Isolation and Characterization of Protein Fractions for Valorization of Sacha Inchi Oil Press-Cake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SIPC Adequacy
2.3. Proximal Composition
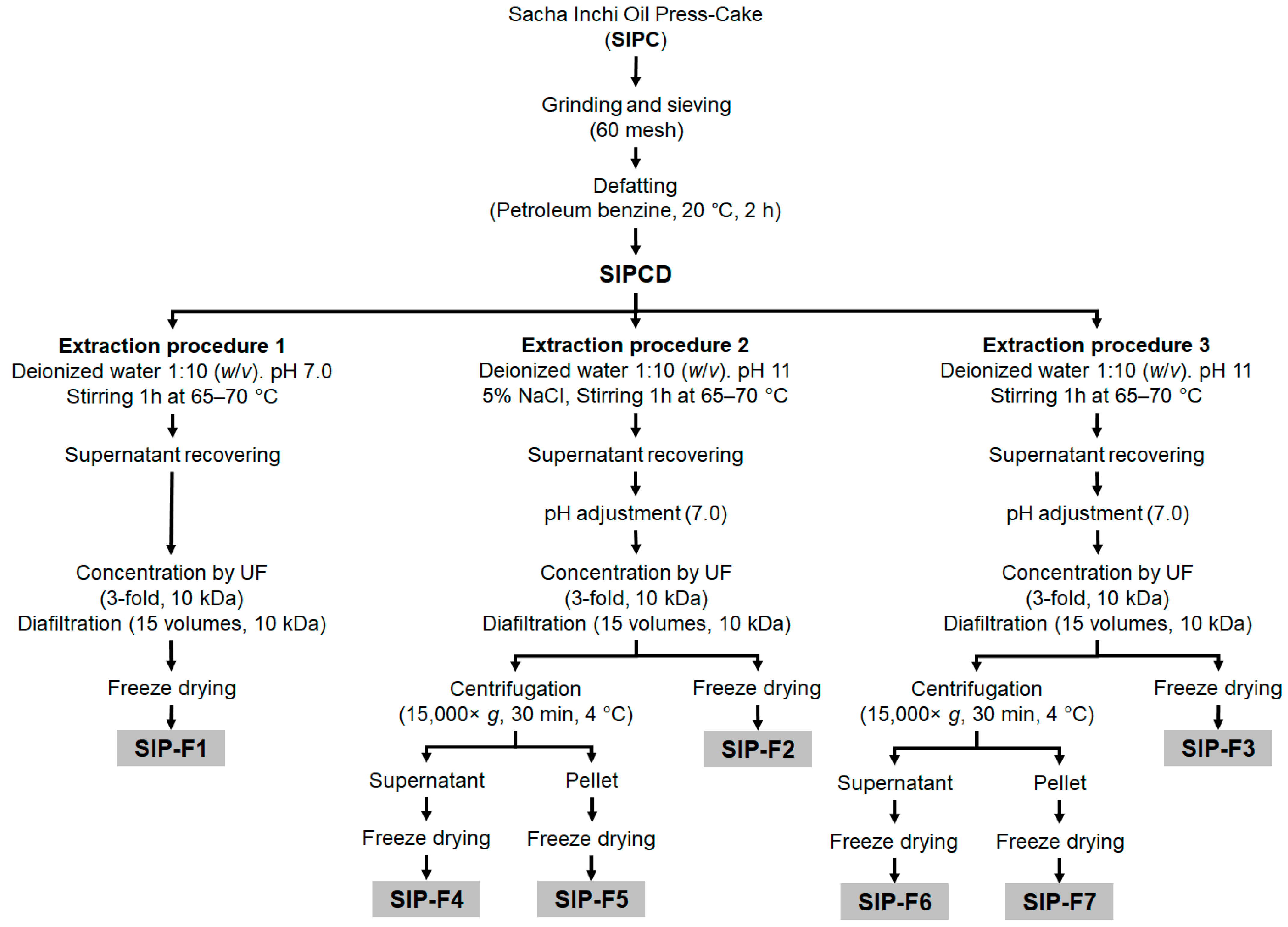
2.4. Isolation of the Protein Fractions
2.5. Characterization of the Protein Fractions
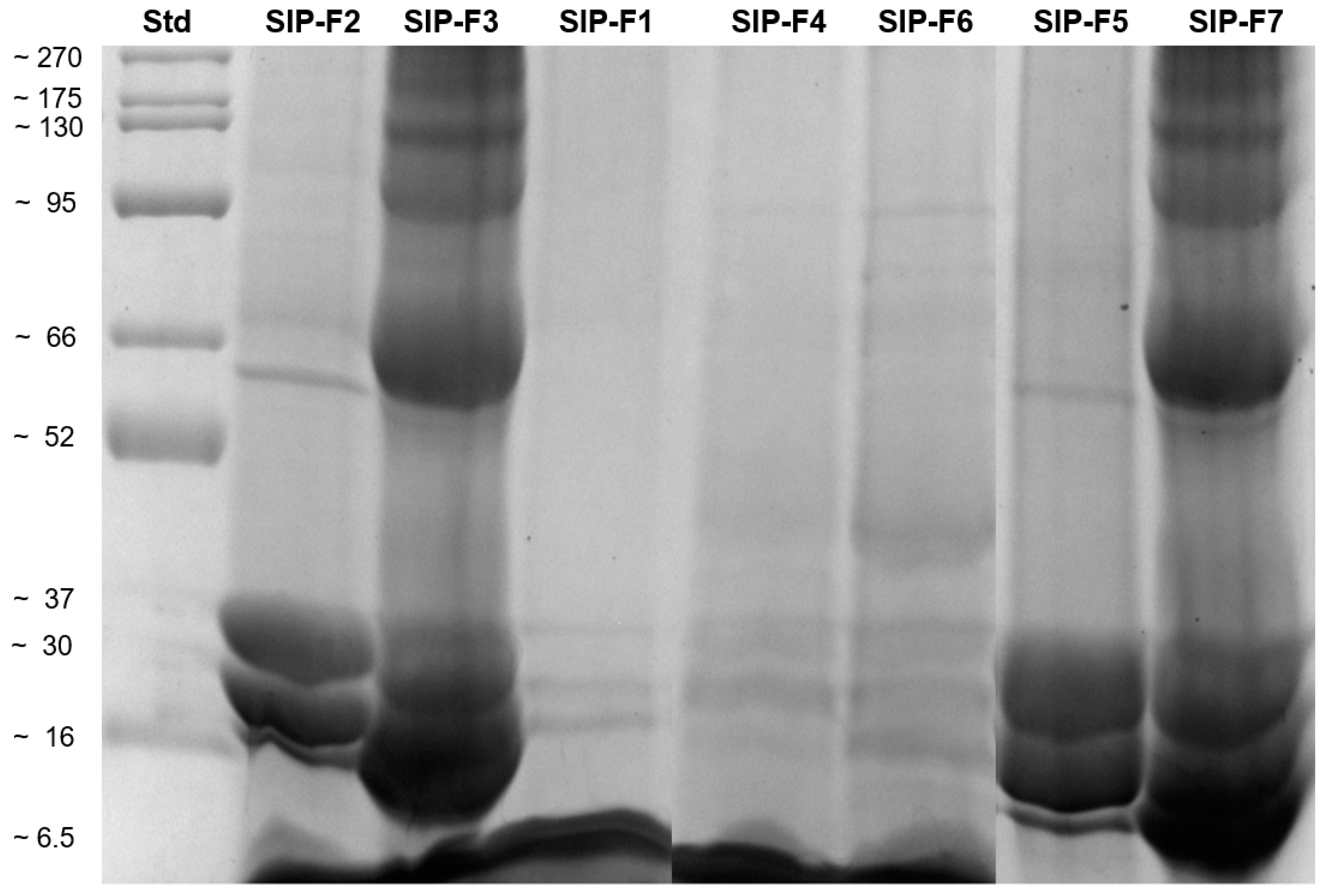
2.5.1. Sodium Dodecyl Sulfate–Polyacrylamide Gel E (SDS-PAGE)
2.5.2. Protein Secondary Structure Analysis
2.5.3. Oil Absorption Capacity (OAC)
2.5.4. Water Absorption Index (WAI) and Water Solubility Index (WSI)
2.5.5. Foaming Properties
2.5.6. Emulsifying Properties
2.5.7. Color
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Proximal Composition
3.2. Isolation of the Protein Fractions
3.3. Characteristics of the Protein Fractions
3.3.1. Electrophoretic Profiles
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.4. Functional Properties of the SIPF
3.4.1. OAC, WAI, and WSI
3.4.2. FA, FS, and FD
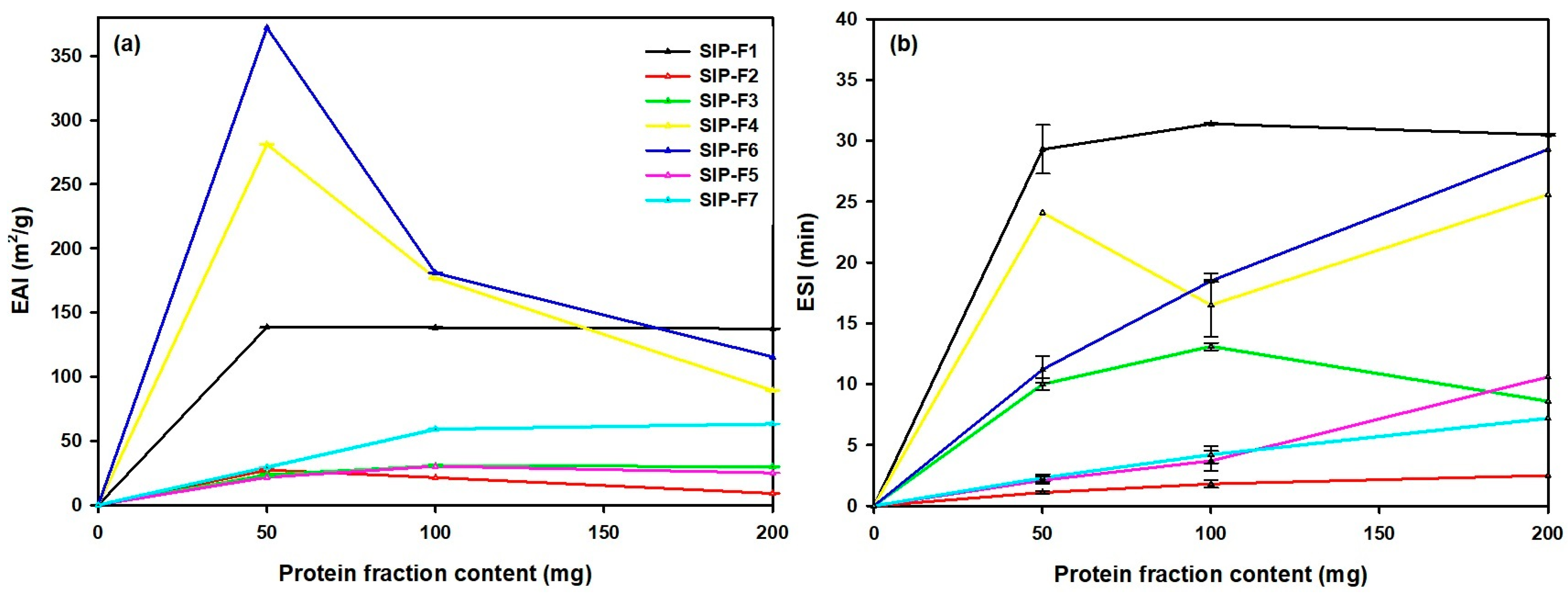
3.4.3. EAI and ESI
3.4.4. Color
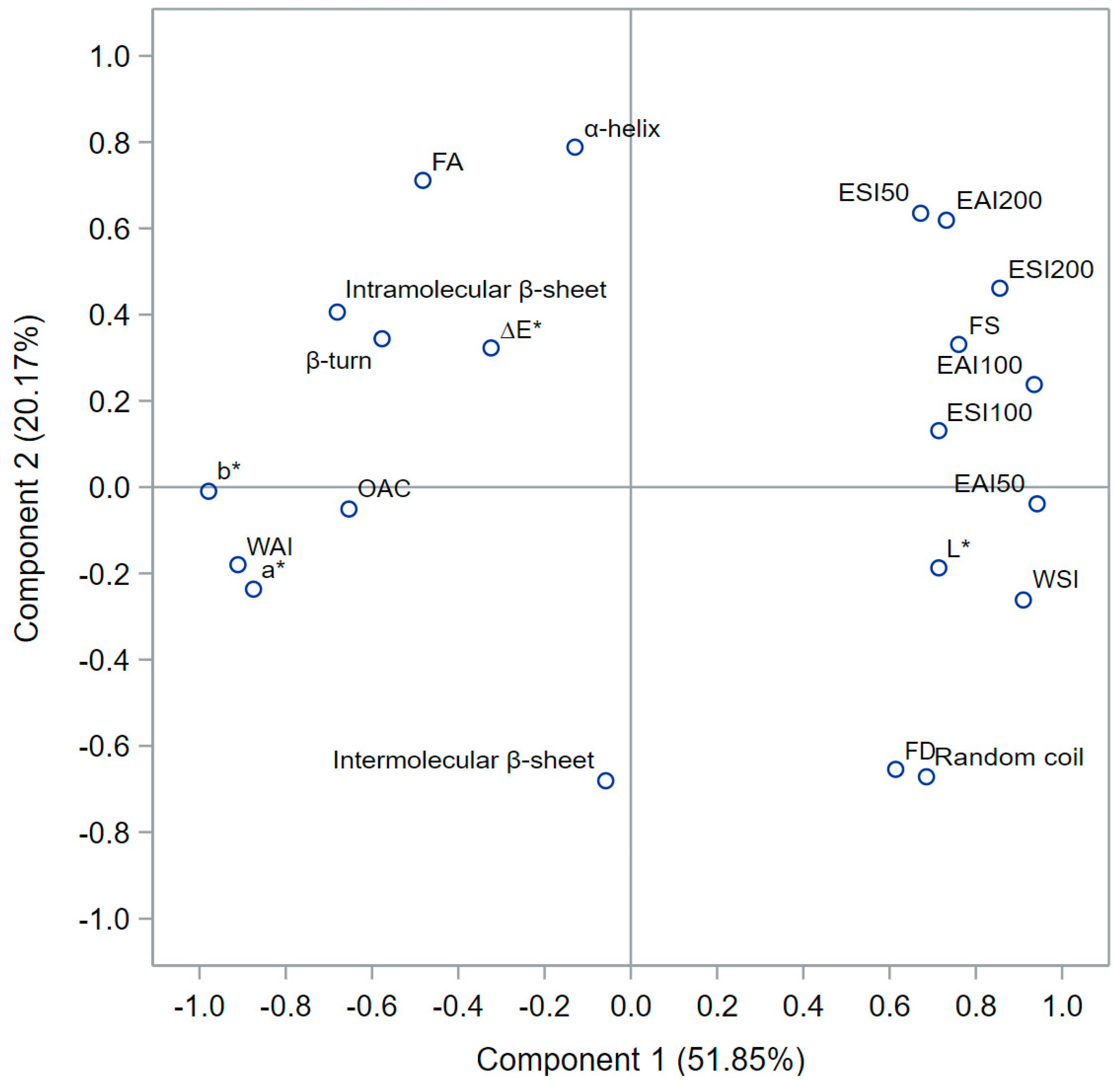
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawdkuen, S.; Ketnawa, S. Extraction, characterization, and application of agricultural and food processing by-products. In Food Preservation and Waste Exploitation; Socaci, S.A., Farcas, A.C., Aussenac, T., Laguerre, J.-C., Eds.; IntechOpen: London, UK, 2019; pp. 1–32. [Google Scholar]
- Sanchez-Reinoso, Z.; Gutiérrez, L.F. Effects of the Emulsion Composition on the Physical Properties and Oxidative Stability of Sacha Inchi (Plukenetia volubilis L.) Oil Microcapsules Produced by Spray Drying. Food Bioprocess Technol. 2017, 10, 1354–1366. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Quiñones-segura, Y.; Sanchez-Reinoso, Z.; Díaz, D.L.; Abril, J.I. Physicochemical Properties of Oils Extracted from γ-Irradiated Sacha Inchi (Plukenetia volubilis L.) Seeds. Food Chem. 2017, 237, 581–587. [Google Scholar] [CrossRef]
- Vanegas-Azuero, A.M.; Gutiérrez, L.F. Physicochemical and Sensory Properties of Yogurts Containing Sacha Inchi (Plukenetia volubilis L.) Seeds and β-Glucans from Ganoderma Lucidum. J. Dairy Sci. 2018, 101, 1020–1033. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, L.-F.; Rosada, L.-M.; Jiménez, Á. Chemical Composition of Sacha Inchi (Plukenetia volubilis L.) Seeds and Characteristics of Their Lipid Fraction. Grasas Aceites 2011, 62, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, L.F.; Sanchez-Reinoso, Z.; Quiñones-Segura, Y. Effects of Dehulling Sacha Inchi (Plukenetia volubilis L.) Seeds on the Physicochemical and Sensory Properties of Oils Extracted by Means of Cold Pressing. J. Am. Oil Chem. Soc. 2019, 96, 1187–1195. [Google Scholar] [CrossRef]
