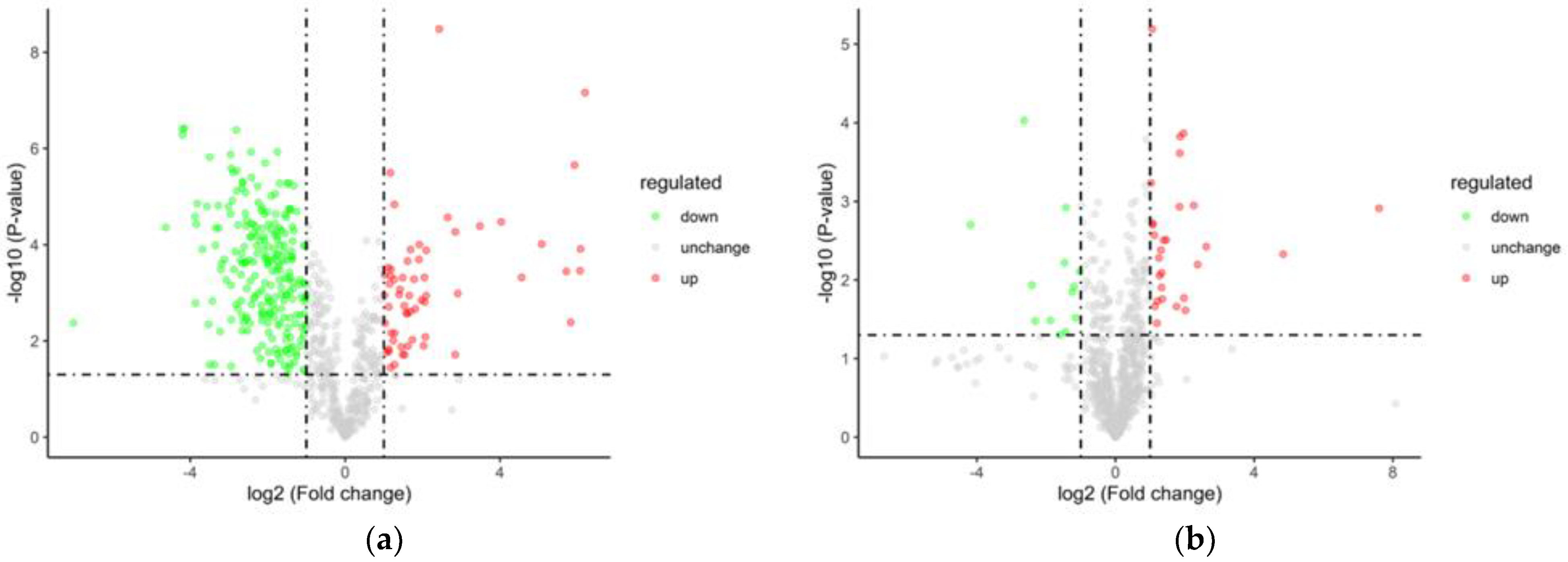
3.6.1. Differential Metabolite Screening
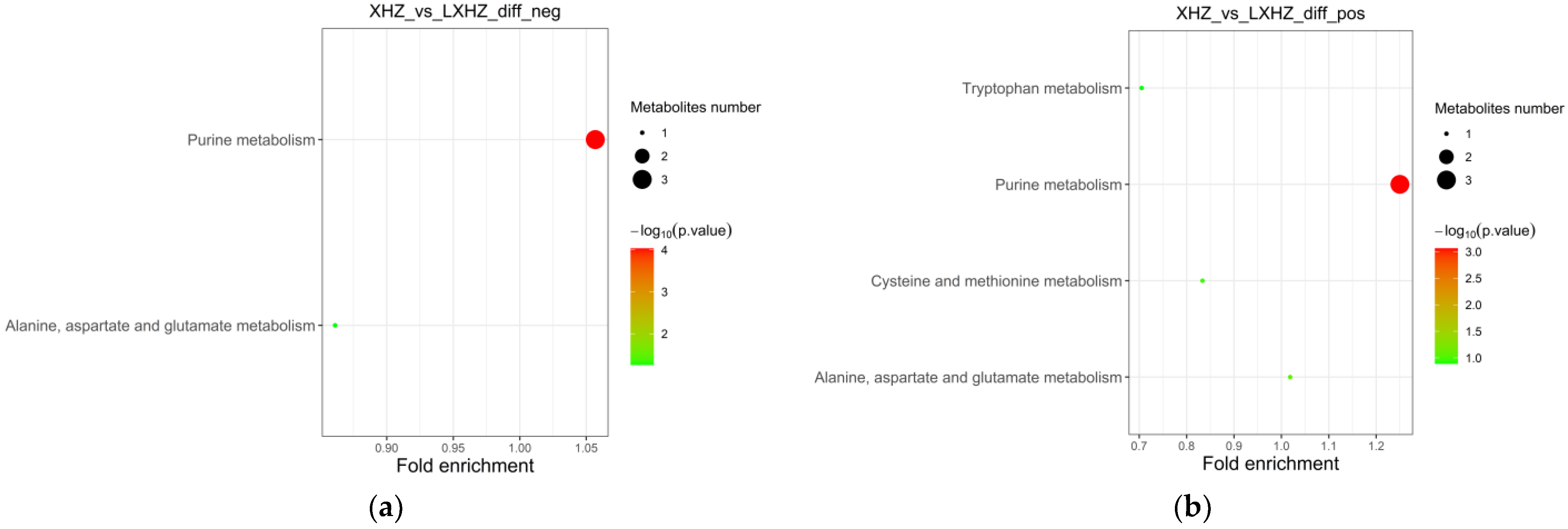
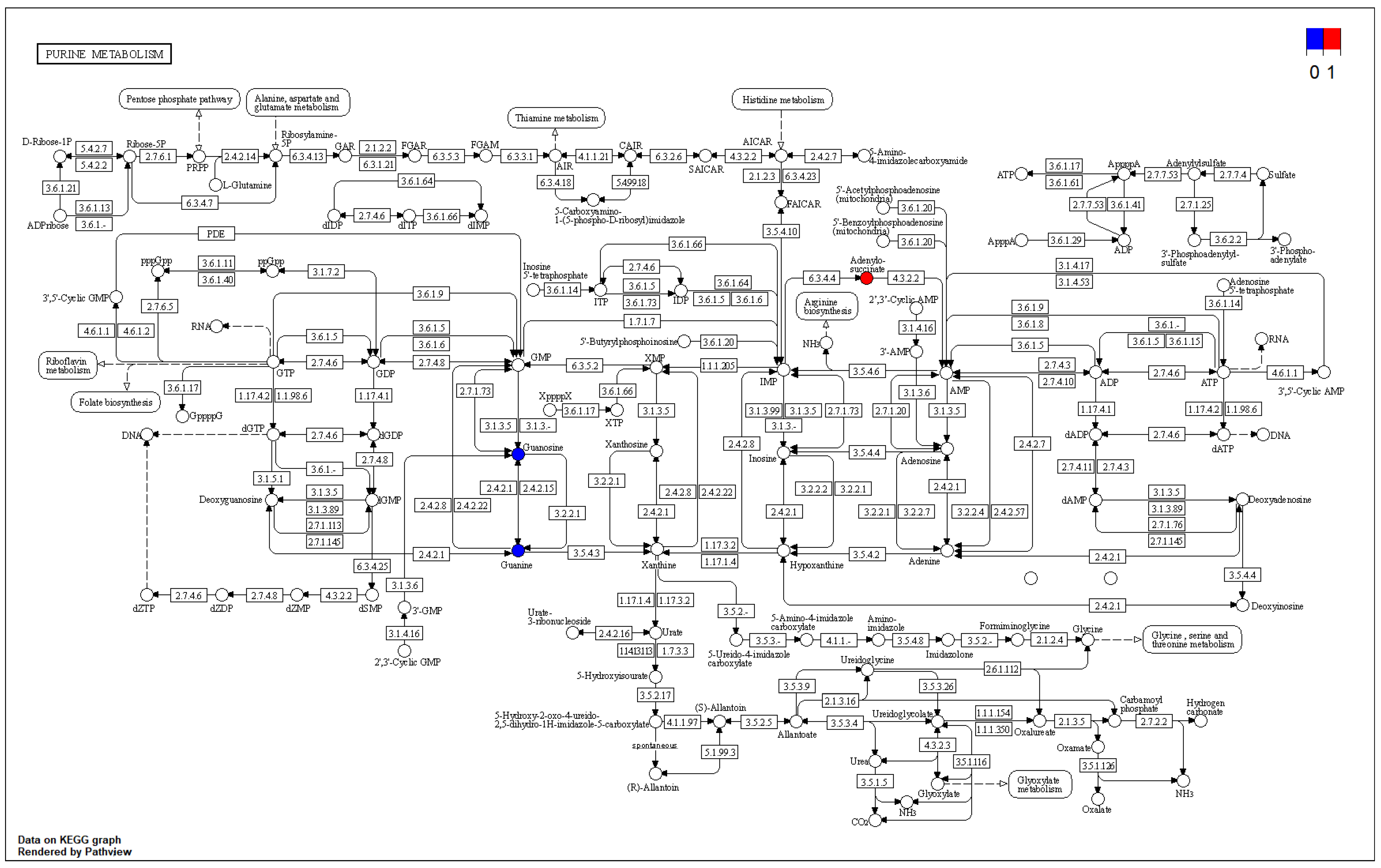
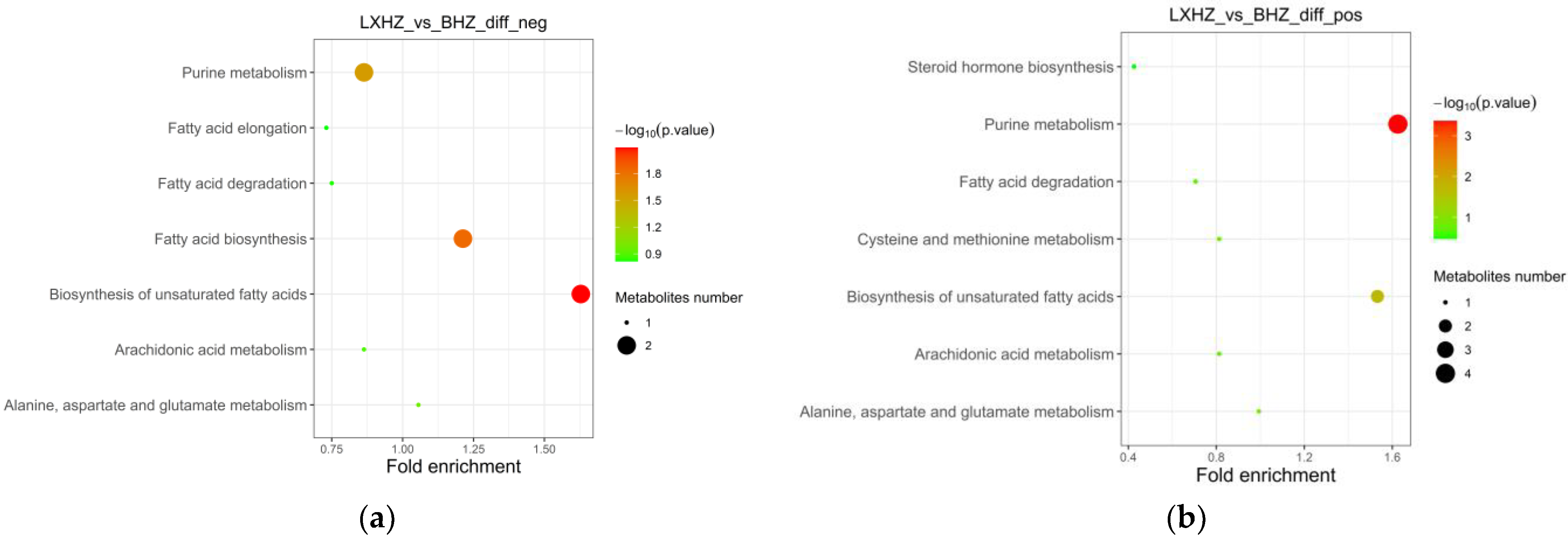
3.6.2. Functional Annotation and Enrichment Analysis of the Differential Metabolite KEGG
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Li, D.; Wang, X.; Wang, Q.; Li, H.; Teng, W.; Liu, X.; Zhou, Z. Reason of massive mortality of Japanese scallop Pationopectin yessoenisis in raft cultivation in coastal Changhai county. Fish. Sci. 2019, 38, 420–427. [Google Scholar]
- Wang, Q.C. Introduction of Pationopectin yessoenisis and the prospect of increasing culture in north China. Fish. Sci. 1984, 4, 24–27. [Google Scholar]
- Guan, B.B.; Chen, B.; Cheng, X.H. Research on characterization and evaluation methods of aquatic products. Fujian Anal. Test. 2019, 28, 15–21. [Google Scholar] [CrossRef]
- Pan, L.; Lin, C.; Zhang, G.; Mu, G.; Wang, Y. The influence of purification of temporary breeding and low temperature water transportation on the quality of scallop. J. Agric. Eng. 2017, 33, 301–307. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, Y.; Sun, X.; Huang, B.; Zhang, C. Key technologies for the transportation of live fish and their processes. Fish. Mod. 2014, 41, 34–39. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Q. Progress in Understanding Environmental Stress and Physiological Regulation Mechanism in Aquatic Animals during Live Transportation. Food Sci. 2021, 42, 319–325. [Google Scholar]
- Pan, L.; Jiang, J.; Zhang, N.; Huang, D.; Gao, X.; Liu, H.; Zhang, G. Effects of different temperatures on quality of live bay scallop argopectenirradians packed by oxygenation. Fish. Sci. 2019, 38, 182–187. [Google Scholar]
