Effects of Glucose and Homogenization Treatment on the Quality of Liquid Whole Eggs
Abstract
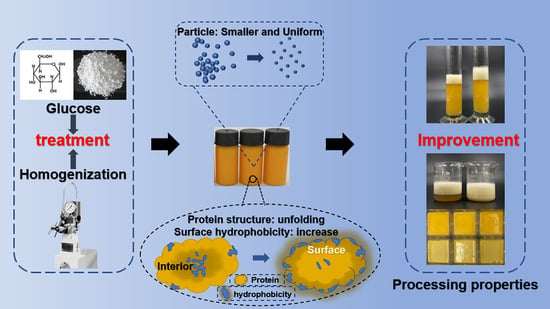
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Turbidity Measurement
2.4. Particle Size Measurement
2.5. Solubility Determination
2.6. Intrinsic Fluorescence Spectroscopy Determination
2.7. Surface Hydrophobicity Determination
2.8. Free Sulfhydryl Content Determination
2.9. Foaming Properties
2.10. Emulsifying Properties
2.11. Gelling Properties
2.12. Statistical Analysis
3. Results and Discussion
3.1. Structure Analysis of Protein in LWE
3.1.1. Intrinsic Fluorescence Spectroscopy
3.1.2. Surface Hydrophobicity
3.1.3. Free Sulfhydryl
3.2. Physicochemical Properties of LWE
3.2.1. Particle Size
3.2.2. Turbidity
3.2.3. Solubility
3.3. Processing Properties of LWE
3.3.1. Foaming Properties
3.3.2. Emulsifying Properties
3.3.3. Gelling Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Necidová, L.; Bursová, Š.; Ježek, F.; Harustiakova, D.; Vorlová, L.; Golian, J. Effect of preservatives on the shelf-life and sensory characteristics of pasteurized liquid whole egg stored at 4 °C. Poult. Sci. 2019, 98, 5940–5948. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Casiraghi, E.; Primavesi, L.; Pompei, C.; Hidalgo, A. Functional properties of pasteurised liquid whole egg products as affected by the hygienic quality of the raw eggs. LWT-Food Sci. Technol. 2010, 43, 436–441. [Google Scholar] [CrossRef]
- Lechevalier, V.; Guérin-Dubiard, C.; Anton, M.; Beaumal, V.; Briand, E.D.; Gillard, A.; Le Gouar, Y.; Musikaphun, N.; Tanguy, G.; Pasco, M.; et al. Pasteurisation of liquid whole egg: Optimal heat treatments in relation to its functional, nutritional and allergenic properties. J. Food Eng. 2017, 195, 137–149. [Google Scholar] [CrossRef]
- Patrignani, F.; Lanciotti, R. Applications of High and Ultra High Pressure Homogenization for Food Safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillard, R.; Marciniak, A.; Brisson, G.; Perreault, V.; House, J.D.; Pouliot, Y.; Doyen, A. Impact of Ultra-High Pressure Homogenization on the Structural Properties of Egg Yolk Granule. Foods 2022, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Feng, X.; Yang, Y.; Chen, Y.; Wang, J.; Wei, S.; Li, S. Effects of high-speed shear homogenization on properties and structure of the chicken myofibrillar protein and low-fat mixed gel. LWT 2019, 110, 19–24. [Google Scholar] [CrossRef]
- Ma, L.; Li, A.; Li, T.; Li, M.; Wang, X.; Hussain, M.A.; Qayum, A.; Jiang, Z.; Hou, J. Structure and characterization of laccase-crosslinked α-lactalbumin: Impacts of high pressure homogenization pretreatment. LWT 2020, 118, 108843. [Google Scholar] [CrossRef]
- Wu, F.; Shi, X.; Zou, H.; Zhang, T.; Dong, X.; Zhu, R.; Yu, C. Effects of high-pressure homogenization on physicochemical, rheological and emulsifying properties of myofibrillar protein. J. Food Eng. 2019, 263, 272–279. [Google Scholar] [CrossRef]
- Saricaoglu, F.T. Application of high-pressure homogenization (HPH) to modify functional, structural and rheological properties of lentil (Lens culinaris) proteins. Int. J. Biol. Macromol. 2020, 144, 760–769. [Google Scholar] [CrossRef]
- Babaei, J.; Khodaiyan, F.; Mohammadian, M. Effects of enriching with gellan gum on the structural, functional, and degradation properties of egg white heat-induced hydrogels. Int. J. Biol. Macromol. 2019, 128, 94–100. [Google Scholar] [CrossRef]