- Torres Sánchez, E.G.; Hernández-Ledesma, B.; Gutiérrez, L.-F. Sacha Inchi Oil Press-Cake: Physicochemical Characteristics, Food-Related Applications and Biological Activity. Food Rev. Int. 2023, 39, 148–159. [Google Scholar] [CrossRef]
- Ibáñez, M.A.; de Blas, C.; Cámara, L.; Mateos, G.G. Chemical Composition, Protein Quality and Nutritive Value of Commercial Soybean Meals Produced from Beans from Different Countries: A Meta-Analytical Study. Anim. Feed Sci. Technol. 2020, 267, 114531. [Google Scholar] [CrossRef]
- Rawdkuen, S.; D’Amico, S.; Schoenlechner, R. Physicochemical, Functional, and In Vitro Digestibility of Protein Isolates from Thai and Peru Sacha Inchi (Plukenetia volubilis L.) Oil Press-Cakes. Foods 2022, 11, 1869. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K.; Hamaker, B.R.; Sze-Tao, K.W.C.; Venkatachalam, M. Isolation, Purification, and Biochemical Characterization of a Novel Water Soluble Protein from Inca Peanut (Plukenetia volubilis L.). J. Agric. Food Chem. 2002, 50, 4906–4908. [Google Scholar] [CrossRef]
- Sathe, S.K.; Kshirsagar, H.H.; Sharma, G.M. Solubilization, Fractionation, and Electrophoretic Characterization of Inca Peanut (Plukenetia volubilis L.) Proteins. Plant Foods Hum. Nutr 2012, 67, 247–255. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Rodzi, N.; Pinijsuwan, S. Characterization of Sacha Inchi Protein Hydrolysates Produced by Crude Papain and Calotropis Proteases. LWT Food Sci. Technol. 2018, 98, 18–24. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Horwitz, W., Ed.; Association of Official Analytical Chemists (AOAC) International: Rockville, MD, USA, 2005. [Google Scholar]
- Suwanangul, S.; Sangsawad, P.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Chittrakorn, S.; Ruttarattanamongkol, K. Antioxidant Activities of Sacha Inchi (Plukenetia volubilis L.) Protein Isolate and Its Hydrolysates Produced with Different Proteases. Maejo Int. J. Sci. Technol. 2021, 15, 48–60. [Google Scholar]