- Konstantinov, A.S.; Pushkar, V.Y.; Averyanova, O.V. Effects of fluctuations of abiotic factors on the metabolism of some hydrobionts. Biol. Bull. Russ. Acad. Sci. 2003, 30, 610–616. [Google Scholar] [CrossRef]
- Lin, H.; Gao, J.; Liang, Z.; Fan, X.; Lin, H.; Cao, W.; Huang, Y.; Qin, X. The effect of cold stress mode on current oxidative stress and energy consumption of Pacific oysters. J. Ocean Univ. Guangdong 2022, 42, 95–103. [Google Scholar]
- Yan, L.; Tian, Y.; Jiang, M.; Liu, R.; Xu, T. Changes of vitality and flavor characteristics of shrimp scallop in waterless transportation—Wet storage sales. Aquat. Sci. 2022, 41, 44–51. [Google Scholar] [CrossRef]
- Zhang, W.; Lv, Z.; Zhang, Y.; Chen, J.; Li, F.; Zheng, L.; Cong, X. Influence of hypoxia stress on physiological metabolism of Ruditapes philippinarum. Chin. J. Ecol. 2014, 33, 2448–2453. [Google Scholar]
- Yang, C.; Wang, X.; Zhou, K.; Jiang, D.; Shan, Y.; Wang, L.; Song, L. Effect of high temperature stress on glycogen metabolism in gills of Yesso scallop Patinopecten yessoensis. Fish Shellfish Immunol. 2023, 138, 108786. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhou, J.; Zhou, Q.; Tian, Y.; Xu, T. Effects of tied-up relaying on stress-reduction and storage-stability of live dived Patinopecten yessoensis. J. Fish. China 2022, 46, 605–615. [Google Scholar]
- Bakhmet, I.; Aristov, D.; Marchenko, J.; Nikolaev, K. Handling the heat: Changes in the heart rate of two congeneric blue mussel species and their hybrids in response to water temperature. J. Sea Res. 2022, 185, 102218. [Google Scholar] [CrossRef]
- Chang, X.Y.; Jiang, P.H.; Deng, J.; Fan, X.P.; Qin, X.M. The influence of chilling stress induced dormancy on the life characteristics and nutritional quality indexes of shrimp scallop. South China Fish. Sci. 2023, 19, 129–139. [Google Scholar]
