Advances in Starch Nanoparticle for Emulsion Stabilization
Abstract
:1. Introduction
2. Multiscale Structure of Starch and Starch Nanoparticle
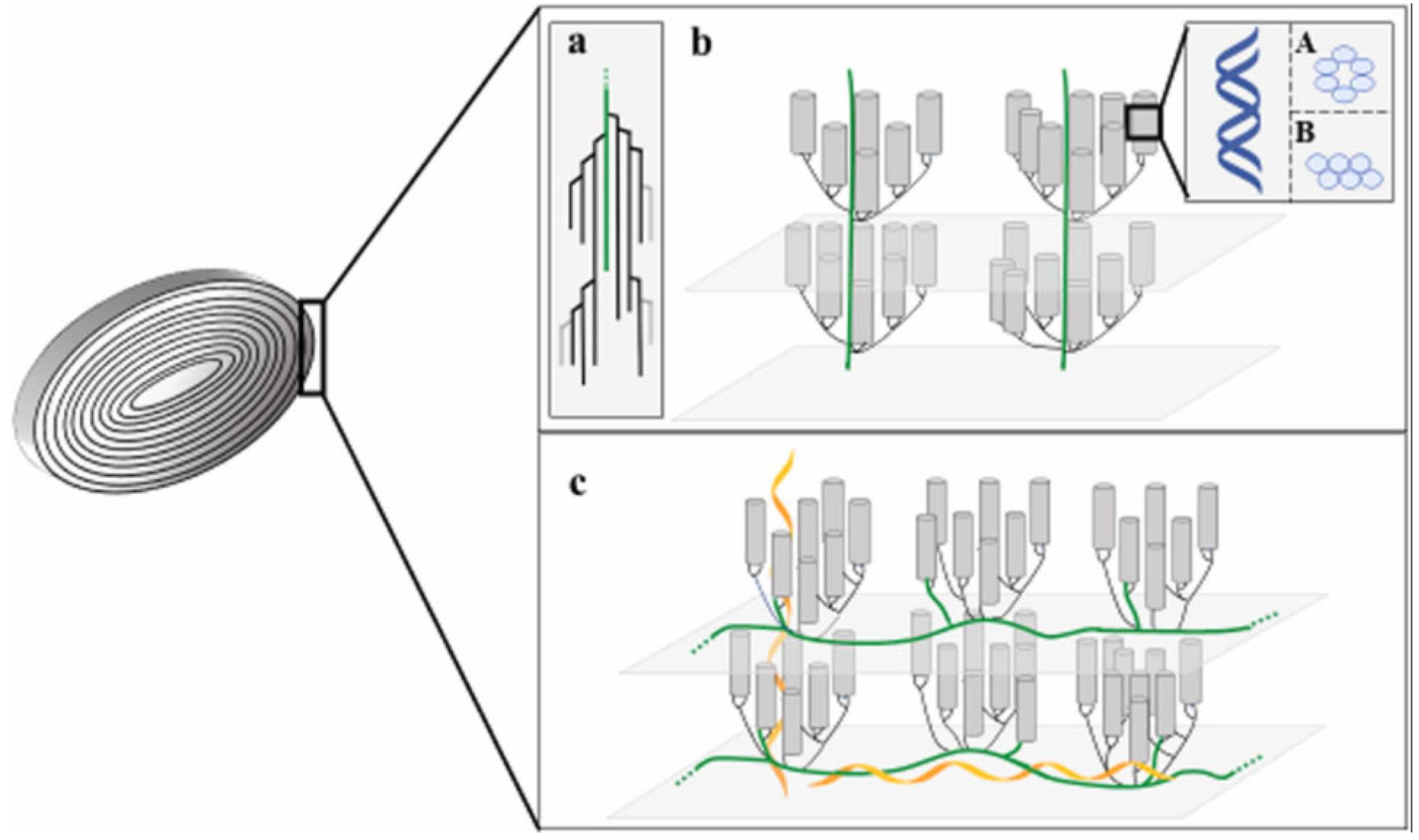
2.1. Molecular Chain, Crystal, and Granule Structure of Starch
2.2. Starch Nanoparticle
2.2.1. Preparation Methods of SNP
2.2.2. Properties of Nano-Starch
3. Application of Starch Nanoparticle in Emulsion
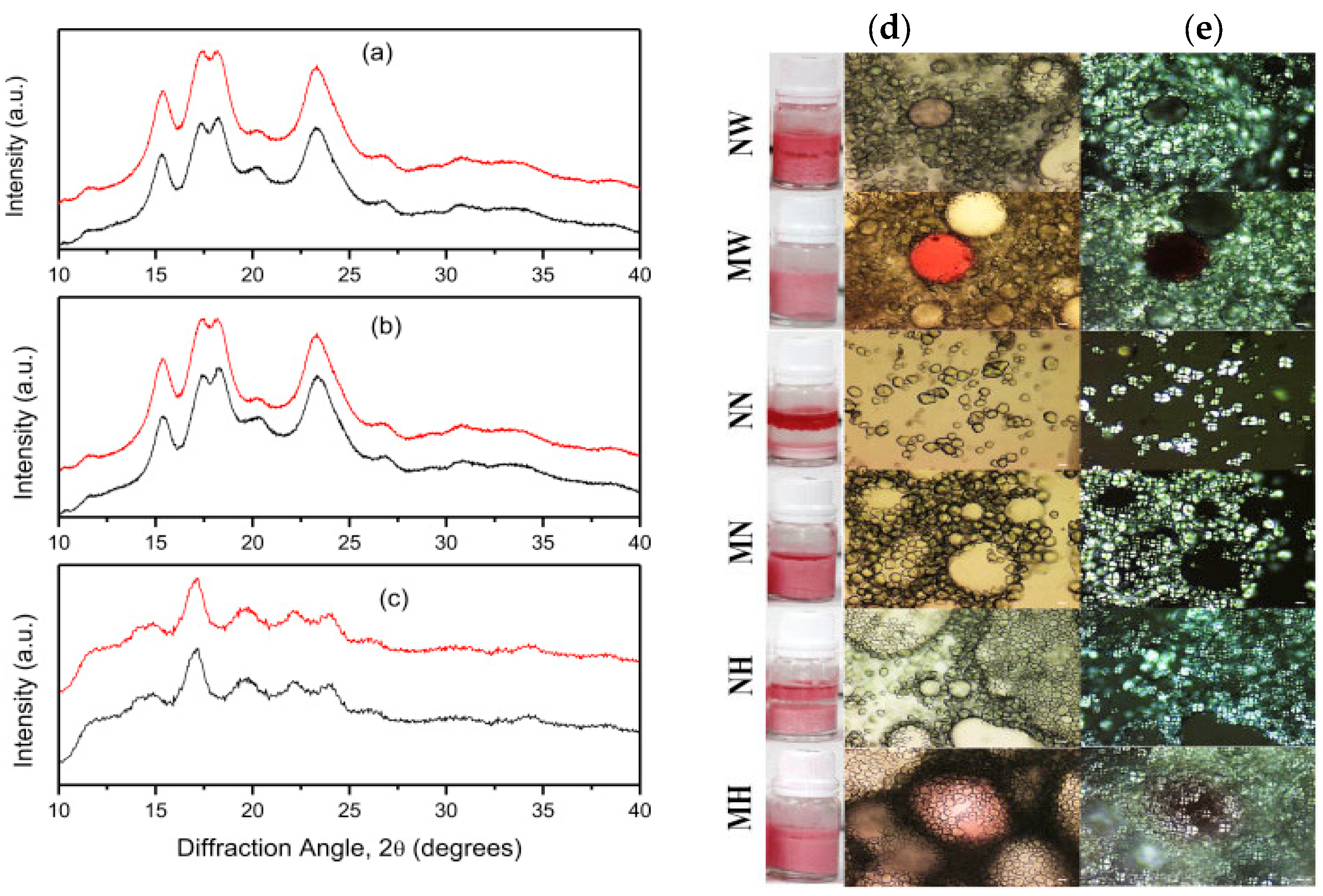
3.1. Pickering Emulsifier Based on Starch Modification
3.2. Pickering Emulsifier Based on Starch Complex
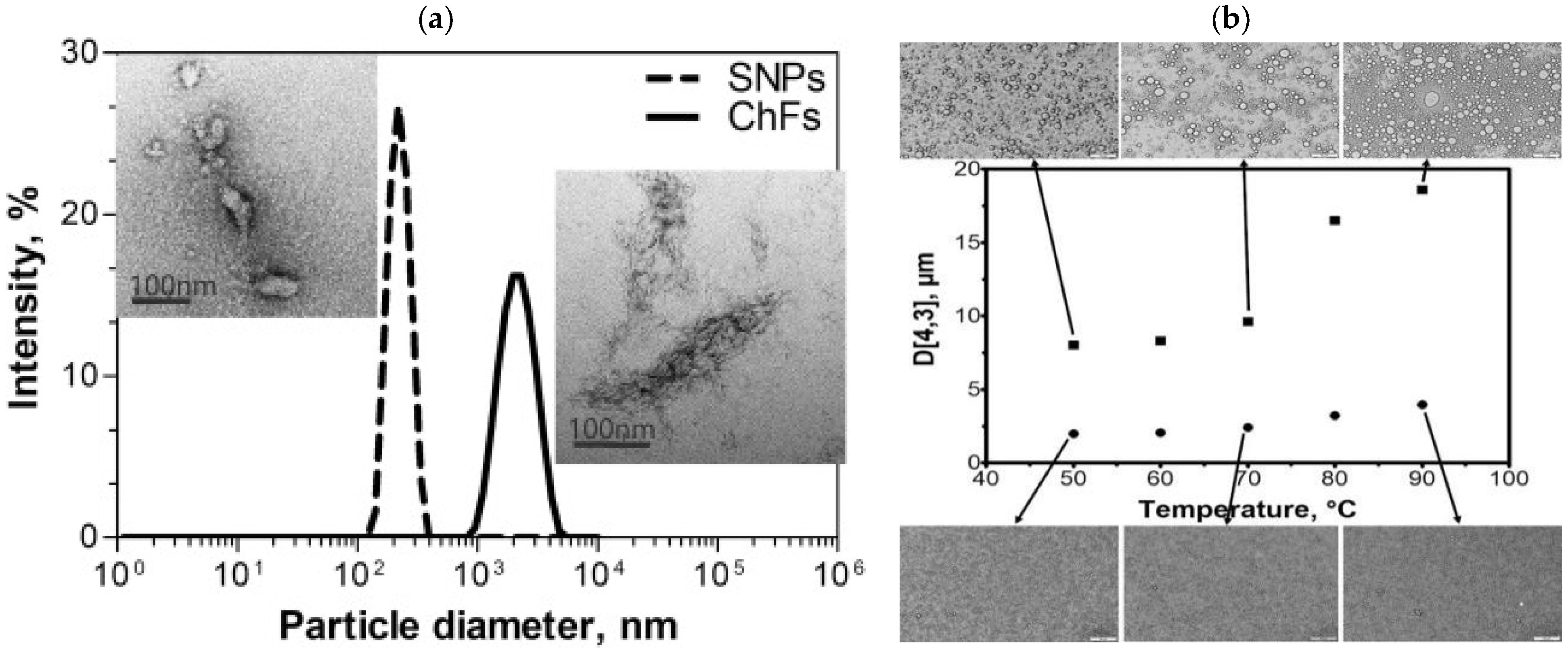
3.3. SNP-Based Pickering Emulsifier
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Canxin, C. Preparation, Properties and Application of Resistant Starch by Extrusion; Jiangnan University: Wuxi, China, 2021; Volume 132. [Google Scholar]
- Ai, Y.; Jane, J.-L. Chapter 3—Understanding Starch Structure and Functionality. In Starch in Food; University, J., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 151–178. [Google Scholar]
- Campelo, P.H.; Sant’Ana, A.S.; Pedrosa Silva Clerici, M.T. Starch nanoparticles: Production methods, structure, and properties for food applications. Curr. Opin. Food Sci. 2020, 33, 136–140. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch nanoparticles: A review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Kumari, S.; Yadav, B.S.; Yadav, R.B. Synthesis and modification approaches for starch nanoparticles for their emerging food industrial applications: A review. Food Res. Int. 2020, 128, 108765. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Synthesis, characteristics, and applications of modified starch nanoparticles: A review. Int. J. Biol. Bacromol. 2022, 194, 289–305. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Pal, R. Rheology of emulsions thickened by starch nanoparticles. Nanomaterials 2022, 12, 2391. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Derry, M.J.; Hatton, F.L.; Armes, S.P. Long-term stability of n-alkane-in-water pickering nanoemulsions: Effect of aqueous solubility of droplet phase on ostwald ripening. Langmuir 2018, 34, 9289–9297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sileola, B.O.; Rajinder, P.; Kaveh, S. Effects of starch nanoparticles on phase inversion of Pickering emulsions. Can. J. Chem. Eng. 2018, 96, 1089–1097. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Guida, C.; Aguiar, A.C.; Cunha, R.L. Green techniques for starch modification to stabilize Pickering emulsions: A current review and future perspectives. Curr. Opin. Food Sci. 2021, 38, 52–61. [Google Scholar] [CrossRef]
