Virtual Cold Chain Method to Evaluate the Effect of Rising Temperature on the Quality Evolution of Peach Fruit
Abstract
:1. Introduction
2. Materials and Methods
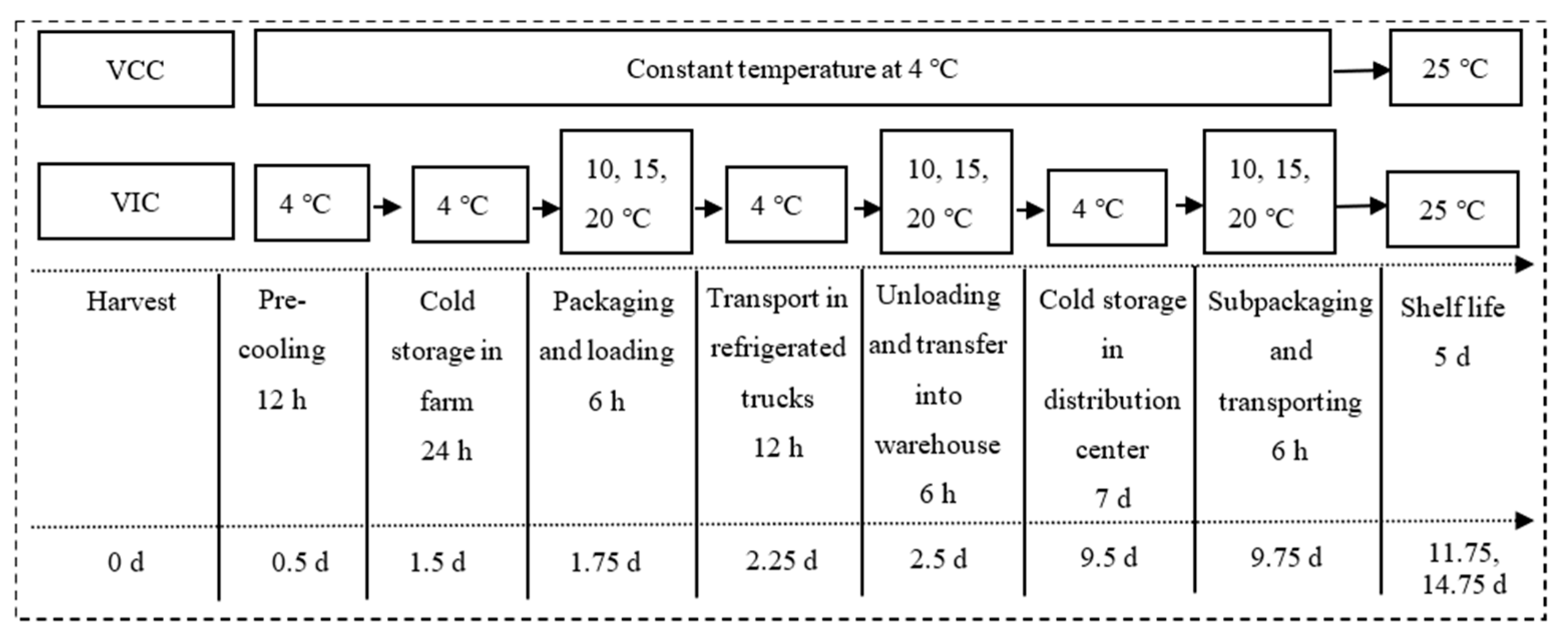
2.1. Description of Virtual Supply Chains
2.2. Material and Sample Preparation
2.3. Fruit Core Temperature
2.4. Respiratory Rate and Ethylene Production Rate
2.5. Firmness, SSC and TA Content
2.6. Malondialdehyde (MDA) Content
2.7. Total Phenol and Total Flavonoid Content
2.8. SOD, POD, and CAT Activity
2.9. Statistical Analysis
3. Results and Discussion
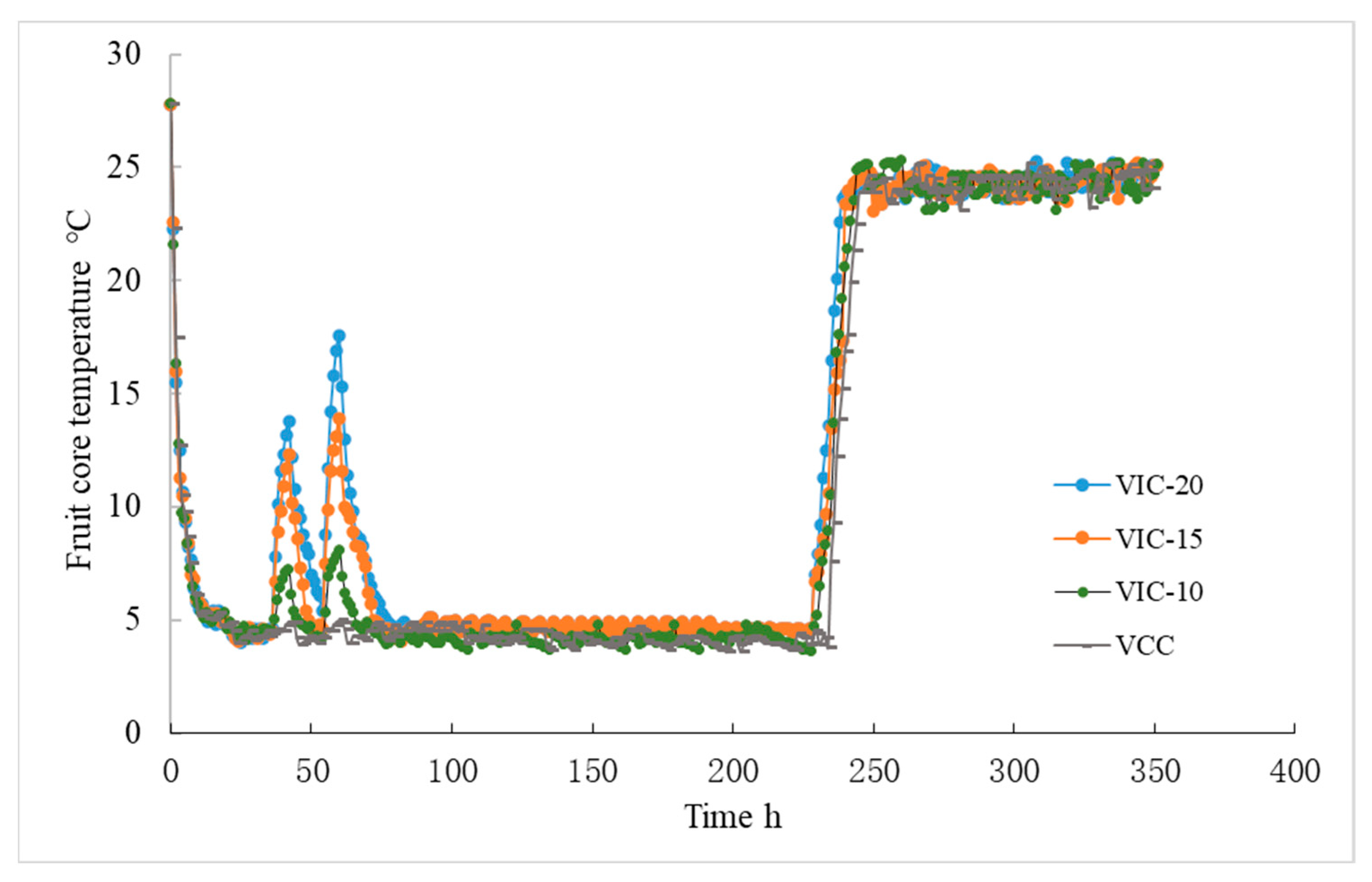
3.1. Fruit Core Temperature Changes in Different Virtual Cold Chains
3.2. Respiratory Rate and Ethylene Production Rate of Peach Fruit in Different Virtual Cold Chains
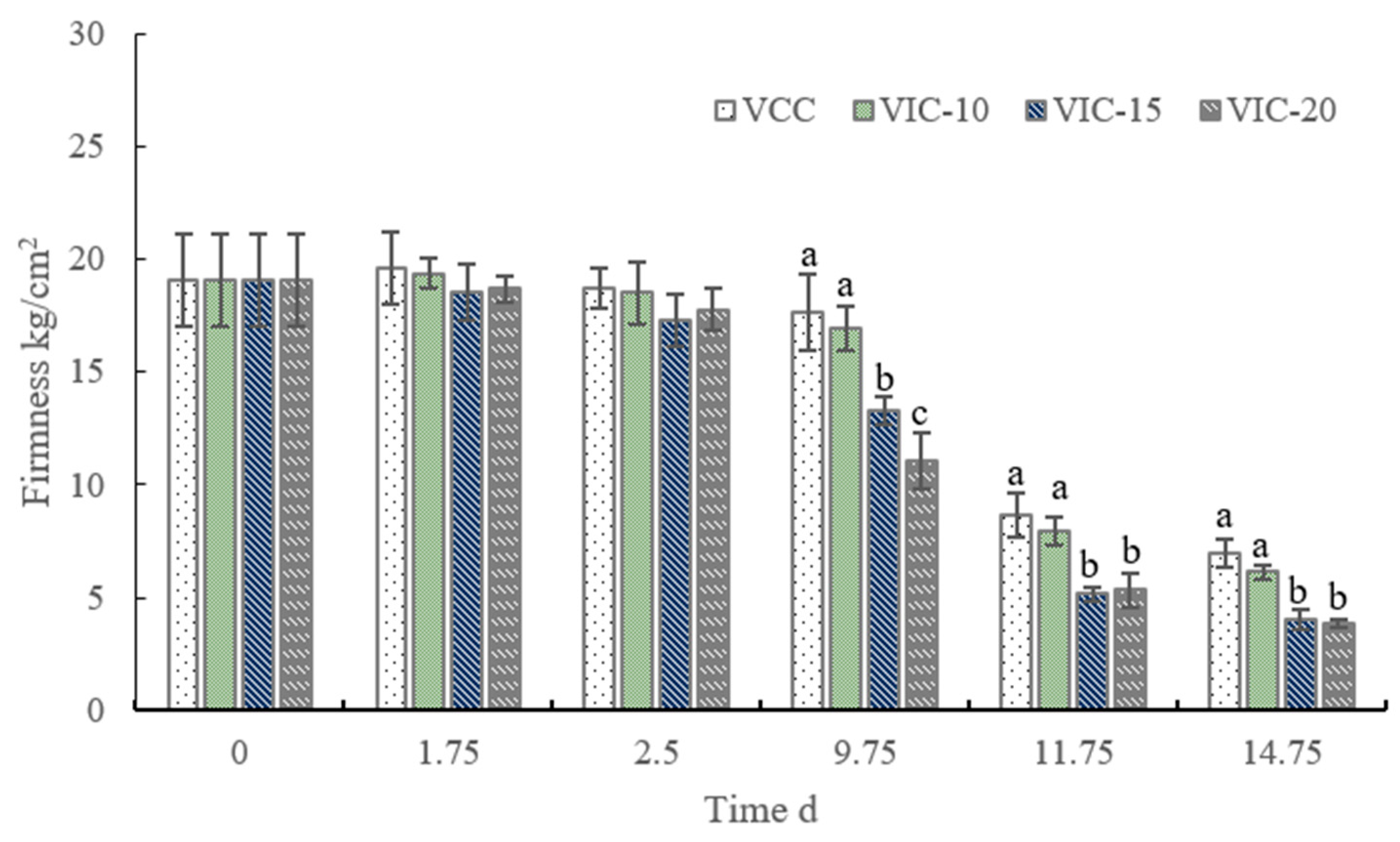
3.3. Firmness of Peach Fruit in Different Virtual Cold Chains
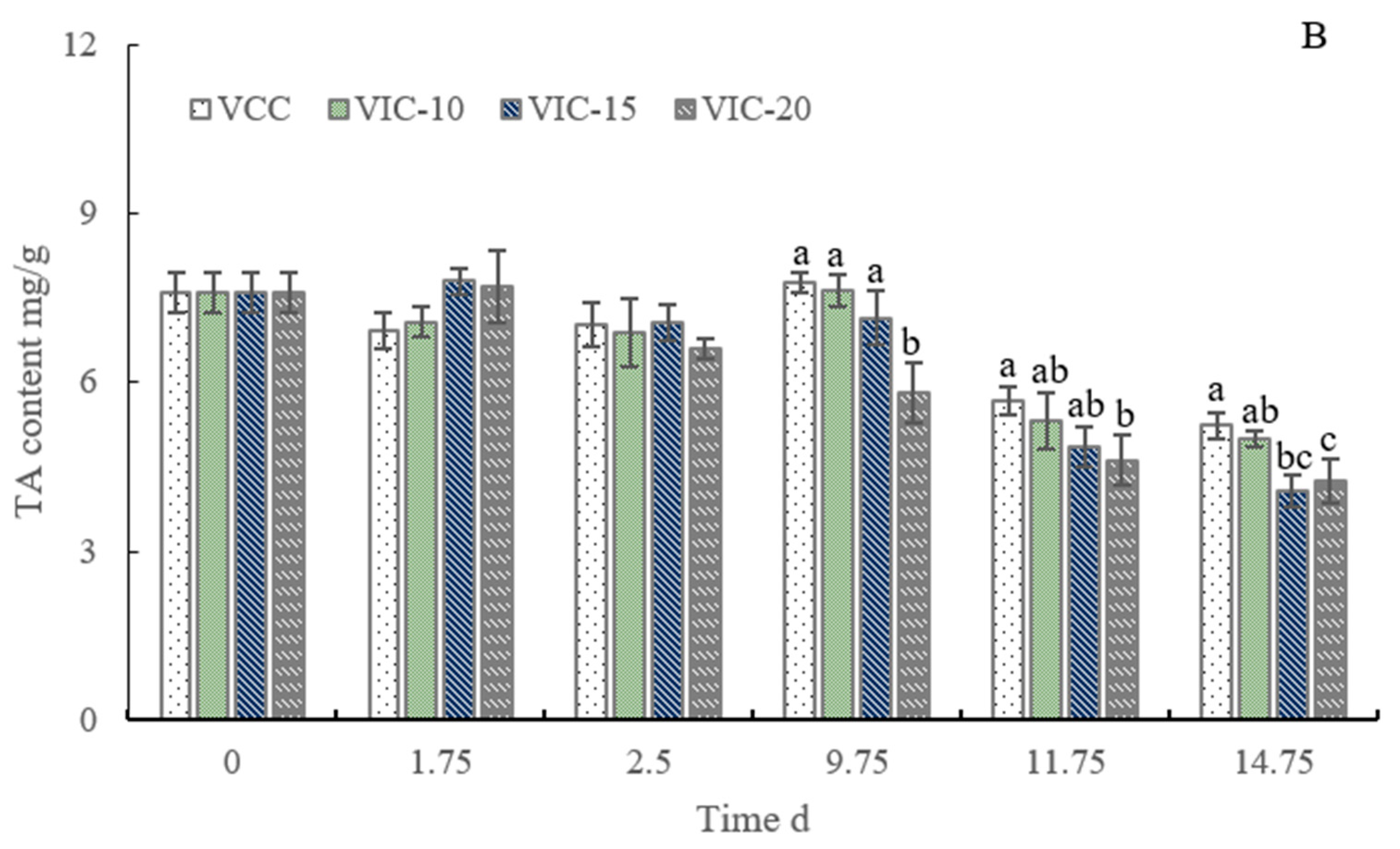
3.4. SSC and TA Content of Peach Fruit in Different Virtual Cold Chains
3.5. MDA Content of Peach Fruit in Different Virtual Cold Chains
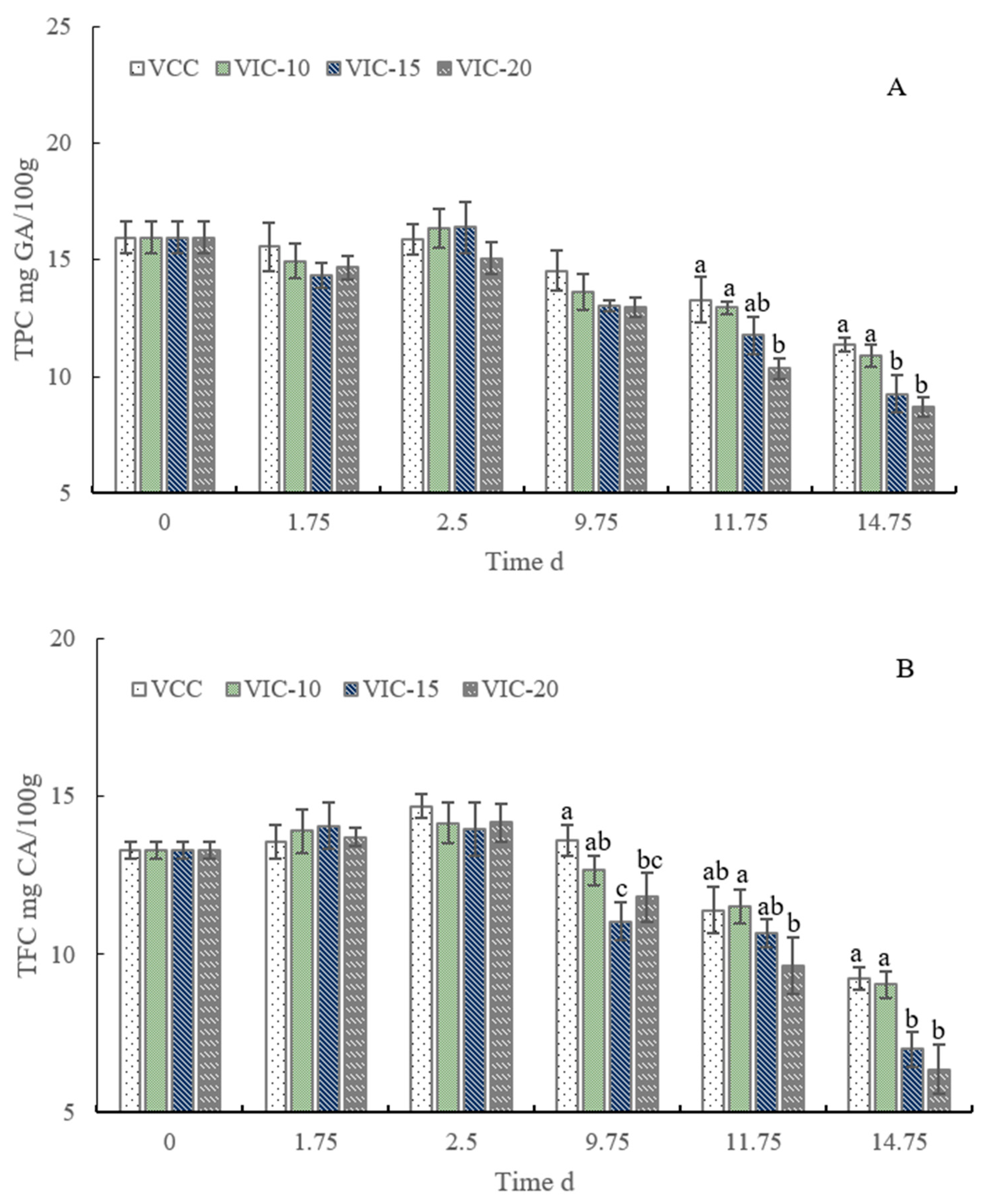
3.6. Total Phenol and Flavonoid Content of Peach Fruit in Different Virtual Cold Chains
3.7. SOD, POD, and CAT Activities of Peach Fruit in Different Virtual Cold Chains
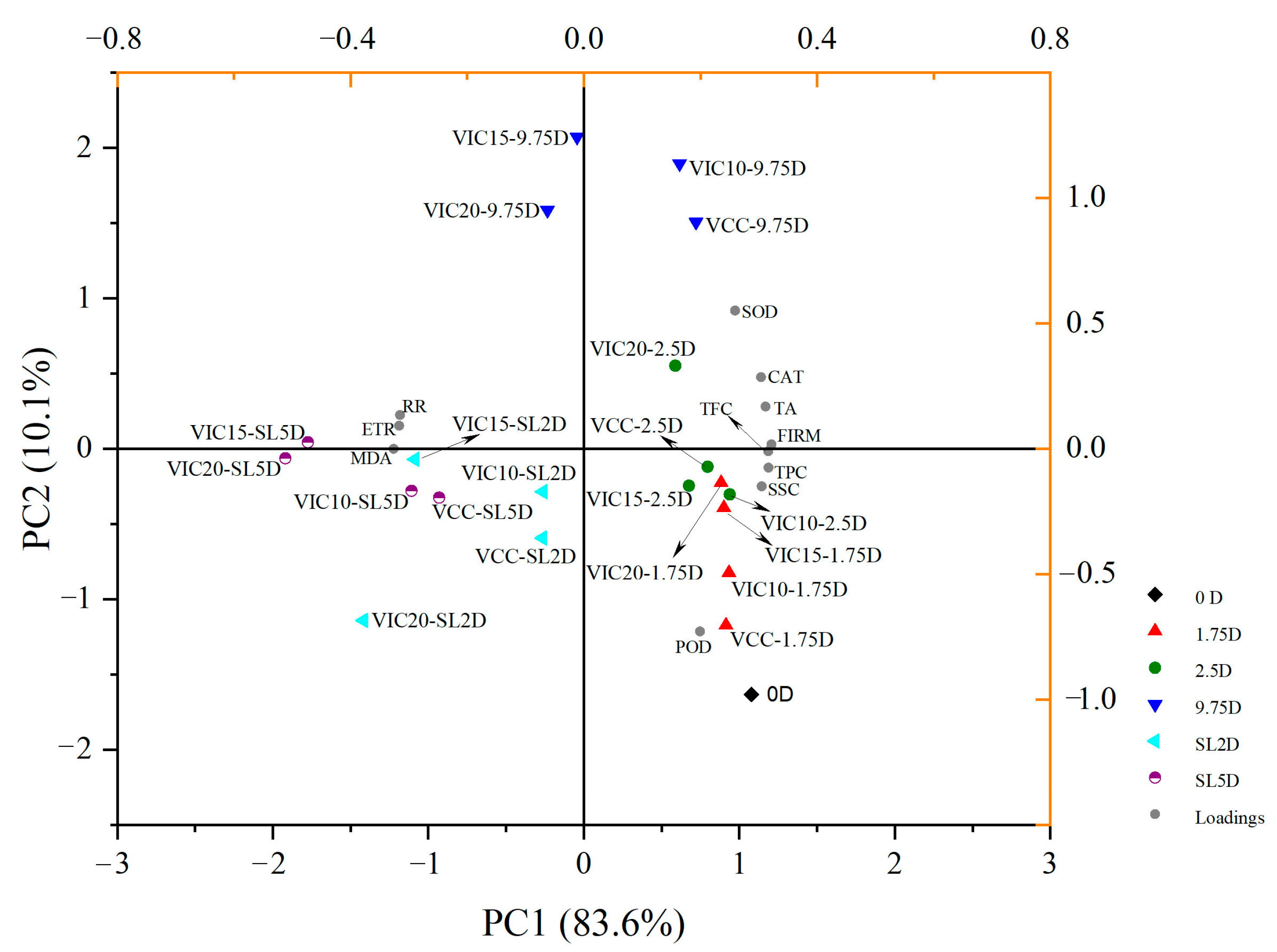
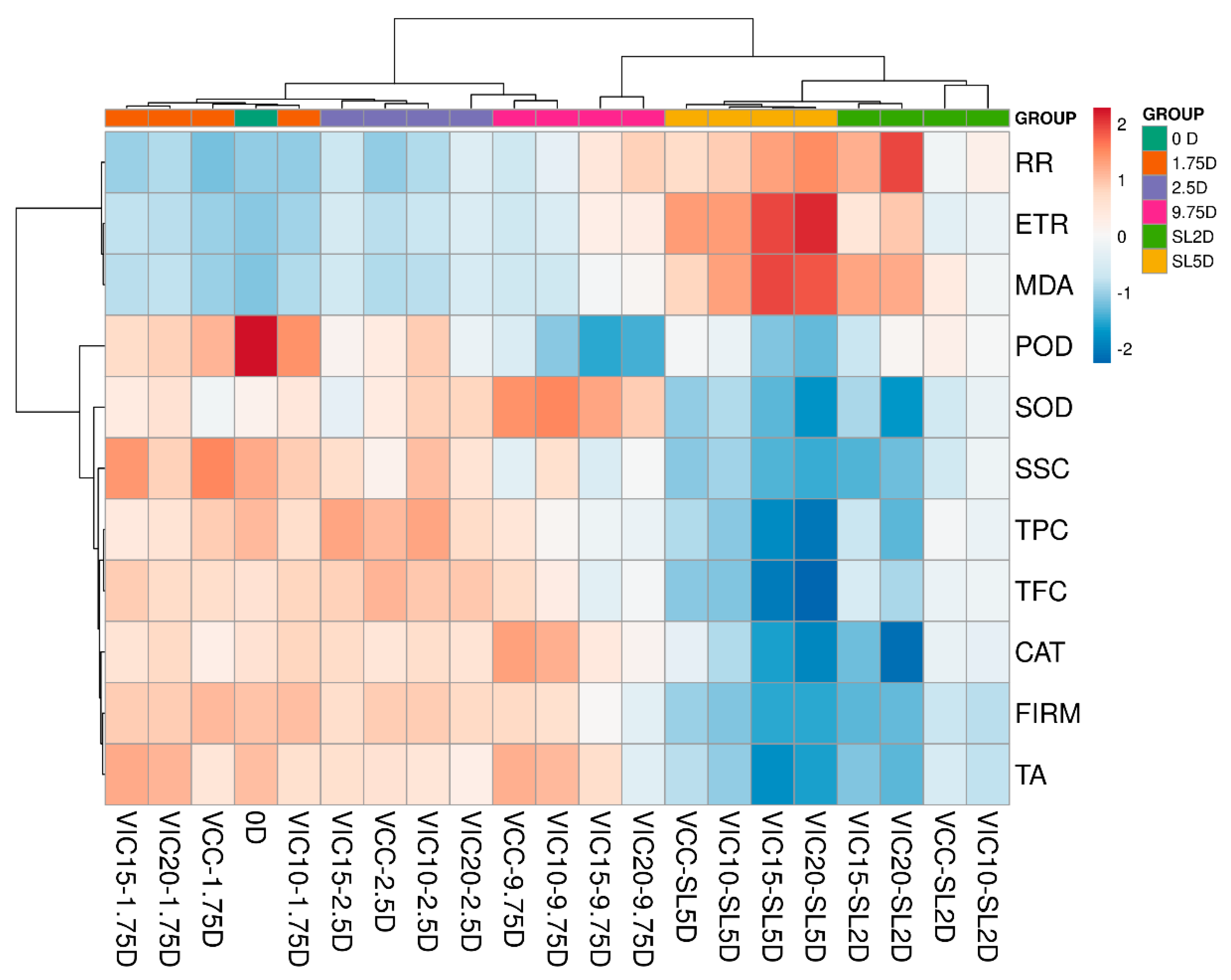
3.8. Principal Component Analysis and Heatmaps of Peach Fruit in Different Virtual Cold Chains