- Duan, Y.; Zhang, J.; Dong, H.; Wang, Y.; Liu, Q.; Li, H. Effect of desiccation and resubmersion on the oxidative stress response of the kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2016, 49, 91–99. [Google Scholar] [CrossRef]
- Lee, A.-C.; Lee, K.-T. The enzyme activities of opine and lactate dehydrogenases in the gills, mantle, foot, and adductor of the hard clam Meretrix lusoria. J. Mar. Sci. Technol. 2011, 19, 4. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The Importance of ATP-related Compounds for the Freshness and Flavor of Post-mortem Fish and Shellfish Muscle: A Review. CRC Crit. Rev. Food Technol. 2015, 57, 1787–1798. [Google Scholar]
- Hiltz, D.F.; Dyer, W.J. Octopine in postmortem adductor muscle of the sea scallop (Placopecten magellanicus). J. Fish. Res. Board Can. 1971, 28, 869–874. [Google Scholar] [CrossRef]
- Atkinson, D.E. Energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef]
- Duncan, P.F. Post-Harvest Physiology of the Scallop Pecten maximus (L.). Ph.D. Thesis, University of Glasgow, Glasgow, UK, 1993. [Google Scholar]
- Vallé, M.; Malle, P.; Bouquelet, S. Evaluation of fish decomposition by liquid chromatographic assay of ATP degradation product. J. AOAC Int. 1998, 81, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Aguilar, R.; Marquez-Ríos, E.; Lugo-Sánchez, M.E.; García-Sanchez, G.; Maeda-Martínez, A.N.; Ocaño-Higuera, V.M. Postmortem changes in the adductor muscle of Pacific lions-paw scallop (Nodipecten subnodosus) during ice storage. Food Chem. 2008, 106, 253–259. [Google Scholar] [CrossRef]
- Sanders, B.M.; Hope, C.; Pascoe, V.M.; Martin, L.S. Characterization of the Stress Protein in Response Two with Different Species of Collisella Limpets Tolerances Temperature. Physiol. Zool. 2012, 64, 1471–1489. [Google Scholar] [CrossRef]