- Suwanangul, S.; Alashi, M.A.; Aluko, R.E.; Tochampa, W.; Ruttarattanamongkol, K. Inhibition of α-Amylase, α-Glucosidase and Pancreatic Lipase Activities in Vitro by Sacha Inchi (Plukenetia volubilis L.) Protein Hydrolysates and Their Fractionated Peptides. Maejo Int. J. Sci. Technol. 2021, 15, 13–26. [Google Scholar]
- Zhan, Q.; Wang, Q.; Liu, Q.; Guo, Y.; Gong, F.; Hao, L.; Wu, H. The Antioxidant Activity of Protein Fractions from Sacha Inchi Seeds after a Simulated Gastrointestinal Digestion. LWT 2021, 145, 111356. [Google Scholar] [CrossRef]
- Sari, Y.W.; Mulder, W.J.; Sanders, J.P.M.; Bruins, M.E. Towards Plant Protein Refinery: Review on Protein Extraction Using Alkali and Potential Enzymatic Assistance. Biotechnol. J. 2015, 10, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Glassford, S.E.; Byrne, B.; Kazarian, S.G. Recent Applications of ATR FTIR Spectroscopy and Imaging to Proteins. Biochim. Biophys. Acta 2013, 1834, 2849–2858. [Google Scholar] [CrossRef] [Green Version]
- Agustika, D.K.; Mercuriani, I.; Purnomo, C.W.; Hartono, S.; Triyana, K.; Iliescu, D.D.; Leeson, M.S. Fourier Transform Infrared Spectrum Pre-Processing Technique Selection for Detecting PYLCV-Infected Chilli Plants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 278, 121339. [Google Scholar] [CrossRef]
- Singh, B.; Velázquez, D.; Terry, J.; Linford, M.R. The Equivalent Width as a Figure of Merit for XPS Narrow Scans. J. Electron Spectros. Relat. Phenom. 2014, 197, 56–63. [Google Scholar] [CrossRef]
- Sadat, A.; Joye, I.J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining Information about Protein Secondary Structures in Aqueous Solution Using Fourier Transform IR Spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, X.; Tang, C.; Li, L.; Ahmad, I. Preparation of Soluble Soybean Protein Aggregates (SSPA) from Insoluble Soybean Protein Concentrates (SPC) and Its Functional Properties. Food Res. Int. 2008, 41, 154–164. [Google Scholar] [CrossRef]
- Jiapong, S.; Ruttarattanamongkol, K. Development of Direct Expanded High Protein Snack Products Fortified with Sacha Inchi Seed Meal. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 680–684. [Google Scholar] [CrossRef]
- Haque, Z.; Kito, M. Lipophilization of As1-Casein. 2. Conformational and Functional Effects. J. Agric. Food Chem. 1983, 31, 1231–1237. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Anantanasuwong, S.; Adachi, S. Emulsifying and Foaming Properties of Defatted Soy Meal Extracts Obtained by Subcritical Water Treatment. Int. J. Food Prop. 2011, 14, 9–16. [Google Scholar] [CrossRef]