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Liu, S.; Tian, C.; Yan, G.; Wang, D. An overview of current status of cold chain in China. Int. J. Refrig. 2018, 88, 483–495. [Google Scholar] [CrossRef]
- Liu, G. Food Losses and Food Waste in China: A First Estimate; OECD Food, Agriculture and Fisheries Papers; No. 66; OECD Publishing: Paris, France, 2014. [Google Scholar] [CrossRef]
- Fu, Y. Fresh Produce Suppliers Focus on Cold Chain Logistics. Economic Information Daily, 19 April 2016. Available online: http://dz.jjckb.cn/www/pages/webpage2009/html/2016-04/19/content_17751.htm(accessed on 6 January 2023). (In Chinese)
- Wu, W.; Cronje, P.; Verboven, P.; Defraeye, T. Unveiling how ventilated packaging design and cold chain scenarios affect the cooling kinetics and fruit quality for each single fruit in an entire pallet. Food Packag. Shelf Life 2019, 21, 100369. [Google Scholar] [CrossRef]
- Defraeye, T.; Wu, W.; Prawiranto, K.; Fortunato, G.; Kemp, S.; Hartmann, S.; Cronje, P.; Verboven, P.; Nicolai, B. Artificial fruit for monitoring the thermal history of horticultural produce in the cold chain. J. Food Eng. 2017, 215, 51–60. [Google Scholar] [CrossRef]
- Hsiao, H.I.; Huang, K.L. Time-temperature transparency in the cold chain. Food Control 2016, 64, 181–188. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, Z.; Zhang, Y.; Shi, X.; Chen, F.; Liu, K. Effect of temperature fluctuation on colour change and softening of postharvest sweet cherry. RSC Adv. 2021, 11, 22969–22982. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Emond, J.P. Quality of strawberry after storage in constant or fluctuating temperatures. In Proceedings of the 20th International Congress of Refrigeration, IIR/IIF, Sydney, Australia, 19 September 1999. Paper No. 205; 9p. [Google Scholar]
- Nunes, M.C.N.; Emond, J.; Brecht, J. Quality of strawberries as affected by temperature abuse during ground, in-flight and retail handling operations. Acta Hortic. 2003, 604, 239–246. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Emond, J.P.; Brecht, J.K. Brief deviations from set point temperatures during normal airport handling operations negatively affect the quality of papaya (Carica papaya L.) fruit. Postharv. Biol. Technol. 2006, 4, 328–340. [Google Scholar] [CrossRef]
- Dea, S.; Brecht, J.K.; Nunes, M.C.N.; Émond, J.P.; Chau, K.V. Impact of environmental conditions during distribution on tomato fruit quality and decay. Proc. Fla. State Hortic. Soc. 2008, 121, 289–296. [Google Scholar]
- Kelly, K.; Robert, M.; Emond, J.P.; Nunes, M.C.N. A novel approach to determine the impact level of each step along the supply chain on strawberry quality. Postharv. Biol. Technol. 2019, 147, 78–88. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Donnell, C.P.; Emond, J.P.; Nunes, M.C.N. Blueberry supply chain: Critical steps impacting fruit quality and application of a boosted regression tree model to predict weight loss. Postharv. Biol. Technol. 2021, 179, 111590. [Google Scholar] [CrossRef]