- Tomanek, L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 2010, 213, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Tomanek, L.; Somero, G.N. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): Implications for regulation of hsp gene expression. J. Exp. Biol. 2002, 205 Pt 5, 677–685. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Chen, H.; Wang, M.; Zhou, Z.; Qiu, L.; Wang, L.; Song, L. The transcriptional response of the Pacific oyster Crassostrea gigas under simultaneous bacterial and heat stresses. Dev. Comp. Immunol. Ontog. Phylogeny Aging 2019, 94, 1–10. [Google Scholar] [CrossRef]
- Yang, C.; Gao, Q.; Liu, C.; Wang, L.; Zhou, Z.; Gong, C.; Zhang, A.; Zhang, H.; Qiu, L.; Song, L. The transcriptional response of the Pacific oyster Crassostrea gigas against acute heat stress. Fish Shellfish Immunol. 2017, 68, 132–143. [Google Scholar] [CrossRef]
- Zhang, S.; Han, G.; Dong, Y. Temporal patterns of cardiac performance and genes encoding heat shock proteins and metabolic sensors of an intertidal limpet Cellana toreuma during sublethal heat stress. J. Therm. Biol. 2014, 41, 31–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, M.; Tian, Y.; Xu, M.; Leng, H.; Liu, J. A Relevance of Adductor Protein Solubility to ATP in Yesso Scallop Patinopecten yessoensis. Fish. Sci. 2020, 39, 476–482. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Tian, Y.; Zhang, L.; Liu, H.; Li, D. Effects of post-harvest handling on biochemical metabolism of bottom cultured live scallop (Patinopecten yessoensis). J. Fish. China 2017, 41, 81–87. [Google Scholar]
- Pampanin, D.M.; Ballarin, L.; Carotenuto, L.; Marin, M.G. Air exposure and functionality of Chamelea gallina hemocytes: Effects on haematocrit, adhesion, phagocytosis and enzyme contents. Comp. Biochem. Phys. 2002, 131A, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Tian, Y.; Li, Y.; Liu, Y. UPLC-MS based metabolomic study of live scallop Mizuhopecten yessoensis during quality determination period. J. Dalian Ocean Univ. 2021, 4, 637–645. [Google Scholar]