- Sisak, C.; Csanádi, Z.; Rónay, E.; Szajáni, B. Elimination of glucose in egg white using immobilized glucose oxidase. Enzym. Microb. Technol. 2006, 39, 1002–1007. [Google Scholar] [CrossRef]
- Li, R.; Hettiarachchy, N.; Rayaprolu, S.; Davis, M.; Eswaranandam, S.; Jha, A.; Chen, P. Improved functional properties of glycosylated soy protein isolate using D-glucose and xanthan gum. J. Food Sci. Technol. 2015, 52, 6067–6072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasiri, R.; Bahrami, H.; Zahedi, M.; Moosavi-Movahedi, A.A.; Sattarahmady, N. A theoretical elucidation of glucose interaction with HSA’s domains. J. Biomol. Struct. Dyn. 2010, 28, 211–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, D.; Pawar, N.; Gupta, A.N. Glucose-induced structural changes and anomalous diffusion of elastin. Colloids Surf. B Biointerfaces 2020, 188, 110776. [Google Scholar] [CrossRef]
- Martínez-Padilla, L.P.; García-Rivera, J.L.; Romero-Arreola, V.; Casas-Alencáster, N.B. Effects of xanthan gum rheology on the foaming properties of whey protein concentrate. J. Food Eng. 2015, 156, 22–30. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Shen, J.; Wang, Y.; Reaney, M.J.T. Whey protein isolate and flaxseed (Linum usitatissimum L.) gum electrostatic coacervates: Turbidity and rheology. Food Hydrocoll. 2017, 64, 18–27. [Google Scholar] [CrossRef]
- Maghamian, N.; Goli, M.; Najarian, A. Ultrasound-assisted preparation of double nano-emulsions loaded with glycyrrhizic acid in the internal aqueous phase and skim milk as the external aqueous phase. LWT 2021, 141, 110850. [Google Scholar] [CrossRef]
- Steiner-Browne, M.; Elcoroaristizabal, S.; Ryder, A.G. Using polarized Total Synchronous Fluorescence Spectroscopy (pTSFS) with PARAFAC analysis for characterizing intrinsic protein emission. Chemom. Intell. Lab. Syst. 2019, 194, 103871. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Chang, C.; Wang, C.; Li, X.; Su, Y.; Yang, Y. Effect of egg yolk on the textural, rheology and structural properties of egg gels. J. Food Eng. 2019, 246, 1–6. [Google Scholar] [CrossRef]
- Sheng, L.; Ye, S.; Han, K.; Zhu, G.; Ma, M.; Cai, Z. Consequences of phosphorylation on the structural and foaming properties of ovalbumin under wet-heating conditions. Food Hydrocoll. 2019, 91, 166–173. [Google Scholar] [CrossRef]
- Gouda, M.; Zu, L.; Ma, S.; Sheng, L.; Ma, M. Influence of bio-active terpenes on the characteristics and functional properties of egg yolk. Food Hydrocoll. 2018, 80, 222–230. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Milani, E.; Varidi, M. Extruded soy protein as a novel emulsifier: Structure, interfacial activity and emulsifying property. Food Hydrocoll. 2019, 93, 361–373. [Google Scholar] [CrossRef]
- Cheng, Y.; Donkor, P.O.; Ren, X.; Wu, J.; Agyemang, K.; Ayim, I.; Ma, H. Effect of ultrasound pretreatment with mono-frequency and simultaneous dual frequency on the mechanical properties and microstructure of whey protein emulsion gels. Food Hydrocoll. 2019, 89, 434–442. [Google Scholar] [CrossRef]
- Khemakhem, M.; Attia, H.; Ayadi, M.A. The effect of pH, sucrose, salt and hydrocolloid gums on the gelling properties and water holding capacity of egg white gel. Food Hydrocoll. 2019, 87, 11–19. [Google Scholar] [CrossRef]