- Lai, Y.; Emond, J.P.; Nunes, M.C.N. Environmental conditions encountered during distribution from the field to the store affect the quality of strawberry (‘Albion’). Proc. Fla. State Hortic. Soc. 2011, 124, 213–220. [Google Scholar]
- Rediers, H.; Claes, M.; Peeters, L.; Willems, K.A. Evaluation of the cold chain of fresh-cut endive from farmer to plate. Postharv. Biol. Technol. 2009, 51, 257–262. [Google Scholar] [CrossRef]
- Derens-Bertheau, E.; Osswald, V.; Laguerre, O.; Alvarez, G. Cold chain of chilled food in France. Int. J. Refrig. 2015, 52, 161–167. [Google Scholar] [CrossRef]
- Caro, M.P.; Ali, M.S.; Vecchio, M.; Giaffreda, R. Blockchain-based traceability in agri-food supply chain management: A practical implementation. In Proceedings of the 2018 IoT Vertical and Topical Summit on Agriculture-Tuscany (IOT Tuscany), Tuscany, Italy, 8–9 May 2018; pp. 1–4. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Lv, Z.; Yang, W.; Zhang, C.; Liu, J.; Jiao, Z. Effect of simulated circulation modes at different temperatures on firmness, sugar and acid composition of peach fruit. Food Sci. 2021, 42, 199–205. (In Chinese) [Google Scholar]
- Liu, B.; Jiao, W.; Wang, B.; Shen, J.; Zhao, H.; Jiang, W. Near freezing point storage compared with conventional low temperature storage on apricot fruit flavor quality (volatile, sugar, organic acid) promotion during storage and related shelf life. Sci. Hortic. 2019, 249, 100–109. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Pulmb-Dhindsa, P.; Thorpe, T.A. Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Lv, Z.; Yang, W.; Zhang, C.; Chen, D.; Jiao, Z. Effect of dehydration techniques on bioactive compounds in hawthorn slices and their correlations with antioxidant properties. J. Food Sci. Technol. 2019, 56, 2446–2457. [Google Scholar] [CrossRef]
- Tauno, M.; Jaak, V. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Chang, E.H.; Lee, J.S.; Kim, J.G. Cell wall degrading enzymes activity is altered by high carbon dioxide treatment in postharvest ‘Mihong’ peach fruit. Sci. Hortic. 2017, 225, 399–407. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Yu, M.; Ma, R.; Yu, Z. Exogenous nitric oxide fumigation promoted the emission of volatile organic compounds in peach fruit during shelf life after long-term cold storage. Food Res. Int. 2019, 133, 109135. [Google Scholar] [CrossRef]
- Tatsuki, M.; Sawamura, Y.; Yaegaki, H.; Suesada, Y.; Nakajima, N. The storage temperature affects flesh firmness and gene expression patterns of cell wall-modifying enzymes in stony hard peach. Postharv. Biol. Technol. 2021, 181, 111658. [Google Scholar] [CrossRef]
- Budde, C.O.; Polenta, G.; Lucangeli, C.D.; Murray, R.E. Air and immersion heat treatments affect ethylene production and organoleptic quality of ‘Dixiland’ peaches. Postharv. Biol. Technol. 2006, 41, 32–37. [Google Scholar] [CrossRef]
- An, X.; Xu, Y.; Jiang, L.; Huang, C.; Yu, Z. Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8). Sci. Hortic. 2019, 245, 178–184. [Google Scholar] [CrossRef]