- Fatemeh, K.; Nasser, N. Highly interconnected macroporous structures made from starch nanoparticle-stabilized medium internal phase emulsion polymerization for use in cell culture. Polymer 2019, 180, 121744. [Google Scholar] [CrossRef]
- Amol, C.; Yuanjie, P.; Nitin, N. Beverage emulsions: Comparison among nanoparticle stabilized emulsion with starch and surfactant stabilized emulsions. Food Res. Int. 2015, 69, 156–163. [Google Scholar] [CrossRef]
- Hao, L.; Yaoqi, T. Nanostarch: Preparation, modification, and application in pickering emulsions. J. Agric. Food Chem. 2021, 69, 6929–6942. [Google Scholar] [CrossRef]
- Yun, W.; Genyi, Z. The preparation of modified nano-starch and its application in food industry. Food Res. Int. 2021, 140, 110009. [Google Scholar] [CrossRef]
- Hassan, N.A.; Darwesh, O.M.; Smuda, S.S.; Altemimi, A.B.; Hu, A.; Cacciola, F.; Haoujar, I.; Abedelmaksoud, T.G. Recent trends in the preparation of nano-starch particles. Molecules 2022, 27, 5497. [Google Scholar] [CrossRef]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Technol. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- French, D. Fine structure of starch and its relationship to the organization of starch granules. J. Jpn. Soc. Starch Sci. 1972, 19, 8–25. [Google Scholar] [CrossRef] [Green Version]
- Robin, J.P.; Mercier, C.; Charbonniere, R.; Guilbot, A. Lintnerized starches. Gel filtration and enzymatic studies of insoluble residues from prolonged acid treatment of potato starch. Cereal Chem. 1974, 51, 389–406. [Google Scholar]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch Stärke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef]
- Bertoft, E. Fine structure of amylopectin. In Starch; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–40. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, S.S.; Lim, S.-T. Preparation, characterization and utilization of starch nanoparticles. Colloids Surf. B 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Kong, L.Y.; Lee, C.; Kim, S.H.; Ziegler, G.R. Characterization of starch polymorphic structures using vibrational sum frequency generation spectroscopy. J. Phys. Chem. B 2014, 118, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; Bello-Pérez, L.A. Self-assembled and assembled starch V-type complexes for the development of functional foodstuffs: A review. Food Hydrocoll. 2022, 125, 107453. [Google Scholar] [CrossRef]