- Hata, H.; Nishiyama, M.; Kitao, A. Molecular dynamics simulation of proteins under high pressure: Structure, function and thermodynamics. Biochim. Biophys. Acta-Gen. Subj. 2020, 1864, 129395. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Cai, Y.; Liu, T.; Long, Z.; Huang, L.; Deng, X.; Zhao, Q.; Zhao, M. Effects of pretreatments on the structure and functional properties of okara protein. Food Hydrocoll. 2019, 90, 394–402. [Google Scholar] [CrossRef]
- Xi, C.; Kang, N.; Zhao, C.; Liu, Y.; Sun, Z.; Zhang, T. Effects of pH and different sugars on the structures and emulsification properties of whey protein isolate-sugar conjugates. Food Biosci. 2020, 33, 100507. [Google Scholar] [CrossRef]
- Yang, X.; Su, Y.; Li, L. Study of soybean gel induced by Lactobacillus plantarum: Protein structure and intermolecular interaction. LWT 2020, 119, 108794. [Google Scholar] [CrossRef]
- Ai, M.; Xiao, N.; Jiang, A. Molecular structural modification of duck egg white protein conjugates with monosaccharides for improving emulsifying capacity. Food Hydrocoll. 2021, 111, 106271. [Google Scholar] [CrossRef]
- Chen, G.; Wang, S.; Feng, B.; Jiang, B.; Miao, M. Interaction between soybean protein and tea polyphenols under high pressure. Food Chem. 2019, 277, 632–638. [Google Scholar] [CrossRef]
- Barros, A.E.B.; Carvalho, F.A.O.; Alves, F.R.; Carvalho, J.W.P.; Tabak, M. Denaturant effects on HbGp hemoglobin as monitored by 8-anilino-1-naphtalene-sulfonic acid (ANS) probe. Int. J. Biol. Macromol. 2015, 74, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Yan, L.; Huang, G.; Dong, Z. Effect of oxidization and chitosan on the surface activity of soy protein isolate. Carbohydr. Polym. 2016, 151, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wu, F.; Cha, Y.; Zou, H.; Bao, J.; Xu, R.; Du, M. Effects of high-pressure homogenization on functional properties and structure of mussel (Mytilus edulis) myofibrillar proteins. Int. J. Biol. Macromol. 2018, 118, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Y.; Wang, X.-X.; Ma, F.; Xu, B.-C.; Li, P.-J.; Chen, C.-G. Origin of high-pressure induced changes in the properties of reduced-sodium chicken myofibrillar protein gels containing CaCl2: Physicochemical and molecular modification perspectives. Food Chem. 2020, 319, 126535. [Google Scholar] [CrossRef]
- Báez, G.D.; Busti, P.A.; Verdini, R.; Delorenzi, N.J. Glycation of heat-treated β-lactoglobulin: Effects on foaming properties. Food Res. Int. 2013, 54, 902–909. [Google Scholar] [CrossRef]
- Jiang, W.; He, X.; Yang, H.; Xiang, X.; Hu, S.; Li, S.; Liu, Y. Histamine reduction by Maillard reaction with glucose. Food Control 2017, 82, 136–144. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, Y.L.; Xu, X. High-pressure homogenization combined with sulfhydryl blockage by hydrogen peroxide enhance the thermal stability of chicken breast myofibrillar protein aqueous solution. Food Chem. 2019, 285, 31–38. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakano, M.; Kato, S.; Yoshihara, D.; Ookawara, T.; Eguchi, H.; Taniguchi, N.; Suzuki, K. Oxidative modification to cysteine sulfonic acid of Cys111 in human copper-zinc superoxide dismutase. J. Biol. Chem. 2007, 282, 35933–35944. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Wang, J.; Wu, D.; Xu, X.; Wu, C.; Du, M. Physicochemical properties and oil/water interfacial adsorption behavior of cod proteins as affected by high-pressure homogenization. Food Hydrocoll. 2020, 100, 105429. [Google Scholar] [CrossRef]