- Hayama, H.; Tatsuki, M.; Ito, A.; Kashimura, Y. Ethylene and fruit softening in the stony hard mutation in peach. Postharv. Biol. Technol. 2006, 41, 16–21. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, W.; Ding, Y.; Wang, Y.; Niu, L.; Yao, J.; Pan, L.; Lu, Z.; Cui, G.; Li, G.; et al. Peach ethylene response factor PpeERF2 represses the expression of ABA biosynthesis and cell wall degradation genes during fruit ripening. Plant Sci. 2019, 283, 116–126. [Google Scholar] [CrossRef]
- Dawson, D.M.; Watkins, C.B.; Melton, L.D. Intermittent warming affects cell wall composition of ‘Fantasia’ nectarines during ripening and storage. J. Am. Soc. Hortic. Sci. 1995, 120, 1057–1062. [Google Scholar] [CrossRef] [Green Version]
- Buescher, R.W.; Furmanski, R.J. Role of pectinesterase and polygalacturonase in the formation of wooliness in peaches. J. Food Sci. 1978, 43, 264–266. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.; Gong, Y.; Zhu, Y.; Wang, H.; Zhang, X.; Yu, D.; Yu, F.; Qiu, Z.; Lu, H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharv. Biol. Technol. 2013, 86, 53–61. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT-Food Sci. Technol. 2004, 37, 687–695. [Google Scholar] [CrossRef]
- Gautier, H.; Rocci, A.; Buret, M.; Grasselly, D.; Causse, M. Fruit load or fruit position alters response to temperature and subsequently cherry tomato quality. J. Sci. Food Agric. 2005, 85, 1009–1016. [Google Scholar] [CrossRef]