- Tran, T.T.B.; Shelat, K.J.; Tang, D.; Li, E.P.; Gilbert, R.G.; Hasjim, J. Milling of rice grains. The degradation on three structural levels of starch in rice flour can be independently controlled during grinding. J. Agric. Food Chem. 2011, 59, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Baldwin, P.M. Microscopy of starch: Evidence of a new level of granule organization. Carbohyd. Polym. 1997, 32, 177–191. [Google Scholar] [CrossRef]
- Qiu, C.; Hu, Y.; Jin, Z.Y.; McClements, D.J.; Qin, Y.; Xu, X.M.; Wang, J.P. A review of green techniques for the synthesis of size-controlled starch-based nanoparticles and their applications as nanodelivery systems. Trends Food Sci. Technol. 2019, 92, 138–151. [Google Scholar] [CrossRef]
- Xu, E.; Campanella, O.H.; Ye, X.; Jin, Z.; Liu, D.; BeMiller, J.N. Advances in conversion of natural biopolymers: A reactive extrusion (REX)–enzyme-combined strategy for starch/protein-based food processing. Trends Food Sci. Technol. 2020, 99, 167–180. [Google Scholar] [CrossRef]
- Song, D.; Thio, Y.S.; Deng, Y. Starch nanoparticle formation via reactive extrusion and related mechanism study. Carbohyd. Polym. 2011, 85, 208–214. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Masoodi, F.A.; Rizvi, S.H. Influence of ball milling on the production of starch nanoparticles and its effect on structural, thermal and functional properties. Int. J. Biol. Macromol. 2020, 151, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Qin, L.Z.; Hong, H.; Li, Q. Preparation of starch nanoparticles via high-energy ball milling. J. Nano Res. 2016, 40, 174–179. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, C.; Han, Z.; Xiong, L.; Sun, Q. Evaluation of rheological behavior of starch nanocrystals by acid hydrolysis and starch nanoparticles by self-assembly: A comparative study. Food Hydrocoll. 2016, 52, 914–922. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Arsad, A.; Abbas, A.; Gbadamosi, A.; Azli, N.B.; Oseh, J. Ultrasound-assisted weak-acid hydrolysis of crystalline starch nanoparticles for chemical enhanced oil recovery. Int. J. Biol. Macromol. 2020, 148, 1251–1271. [Google Scholar] [CrossRef]