- Primozic, M.; Duchek, A.; Nickerson, M.; Ghosh, S. Formation, stability and in vitro digestibility of nanoemulsions stabilized by high-pressure homogenized lentil proteins isolate. Food Hydrocoll. 2018, 77, 126–141. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, S.; Wang, Y.; Zhou, G.; Wang, L.; Wang, X.; Xu, N. Effect of different homogenization pressure on soy protein isolate-vitamin D3 complex. Process Biochem. 2019, 87, 145–150. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Xue, S.; Li, M.; Xu, X.; Han, M.; Zhou, G. Effect of the disruption chamber geometry on the physicochemical and structural properties of water-soluble myofibrillar proteins prepared by high pressure homogenization (HPH). LWT 2019, 105, 215–223. [Google Scholar] [CrossRef]
- Ye, R.; Harte, F. High pressure homogenization to improve the stability of casein—Hydroxypropyl cellulose aqueous systems. Food Hydrocoll. 2014, 35, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Guzey, D.; McClements, D.J.; Weiss, J. Adsorption kinetics of BSA at air–sugar solution interfaces as affected by sugar type and concentration. Food Res. Int. 2003, 36, 649–660. [Google Scholar] [CrossRef]
- Herceg, Z.; Režek, A.; Lelas, V.; Krešić, G.; Franetović, M. Effect of carbohydrates on the emulsifying, foaming and freezing properties of whey protein suspensions. J. Food Eng. 2007, 79, 279–286. [Google Scholar] [CrossRef]
- Barać, M.B.; Pešić, M.B.; Stanojević, S.P.; Kostić, A.Ž.; Čabrilo, S.B. Techno-functional properties of pea (Pisum sativum) protein isolates: A review. Acta Period. Technol. 2015, 46, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Nasabi, M.; Labbafi, M.; Mousavi, M.E.; Madadlou, A. Effect of salts and nonionic surfactants on thermal characteristics of egg white proteins. Int. J. Biol. Macromol. 2017, 102, 970–976. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. LWT-Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Q.; Tang, C.; Luo, J.; Wu, X.; Lu, L.; Cai, Z. Study on structural, rheological and foaming properties of ovalbumin by ultrasound-assisted glycation with xylose. Ultrason. Sonochem. 2019, 58, 104644. [Google Scholar] [CrossRef]
- Shi, X.; Zou, H.; Sun, S.; Lu, Z.; Zhang, T.; Gao, J.; Yu, C. Application of high-pressure homogenization for improving the physicochemical, functional and rheological properties of myofibrillar protein. Int. J. Biol. Macromol. 2019, 138, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Narsimhan, G. Evolution of liquid holdup profile in a standing protein stabilized foam. J. Colloid Interface Sci. 2004, 280, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Chang, C.; Wang, C.; Zhang, M.; Su, Y.; Yang, Y. Foaming characterization of fresh egg white proteins as a function of different proportions of egg yolk fractions. Food Hydrocoll. 2019, 90, 118–125. [Google Scholar] [CrossRef]
- Raikos, V.; Campbell, L.; Euston, S.R. Rheology and texture of hen’s egg protein heat-set gels as affected by pH and the addition of sugar and/or salt. Food Hydrocoll. 2007, 21, 237–244. [Google Scholar] [CrossRef]
- Asghari, A.K.; Norton, I.; Mills, T.; Sadd, P.; Spyropoulos, F. Interfacial and foaming characterisation of mixed protein-starch particle systems for food-foam applications. Food Hydrocoll. 2016, 53, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hu, X.; Ye, Y.; Wang, M.; Wang, J. Emulsifying properties of wheat germ:Influence of pH and NaCl. Food Hydrocoll. 2020, 100, 105431. [Google Scholar] [CrossRef]