- Cordero-Clavijo, L.M.; Serna-Saldívar, S.O.; Lazo-Vélez, M.A.; González, J.F.A.; Panata-Saquicilí, D.; Briones-Garcia, M. Characterization, Functional and Biological Value of Protein-Enriched Defatted Meals from Sacha Inchi (Plukenetia volubilis) and Chocho (Lupinus mutabilis). J. Food Meas. Charact. 2021, 15, 5071–5077. [Google Scholar] [CrossRef]
- Jagersberger, J. Development of Novel Products on Basis of Sacha Inchi-Use of Press Cakes and Hulls. Master’s Thesis, Universität Wien, Vienna, Austria, 2013. [Google Scholar]
- Rawdkuen, S.; Murdayanti, D.; Ketnawa, S.; Phongthai, S. Chemical Properties and Nutritional Factors of Pressed-Cake from Tea and Sacha Inchi Seeds. Food Biosci. 2016, 15, 64–71. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Wu, W.; Wang, L.; Qin, F.; Xie, W. Effect of Extraction PH on Functional Properties, Structural Properties, and in Vitro Gastrointestinal Digestion of Tartary Buckwheat Protein Isolates. J. Cereal Sci. 2021, 101, 103314. [Google Scholar] [CrossRef]
- Nasabi, M.; Labbafi, M.; Mousavi, M.E.; Madadlou, A. Effect of Salts and Nonionic Surfactants on Thermal Characteristics of Egg White Proteins. Int. J. Biol. Macromol. 2017, 102, 970–976. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Chang, C.; Gu, L.; Su, Y.; Yang, Y. Effects of NaOH/NaCl Pickling on Heat-Induced Gelation Behaviour of Egg White. Food Chem. 2019, 297, 124939. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse Proteins: Secondary Structure, Functionality and Applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Čepková, P.H.; Jágr, M.; Viehmannová, I.; Dvořáček, V.; Huansi, D.C.; Mikšík, I. Diversity in Seed Storage Protein Profile of Oilseed Crop Plukenetia volubilis from Diversity in Seed Storage Protein Profile of Oilseed Crop Plukenetia volubilis from Peruvian Amazon. Int. J. Agric. Biol. 2019, 21, 679–688. [Google Scholar]
- Quinteros, M.; Vilcacundo, R.; Carpio, C.; Carrillo, W. Isolation of Proteins from Sacha Inchi (Plukenetia volubilis L.) in Presence of Water and Salt. Asian J. Pharm. Clin. Res. 2016, 9, 193–196. [Google Scholar]
- Tsomele, G.F.; Meiring, B.; Anyasi, T.A.; Mlambo, V.; Amonsou, E.; Lepule, S.P.; Siwela, M.; Wokadala, O.C. Influence of Amino Acid Profile and Secondary Structure on Nutritional and Functional Properties of Trichilia Emetica and Trichilia Dregeana Protein Concentrates. Int. J. Food Sci. Technol. 2023, 1–12. [Google Scholar] [CrossRef]