- Liu, C.Z.; Li, M.; Ji, N.; Liu, J.; Xiong, L.; Sun, Q.J. Morphology and characteristics of starch nanoparticles self-assembled via a rapid ultrasonication method for peppermint oil encapsulation. J. Agric. Food Chem. 2017, 65, 8363–8373. [Google Scholar] [CrossRef]
- Wei, B.; Cai, C.; Tian, Y. Nano-sized starch: Preparations and applications. In Functional Starch and Applications in Food; Springer: Singapore, 2018; pp. 147–176. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Li, L.L.; Liu, C.; Song, C.L.; Wang, P.X. Fabrication of size-controlled starch-based nanospheres by nanoprecipitation. ACS Appl. Mater. Interfaces 2009, 1, 956–959. [Google Scholar] [CrossRef]
- Liu, G.; Li, T.; Yang, X.; She, Y.; Wang, M.; Wang, J.; Zhang, M.; Wang, S.; Jin, F.; Jin, M. Competitive fluorescence assay for specific recognition of atrazine by magnetic molecularly imprinted polymer based on Fe3O4-chitosan. Carbohyd. Polym. 2016, 137, 75–81. [Google Scholar] [CrossRef]
- Rutkaite, R.; Bendoraitiene, J.; Klimaviciute, R.; Zemaitaitis, A. Cationic starch nanoparticles based on polyelectrolyte complexes. Int. J. Biol. Macromol. 2012, 50, 687–693. [Google Scholar] [CrossRef]
- Wang, W.; Jin, X.; Zhu, Y.; Zhu, C.; Yang, J.; Wang, H.; Lin, T. Effect of vapor-phase glutaraldehyde crosslinking on electrospun starch fibers. Carbohyd. Polym. 2016, 140, 356–361. [Google Scholar] [CrossRef]
- Ghaeb, M.; Tavanai, H.; Kadivar, M. Electrosprayed maize starch and its constituents (amylose and amylopectin) nanoparticles. Polym. Advan. Technol. 2015, 26, 917–923. [Google Scholar] [CrossRef]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharmaceut. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Saad, W.S.; Prud’homme, R.K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today 2016, 11, 212–227. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.Z.; Zou, D.; Hui, Y.; Nigam, K.; Middelberg, A.P.J.; Zhao, C.X. Formulation of nanoparticles using mixing-induced nanoprecipitation for drug delivery. Ind. Eng. Chem. Res. 2020, 59, 4134–4149. [Google Scholar] [CrossRef]
- Iván Martínez-Muñoz, O.; Elizabeth Mora-Huertas, C. Nanoprecipitation technology to prepare carrier systems of interest in pharmaceutics: An overview of patenting. Int. J. Pharmaceut. 2022, 614, 121440. [Google Scholar] [CrossRef] [PubMed]