- Bugaud, C.; Daribo, M.O.; Beauté, M.P.; Telle, N.; Dubois, C. Relative importance of location and period of banana bunch growth in carbohydrate content and mineral composition of fruit. Fruits 2009, 64, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [Green Version]
- Lobit, P.; Génard, M.; Soing, P.; Habib, R. Modelling malic acid accumulation in fruits: Relationships with organic acids, potassium, and temperature. J. Exp. Bot. 2006, 57, 1471–1483. [Google Scholar] [CrossRef] [Green Version]
- Araujo, W.L.; Nunes-nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharv. Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Zhuang, H.; Hildebrand, D.F.; Barth, M.M. Temperature influenced lipid peroxidation and deterioration in broccoli buds during postharvest storage. Postharv. Biol. Technol. 1997, 10, 49–58. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, M.M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Drogoudi, P.; Pantelidis, G.E.; Goulas, V.; Manganaris, G.A.; Manganaris, G.A.; Ziogas, V.; Manganaris, A. The appraisal of qualitative parameters and antioxidant contents during postharvest peach fruit ripening underlines the genotype significance. Postharv. Biol. Technol. 2016, 115, 142–150. [Google Scholar] [CrossRef]
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M.; et al. Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol. 2014, 164, 1191–1203. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Huber, D.J.; Qu, H.; Yun, Z.; Wang, H.; Huang, Z.; Huang, H.; Jiang, Y. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef]