- Law, H.-Y.; Choi, S.-M.; Ma, C.-Y. Study of Conformation of Vicilin from Dolichos Lablab and Phaseolus Calcaratus by Fourier-Transform Infrared Spectroscopy and Differential Scanning Calorimetry. Food Res. Int. 2008, 41, 720–729. [Google Scholar] [CrossRef]
- Song, X.; Si, L.; Sun, X.; Zhu, X.; Li, Z.; Li, Y.; Wang, Y.; Hou, H. Rheological Properties, Thermal Stability and Conformational Changes of Collagen from Sea Cucumber (Apostichopus japonicas). Food Chem. 2022, 389, 133033. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Han, L.; Xu, Z.; Ma, K.; Li, T. Effect of Alkaline PH on the Thermal Aggregation Behavior of Mackerel Myosin. Mod. Food Sci. Technol. 2022, 38, 114–120. [Google Scholar] [CrossRef]
- Withana-Gamage, T.S.; Wanasundara, J.P.; Pietrasik, Z.; Shand, P.J. Physicochemical, Thermal and Functional Characterisation of Protein Isolates from Kabuli and Desi Chickpea (Cicer arietinum L.): A Comparative Study with Soy (Glycine max) and Pea (Pisum sativum L.). J. Sci. Food Agric. 2011, 91, 1022–1031. [Google Scholar] [CrossRef]
- Mao, X.Y.; Hua, Y.F. Chemical Composition, Molecular Weight Distribution, Secondary Structure and Effect of NaCl on Functional Properties of Walnut (Juglans regia L.) Protein Isolates and Concentrates. J. Food Sci. Technol. 2014, 51, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
- da Silva Bambirra Alves, F.E.; Carpiné, D.; Lopes Teixeira, G.; Goedert, A.C.; de Paula Scheer, A.; Ribani, R.H. Valorization of an Abundant Slaughterhouse By-Product as a Source of Highly Technofunctional and Antioxidant Protein Hydrolysates. Waste Biomass Valoriz. 2021, 12, 263–279. [Google Scholar] [CrossRef]
- Sathe, S.K.; Desphande, S.S.; Salunkhe, D.K. Functional Properties of Winged Bean Proteins. J. Food Sci. 1982, 42, 503–509. [Google Scholar] [CrossRef]
- Mercado, R.J.L.; Elías, P.C.C.A.; Pascual, C.G.J. Obtención de Un Aislado Proteico de Torta de Sacha Inchi (Plukenetia volubilis L.) y Evaluación de Sus Propiedades Tecno-Funcionales. An. Científicos 2015, 76, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.V.; García, N.H.P.; Amaya-Farfán, J. Physico-Chemical Properties of the Flour, Protein Concentrate, and Protein Isolate of the Cupuassu (Theobroma Grandiflorum Schum) Seed. J. Food Sci. 2006, 71, S573–S578. [Google Scholar] [CrossRef]