- Abushrida, A.; Elhuni, I.; Taresco, V.; Marciani, L.; Stolnik, S.; Garnett, M.C. A simple and efficient method for polymer coating of iron oxide nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 55, 101460. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification–diffusion methods: Critical comparison. Adv. Colloid Interface Sci. 2011, 163, 90–122. [Google Scholar] [CrossRef] [PubMed]
- Markwalter, C.E.; Pagels, R.F.; Hejazi, A.N.; Gordon, A.G.R.; Thompson, A.L.; Prud’homme, R.K. Polymeric nanocarrier formulations of biologics using inverse flash nanoprecipitation. AAPS J. 2020, 22, 18. [Google Scholar] [CrossRef]
- Chin, S.F.; Azman, A.; Pang, S.C. Size controlled synthesis of starch nanoparticles by a microemulsion method. J. Nanomater. 2014, 2014, 763736. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Yang, J.; Jiang, L.; Ren, L.; Zhou, J. Chain length distribution of β-amylase treated potato starch and its effect on properties of starch nanoparticles obtained by nanoprecipitation. Starch Stärke 2019, 71, 1800321. [Google Scholar] [CrossRef]
- Yan, X.; Diao, M.; Yu, Y.; Gao, F.; Wang, E.; Wang, Z.; Zhang, T.; Zhao, P. Characterization of resistant starch nanoparticles prepared via debranching and nanoprecipitation. Food Chem. 2022, 369, 130824. [Google Scholar] [CrossRef] [PubMed]
- Caldonazo, A.; Almeida, S.L.; Bonetti, A.F.; Lazo, R.E.L.; Mengarda, M.; Murakami, F.S. Pharmaceutical applications of starch nanoparticles: A scoping review. Int. J. Biol. Macromol. 2021, 181, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Ramalingam, T.; Nooruddin, T.; Shanmuganathan, R.; Arivalagan, P.; Natarajan, S. Polyherbal drug loaded starch nanoparticles as promising drug delivery system: Antimicrobial, antibiofilm and neuroprotective studies. Process Biochem. 2020, 92, 355–364. [Google Scholar] [CrossRef]
- Fu, Y.J.; Yang, J.D.; Jiang, L.W.; Ren, L.L.; Zhou, J. Encapsulation of lutein into starch nanoparticles to improve its dispersity in water and enhance stability of chemical oxidation. Starch Stärke 2019, 71, 100248. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Alp, E.; Damkaci, F.; Guven, E.; Tenniswood, M. Starch nanoparticles for delivery of the histone deacetylase inhibitor CG-1521 in breast cancer treatment. Int. J. Nanomed. 2019, 14, 1335–1346. [Google Scholar] [CrossRef] [Green Version]