- Liang, H.-N.; Tang, C.-H. pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef]
- Marco-Molés, R.; Hernando, I.; Llorca, E.; Pérez-Munuera, I. Influence of high pressure homogenization (HPH) on the structural stability of an egg/dairy emulsion. J. Food Eng. 2012, 109, 652–658. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Ferrari, M.; Handgraaf, J.-W.; Boccardo, G.; Buffo, A.; Vanni, M.; Marchisio, D.L. Molecular modeling of the interface of an egg yolk protein-based emulsion. Phys. Fluids 2022, 34, 021903. [Google Scholar] [CrossRef]
- Jourdain, L.; Leser, M.E.; Schmitt, C.; Michel, M.; Dickinson, E. Stability of emulsions containing sodium caseinate and dextran sulfate: Relationship to complexation in solution. Food Hydrocoll. 2008, 22, 647–659. [Google Scholar] [CrossRef]
- Wooster, T.J.; Augustin, M.A. β-lactoglobulin-dextran Maillard conjugates: Their effect on interfacial thickness and emulsion stability. J Colloid Interface Sci 2006, 303, 564–572. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, Q. Glycation of whey protein to provide steric hindrance against thermal aggregation. J. Agric. Food Chem. 2012, 60, 9754–9762. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Rousseau, D. Stabilization of acidic soy protein-based dispersions and emulsions by soy soluble polysaccharides. Food Hydrocoll. 2013, 30, 382–392. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, S.; Ye, H.; Luo, W.; Huang, Q.; Geng, F. Ovomucin may be the key protein involved in the early formation of egg-white thermal gel. Food Chem. 2022, 366, 130596. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, F.; Yang, Y.; Xiong, C.; Xu, M.; Tu, Y. Gelation behavior of egg yolk under physical and chemical induction: A review. Food Chem. 2021, 355, 129569. [Google Scholar] [CrossRef]
- Dou, H.; Magnusson, E.; Choi, J.; Duan, F.; Nilsson, L.; Lee, S. Study on aggregation behavior of low density lipoprotein in hen egg yolk plasma by asymmetrical flow field-flow fractionation coupled with multiple detectors. Food Chem. 2016, 192, 228–234. [Google Scholar] [CrossRef]
- Kalkani, A.; Paraskevopoulou, A.; Kiosseoglou, V. Protein interactions and filler effects in heat-set gels based on egg. Food Hydrocoll. 2007, 21, 191–197. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Su, Y.; Chang, C.; Li, X.; Yang, Y.; Gu, L. Preparation and characterization of hen egg proteins-soybean protein isolate composite gels. Food Hydrocoll. 2019, 97, 105191. [Google Scholar] [CrossRef]
- Semenova, M.G.; Antipova, A.S.; Belyakova, L.E. Food protein interactions in sugar solutions. Curr. Opin. Colloid Interface Sci. 2002, 7, 438–444. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Wu, Y.; Chen, H.; Gao, J.; Tong, P. Effects of Glucose and Homogenization Treatment on the Quality of Liquid Whole Eggs. Foods 2022, 11, 2521. https://doi.org/10.3390/foods11162521
Hu W, Wu Y, Chen H, Gao J, Tong P. Effects of Glucose and Homogenization Treatment on the Quality of Liquid Whole Eggs. Foods. 2022; 11(16):2521. https://doi.org/10.3390/foods11162521
Chicago/Turabian StyleHu, Wei, Yong Wu, Hongbing Chen, Jinyan Gao, and Ping Tong. 2022. "Effects of Glucose and Homogenization Treatment on the Quality of Liquid Whole Eggs" Foods 11, no. 16: 2521. https://doi.org/10.3390/foods11162521