- Rayan, A.M.; Swailam, H.M.; Hamed, Y.S. Composition, Structure, and Techno-Functional Characteristics of the Flour, Protein Concentrate, and Protein Isolate from Purslane (Portulaca Oleracea L.) Seeds. Plant Foods Hum. Nutr. 2023, 78, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Diasa, F.F.G.; de Moura Bell, J.M.L.N. Understanding the Impact of Enzyme-Assisted Aqueous Extraction on the Structural, Physicochemical, and Functional Properties of Protein Extracts from Full-Fat Almond Flour. Food Hydrocoll. 2022, 127, 107534. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Emannuele, A.; Ribeiro, C.; Oliveira, É.R.; Garcia, M.C.; Soares, M.; Júnior, S.; Caliari, M. Structural and Physicochemical Properties of Freeze-Dried Açaí Pulp (Euterpe Oleracea Mart.). Food Sci. Technol. 2020, 2061, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kornet, R.; Diedericks, C.F.; Yang, Q.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Venema, P.; van der Linden, E.; Sagis, L.M.C. Rethinking Plant Protein Extraction: Albumin—From Side Stream to an Excellent Foaming Ingredient. Food Struct. 2022, 31, 100254. [Google Scholar] [CrossRef]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.; Chen, B.; Rao, J. Plant Proteins from Green Pea and Chickpea: Extraction, Fractionation, Structural Characterization and Functional Properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Innocente, N.; Corradini, C.; Blecker, C.; Paquot, M. Emulsifying Properties of the Total Fraction and the Hydrophobic Fraction of Bovine Milk Proteose-Peptones. Int. Dairy J. 1998, 6946, 981–985. [Google Scholar] [CrossRef]
- Rungrot, K.; Hudthagosol, C.; Sanporkha, P. Effect of Sacha Inchi Pressed-Cake (Plukenetia volubilis L.) on the Physical, Chemical and Sensory Properties of Tuiles. Chiang Mai Univ. J. Nat. Sci. 2021, 20, 1–10. [Google Scholar] [CrossRef]

| Parameter | SIPC | SIPCD |
|---|---|---|
| Dry matter (%) | 90.92 a ± 0.03 | 90.01 b ± 0.01 |
| Protein (%) | 54.47 b ± 0.11 | 56.86 a ± 0.42 |
| Fat (%) | 6.84 a ± 0.02 | 1.70 b ± 0.01 |
| Total dietary fiber (%) | 13.85 b ± 0.25 | 18.17 a ± 0.46 |
| Ash (%) | 6.08 b ± 0.02 | 6.38 a ± 0.02 |
| Carbohydrates (%) | 9.68 a ± 0.34 | 6.62 b ± 0.72 |
| Treatment | Protein Content * (%, N × 5.71) | Extraction Yield (%) | Protein Recovery (%) | Protein Concentration Increase (Fold) |
|---|---|---|---|---|
| SIP-F1 | 60.53 d ± 0.00 | 4.89 e ± 0.36 | 5.21 e ± 0.38 | 1.06 d ± 0.00 |