- Yaseen, T.; Pu, H.; Sun, D.W. Functionalization techniques for improving SERS substrates and their applications in food safety evaluation: A review of recent research trends. Trends Food Sci. Technol. 2018, 72, 162–174. [Google Scholar] [CrossRef]
- Chavan, P.; Sinhmar, A.; Sharma, S.; Dufresne, A.; Thory, R.; Kaur, M.; Sandhu, K.S.; Nehra, M.; Nain, V. Nanocomposite starch films: A new approach for biodegradable packaging materials. Starch Stärke 2022, 74, 5–6. [Google Scholar] [CrossRef]
- Dularia, C.; Sinhmar, A.; Thory, R.; Pathera, A.K.; Nain, V. Development of starch nanoparticles based composite films from non-conventional source—Water chestnut (Trapa bispinosa). Int. J. Biol. Macromol. 2019, 136, 1161–1168. [Google Scholar] [CrossRef]
- Roy, K.; Thory, R.; Sinhmar, A.; Pathera, A.K.; Nain, V. Development and characterization of nano starch-based composite films from mung bean (Vigna radiata). Int. J. Biol. Macromol. 2020, 144, 242–251. [Google Scholar] [CrossRef]
- Sharma, I.; Sinhmar, A.; Thory, R.; Sandhu, K.S.; Kaur, M.; Nain, V.; Pathera, A.K.; Chavan, P. Synthesis and characterization of nano starch-based composite films from kidney bean (Phaseolus vulgaris). J. Food Sci. Technol. 2021, 58, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Silva, M.; Bello-Perez, L.A.; Agama-Acevedo, E.; Alvarez-Ramirez, J. Effect of amylose content in morphological, functional and emulsification properties of OSA modified corn starch. Food Hydrocoll. 2019, 97, 105212. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Huang, Q. Effects of hydrothermal pretreatment on subsequent octenylsuccinic anhydride (OSA) modification of cornstarch. Carbohydr. Polym. 2014, 101, 493–498. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Bello-Perez, L.A. Starch as an emulsions stability: The case of octenyl succinic anhydride (OSA) starch. Curr. Opin. Food Sci. 2017, 13, 78–83. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Tarté, R.; Acevedo, N.C. Synergistic effects of starch nanoparticles and chitin nanofibers on the stability of oil-in-water Pickering emulsions. Food Chem. 2021, 363, 130301. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Y.; Du, Y.; Chen, Y.; Wu, Y.; Zhong, K.; Huang, Y.; Gao, H. Food-grade olive oil Pickering emulsions stabilized by starch/β-cyclodextrin complex nanoparticles: Improved storage stability and regulatory effects on gut microbiota. LWT 2022, 155, 112950. [Google Scholar] [CrossRef]