- Díaz Enrich, M.J.; Ramos Martínez, J.I.; Ibarguren Arizeta, I. Implication of guanosine 3′,5′-cyclic monophosphate, adenosine 3′,5′-cyclic monophosphate, adenosine 5′-mono-, di- and triphosphate and fructose-2,6-bisphosphate in the regulation of the glycolytic pathway in hypoxic/anoxic mussel, Mytilus galloprovincialis. Mol. Cell. Biochem. 2002, 240, 111–118. [Google Scholar] [PubMed]

| Database | Peptides | Unique Peptides | Protein Groups |
|---|---|---|---|
| Patinopecten yessoensis (Mizuhopecten yessoensis_6573) | 4548 | 4134 | 856 |
| Comparisons | Up | Down | All |
|---|---|---|---|
| XHZ_vs_LXHZ | 60 | 237 | 297 |
| LXHZ_vs_BHZ | 28 | 13 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Chen, D.; Chang, X.; Zhang, C.; Fan, X.; Qin, X. Study on the Regulation Mechanism of Quality Deterioration Due to Chilling Stress and Dry Exposure during Anhydrous Storage and Transportation of Yesso Scallop Patinopecten yessoensis. Foods 2023, 12, 2902. https://doi.org/10.3390/foods12152902
Jiang P, Chen D, Chang X, Zhang C, Fan X, Qin X. Study on the Regulation Mechanism of Quality Deterioration Due to Chilling Stress and Dry Exposure during Anhydrous Storage and Transportation of Yesso Scallop Patinopecten yessoensis. Foods. 2023; 12(15):2902. https://doi.org/10.3390/foods12152902
Chicago/Turabian StyleJiang, Peihong, Dongjie Chen, Xiangyang Chang, Changfeng Zhang, Xiuping Fan, and Xiaoming Qin. 2023. "Study on the Regulation Mechanism of Quality Deterioration Due to Chilling Stress and Dry Exposure during Anhydrous Storage and Transportation of Yesso Scallop Patinopecten yessoensis" Foods 12, no. 15: 2902. https://doi.org/10.3390/foods12152902