| SIP-F2 | 85.08 b ± 0.00 | 10.04 c ± 0.81 | 15.39 c ± 1.46 | 1.50 b ± 0.00 |
| SIP-F3 | 83.94 b ± 0.00 | 24.73 a ± 0.06 | 36.50 a ± 0.09 | 1.48 b ± 0.00 |
| SIP-F4 | 74.52 c ± 2.83 | 3.00 e ± 0.03 | 3.93 e ± 0.19 | 1.31 c ± 0.05 |
| SIP-F6 | 74.23 c ± 0.00 | 2.17 e ± 0.12 | 2.83 e ± 0.16 | 1.31 c ± 0.00 |
| SIP-F5 | 93.07 a ± 4.04 | 7.04 d ± 0.03 | 11.52 d ± 0.55 | 1.64 a ± 0.07 |
| SIP-F7 | 83.37 b ± 0.00 | 22.56 b ± 0.12 | 33.08 b ± 0.18 | 1.47 b ± 0.00 |
| Treatment | Intermolecular β-Sheet | Intramolecular β-Sheet | Random Coil | α-Helix | β-Turn |
|---|---|---|---|---|---|
| 1627–1610 cm−1 | 1642–1628 cm−1 | 1650–1643 cm−1 | 1659–1650 cm−1 | 1700–1660 cm−1 | |
| SIP-F1 | 3.95 c ± 3.30 | 28.71 bc ± 10.01 | 0.00 d ± 0.00 | 44.59 a ± 10.82 | 22.75 a ± 3.80 |
| SIP-F2 | 19.53 a ± 2.49 | 0.00 e ± 0.00 | 57.69 b ± 6.22 | 0.00 d ± 0.00 | 22.78 a ± 6.78 |
| SIP-F3 | 0.00 c ± 0.00 | 66.45 a ± 4.46 | 3.82 cd ± 6.61 | 0.42 d ± 0.74 | 29.31 a ± 1.97 |
| SIP-F4 | 11.22 b ± 0.50 | 11.18 d ± 0.65 | 57.79 b ± 1.92 | 4.98 c ± 1.04 | 14.83 b ± 0.73 |
| SIP-F6 | 11.95 b ± 1.10 | 0.00 e ± 0.00 | 70.61 a ± 2.31 | 0.00 d ± 0.00 | 17.43 b ± 1.20 |
| SIP-F5 | 10.79 b ± 1.71 | 39.29 b ± 5.37 | 2.76 cd ± 3.88 | 18.94 b ± 7.31 | 28.22 a ± 10.51 |
| SIP-F7 | 17.06 a ± 3.04 | 31.61 b ± 4.31 | 0.00 d ± 0.01 | 27.25 b ± 8.92 | 24.08 a ± 7.85 |
| Treatment | OAC (w/w) | WAI (w/w) | WSI (%) | FA (%) | FS (%) | FD |
|---|---|---|---|---|---|---|
| SIP-F1 | 4.7 cd ± 0.6 | 0.7 d ± 0.0 | 14.9 b ± 0.0 | 133.4 a ± 3.5 | 85.8 a ± 0.9 | 18.1 c ± 2.5 |
| SIP-F2 | 4.7 c ± 0.7 | 3.7 bc ± 0.4 | 15.8 b ± 1.3 | 87.9 d ± 4.7 | 65.6 b ± 5.0 | 35,6 b ± 3.4 |
| SIP-F3 | 6.6 b ± 1.4 | 4.6 ab ± 0.6 | 4.7 c ± 1.2 | 97.7 c ± 2.6 | 61.8 b ± 5.6 | 27.1 bc ± 0.5 |
| SIP-F4 | 4.3 d ± 0.4 | 0.5 d ± 0.1 | 87.5 a ± 1.4 | 36.4 f ± 0.0 | 89.4 a ± 6.2 | 63.6 a ± 0.0 |
| SIP-F6 | 6.2 bc ± 0.4 | 0.7 d ± 0.1 | 86.3 a ± 1.0 | 56.8 e ± 2.6 | 86.2 a ± 1.5 | 61.6 a ± 1.1 |
| SIP-F5 | 9.0 a ± 0.4 | 3.0 c ± 0.2 | 1.9 c ± 1.0 | 62.1 e ± 2.3 | 81.3 a ± 11.4 | 34.0 b ± 11.7 |
| SIP-F7 | 7.9 ab ± 0.7 | 5.0 a ± 0.8 | 3.0 c ± 0.9 | 122.7 b ± 5.2 | 89.8 a ± 8.6 | 22.9 bc ± 0.8 |
| Treatment | L* | a* | b* | ΔE |
|---|---|---|---|---|
| SIPCD | 85.55 a ± 0.13 | −0.12 bc ± 0.00 | 12.04 b ± 0.01 | 0.0 ± 0.0 |
| SIP-F1 | 84.62 a ± 0.10 | −1.92 e ± 0.02 | 7.40 cd ± 0.01 | 5.06 bc ± 0.02 |