- Guo, B.; Hu, X.; Wu, J.; Chen, R.; Dai, T.; Liu, Y.; Luo, S.; Liu, C. Soluble starch/whey protein isolate complex-stabilized high internal phase emulsion: Interaction and stability. Food Hydrocoll. 2021, 111, 106377. [Google Scholar] [CrossRef]
- Shrestha, S.; Sadiq, M.B.; Anal, A.K. Culled banana resistant starch-soy protein isolate conjugate based emulsion enriched with astaxanthin to enhance its stability. Int. J. Biol. Macromol. 2018, 120, 449–459. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Evans, M.; Ratcliffe, I.; Williams, P.A. Emulsion stabilisation using polysaccharide–protein complexes. Curr. Opin. Colloid Interface Sci. 2013, 18, 272–282. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Lu, X.; Xiao, J.; Huang, Q. Pickering emulsions stabilized by media-milled starch particles. Food Res. Int. 2018, 105, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-D.; Hong, J.S.; Pyo, S.M.; Ko, E.; Shin, H.-Y.; Kim, J.-Y. Starch nanoparticles produced via acidic dry heat treatment as a stabilizer for a Pickering emulsion: Influence of the physical properties of particles. Carbohydr. Polym. 2020, 239, 116241. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Zhang, H.; Niu, B.; Jin, W. Physical stabilities of taro starch nanoparticles stabilized Pickering emulsions and the potential application of encapsulated tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Chen, M.S.; Xu, F.F.; Zhong, F. Stabilizing oil-in-water emulsion with amorphous and granular octenyl succinic anhydride modified starches. J. Agric. Food Chem. 2018, 66, 9301–9308. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Guo, M.; Qin, Y.; Wang, W.; Lv, R.; Xu, E.; Ding, T.; Liu, D.; Wu, Z. Advances in Starch Nanoparticle for Emulsion Stabilization. Foods 2023, 12, 2425. https://doi.org/10.3390/foods12122425
Zhou J, Guo M, Qin Y, Wang W, Lv R, Xu E, Ding T, Liu D, Wu Z. Advances in Starch Nanoparticle for Emulsion Stabilization. Foods. 2023; 12(12):2425. https://doi.org/10.3390/foods12122425
Chicago/Turabian StyleZhou, Jianwei, Meimei Guo, Yu Qin, Wenjun Wang, Ruiling Lv, Enbo Xu, Tian Ding, Donghong Liu, and Zhengzong Wu. 2023. "Advances in Starch Nanoparticle for Emulsion Stabilization" Foods 12, no. 12: 2425. https://doi.org/10.3390/foods12122425