- Min, D.; Dong, L.; Shu, P.; Cui, X.; Zhang, X.; Li, F. The application of carbon dioxide and 1-methylcyclopropene to maintain fruit quality of ‘Niuxin’ persimmon during storage. Sci. Hortic. 2018, 229, 201–206. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Sci. Hortic. 2012, 142, 221–228. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Li, B.; Wang, Q.; Meng, X. Effects of salicylic acid on disease resistance and postharvest decay control of fruits. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar] [CrossRef]
- Xi, J.; Zhao, Q.; Xu, D.; Jin, Y.; Wu, F.; Xu, X. Evolution of volatiles and quality of Chinese steamed bread during storage at different temperatures. Food Chem. 2022, 381, 132213. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Lv, Z.; Yang, W.; Li, A.; Liu, J.; Zhang, Q.; Jiao, Z. Virtual Cold Chain Method to Evaluate the Effect of Rising Temperature on the Quality Evolution of Peach Fruit. Foods 2023, 12, 2403. https://doi.org/10.3390/foods12122403
Liu H, Lv Z, Yang W, Li A, Liu J, Zhang Q, Jiao Z. Virtual Cold Chain Method to Evaluate the Effect of Rising Temperature on the Quality Evolution of Peach Fruit. Foods. 2023; 12(12):2403. https://doi.org/10.3390/foods12122403
Chicago/Turabian StyleLiu, Hui, Zhenzhen Lv, Wenbo Yang, Ang Li, Jiechao Liu, Qiang Zhang, and Zhonggao Jiao. 2023. "Virtual Cold Chain Method to Evaluate the Effect of Rising Temperature on the Quality Evolution of Peach Fruit" Foods 12, no. 12: 2403. https://doi.org/10.3390/foods12122403