| SIP-F2 | 84.45 a ± 0.01 | −0.79 cd ± 0.00 | 10.55 bc ± 0.00 | 1.97 c ± 0.00 |
| SIP-F3 | 77.94 b ± 3.15 | −0.23 c ± 0.29 | 12.84 b ± 1.54 | 7.74 b ± 3.25 |
| SIP-F4 | 84.12 a ± 1.46 | −1.13 d ± 0.30 | 7.26 cd ± 0.97 | 5.30 bc ± 0.76 |
| SIP-F6 | 83.89 a ± 1.42 | −1.25 d ± 0.14 | 5.97 d ± 1.08 | 6.47 bc ± 1.40 |
| SIP-F5 | 78.53 b ± 2.82 | 0.42 ab ± 0.22 | 11.75 b ± 2.19 | 7.42 b ± 2.52 |
| SIP-F7 | 71.58 c ± 0.42 | 0.63 a ± 0.18 | 16.34 a ± 0.47 | 14.63 a ± 0.55 |
| Functional Property | Protein Secondary Structures | ||||
|---|---|---|---|---|---|
| Intermolecular β-Sheet | Intramolecular β-Sheet | Random Coil | A-Helix | β-Turn | |
| OAC | 0.027 | 0.498 | −0.522 | 0.094 | 0.548 |
| WAI | 0.348 | 0.453 | −0.460 | 0.003 | 0.262 |
| WSI | 0.008 | −0.609 | 0.791 | −0.426 | −0.629 |
| FA | −0.253 | 0.372 | −0.707 | 0.714 | 0.370 |
| FS | 0.033 | −0.407 | 0.252 | 0.246 | −0.538 |
| FD | 0.284 | −0.509 | 0.769 | −0.643 | −0.451 |
| L* | −0.022 | −0.624 | 0.588 | −0.109 | −0.280 |
| a* | 0.219 | 0.598 | −0.515 | −0.070 | 0.426 |
| b* | 0.113 | 0.689 | −0.694 | 0.137 | 0.500 |
| ΔE | 0.054 | 0.459 | −0.404 | 0.140 | 0.029 |
| EAI50 | −0.048 | −0.583 | 0.667 | −0.229 | −0.543 |
| EAI100 | −0.156 | −0.516 | 0.459 | 0.068 | −0.508 |
| EAI200 | −0.315 | −0.337 | 0.083 | 0.469 | −0.280 |
| ESI50 | −0.588 | −0.113 | 0.021 | 0.369 | −0.142 |
| ESI100 | −0.201 | −0.403 | 0.456 | −0.124 | −0.256 |
| ESI200 | −0.411 | −0.345 | 0.235 | 0.254 | −0.250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Sánchez, E.; Hernández-Ledesma, B.; Gutiérrez, L.-F. Isolation and Characterization of Protein Fractions for Valorization of Sacha Inchi Oil Press-Cake. Foods 2023, 12, 2401. https://doi.org/10.3390/foods12122401
Torres-Sánchez E, Hernández-Ledesma B, Gutiérrez L-F. Isolation and Characterization of Protein Fractions for Valorization of Sacha Inchi Oil Press-Cake. Foods. 2023; 12(12):2401. https://doi.org/10.3390/foods12122401
Chicago/Turabian StyleTorres-Sánchez, Erwin, Blanca Hernández-Ledesma, and Luis-Felipe Gutiérrez. 2023. "Isolation and Characterization of Protein Fractions for Valorization of Sacha Inchi Oil Press-Cake" Foods 12, no. 12: 2401. https://doi.org/10.3390/foods12122401
APA StyleTorres-Sánchez, E., Hernández-Ledesma, B., & Gutiérrez, L.-F. (2023). Isolation and Characterization of Protein Fractions for Valorization of Sacha Inchi Oil Press-Cake. Foods, 12(12), 2401. https://doi.org/10.3390/foods12122401








