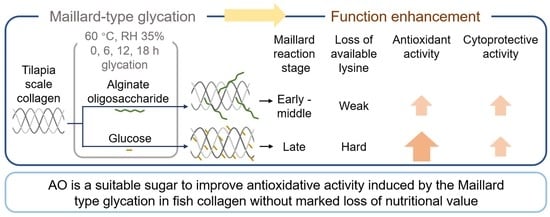
Comparison of Maillard-Type Glycated Collagen with Alginate Oligosaccharide and Glucose: Its Characterization, Antioxidant Activity, and Cytoprotective Activity on H2O2-Induced Cell Oxidative Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glycation of Collagen
2.3. Measurement of UV-Vis Absorbance and Browning Intensity
2.4. Measurement of Available Lysine Content
2.5. Measurement of Sugars Bound to Collagen
2.6. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Simulated Gastrointestinal Digestion In Vitro
2.8. Analysis of Molecular Mass Distribution
2.9. Measurement of Antioxidant Activity
2.9.1. DPPH Radical Scavenging Assay
2.9.2. ABTS Radical Scavenging Assay
2.10. Cell Culture
2.11. Measurement of Cell Viability
2.12. Cytoprotective Effect of Glycated Collagens on H2O2-Induced Oxidative Damage
2.13. Measurement of SOD, CAT, and MDA in H2O2-Induced Raw 264.7 Cells
2.14. Statistical Analysis
3. Results and Discussion
3.1. The Introduction of Reducing Sugars to Collagen through the Maillard Reaction
3.2. The Molecular Mass Distribution of Collagen-Sugar Conjugates and Their Digested Peptides
3.3. Antioxidant Activity of Glycated Collagen
3.4. Comparison of Available Lysine Loss of Antioxidative Collagen between AO- and Glucose-Glycation
3.5. Cytoprotective Activity of Glycated Collagens on H2O2-Induced Cell Oxidative Damage
3.6. Effect of Glycated Collagens on MDA, SOD, and CAT Levels in H2O2-Damaged Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and sealth. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Saeki, H. Protein–saccharide interaction. In Food Proteins and Peptides: Chemistry, Functionality, Interactions, and Commercialization; Hettiarachchy, N.S., Sato, K., Marshall, M.R., Kannan, A., Eds.; CRC Press: New York, NY, USA, 2012; pp. 230–261. ISBN 9781138199002. [Google Scholar]
- Nooshkam, M.; Varidi, M.; Verma, D.K. Functional and biological properties of Maillard conjugates and their potential application in medical and food: A review. Food Res. Int. 2020, 131, 109003. [Google Scholar] [CrossRef]
- Jing, H.; Kitts, D.D. Chemical and biochemical properties of casein-sugar Maillard reaction products. Food Chem. Toxicol. 2002, 40, 1007–1015. [Google Scholar] [CrossRef]
- Jia, C.; Cao, D.; Ji, S.; Lin, W.; Zhang, X.; Muhoza, B. Whey protein isolate conjugated with xylo-oligosaccharides via Maillard reaction: Characterization, antioxidant capacity, and application for lycopene microencapsulation. LWT 2020, 118, 108837. [Google Scholar] [CrossRef]
- Han, M.M.; Yi, Y.; Wang, H.X.; Huang, F. Investigation of the Maillard reaction between polysaccharides and proteins from longan pulp and the improvement in activities. Molecules 2017, 22, 938. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Qi, C.; Yu, W.; Han, X.; Jiarui, Z.; Qing, Z.; Aiguo, J.; Shuliang, S. Advances in research on the bioactivity of alginate oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [Green Version]
- Maitena, U.; Katayama, S.; Sato, R.; Saeki, H. Improved solubility and stability of carp myosin by conjugation with alginate oligosaccharide. Fish. Sci. 2004, 70, 896–902. [Google Scholar] [CrossRef]
- Takeda, H.; Iida, T.; Okada, A.; Ootsuka, H.; Ohshita, T.; Masutani, E.; Katayama, S.; Saeki, H. Feasibility study on water solubilization of spawned out salmon meat by conjugation with alginate oligosaccharide. Fish. Sci. 2007, 73, 924–930. [Google Scholar] [CrossRef]
- Sato, R.; Sawabe, T.; Kishimura, H.; Hayashi, K.; Saeki, H. Preparation of neoglycoprotein from carp myofibrillar protein and alginate oligosaccharide: Improved solubility in low ionic strength medium. J. Agric. Food Chem. 2000, 48, 17–21. [Google Scholar] [CrossRef]
- Sato, R.; Katayama, S.; Sawabe, T.; Saeki, H. Stability and emulsion-forming ability of water-soluble fish myofibrillar protein prepared by conjugation with alginate oligosaccharide. J. Agric. Food Chem. 2003, 51, 4376–4381. [Google Scholar] [CrossRef]
- Sato, R.; Sawabe, T.; Saeki, H. Characterization of fish myofibrillar protein by conjugation with alginate oligosaccharide prepared using genetic recombinant alginate lyase. J. Food Sci. 2005, 70, 58–62. [Google Scholar] [CrossRef]
- Hattori, M.; Ogino, A.; Nakai, H.; Takahashi, K. Functional improvement of β-lactoglobulin by conjugating with alginate lyase-lysate. J. Agric. Food Chem. 1997, 45, 703–708. [Google Scholar] [CrossRef]
- Nishizawa, M.; Saigusa, M.; Saeki, H. Conjugation with alginate oligosaccharide via the controlled Maillard reaction in a dry state is an effective method for the preparation of salmon myofibrillar protein with excellent anti-inflammatory activity. Fish. Sci. 2016, 82, 357–367. [Google Scholar] [CrossRef]
- Saigusa, M.; Nishizawa, M.; Shimizu, Y.; Saeki, H. In vitro and in vivo anti-inflammatory activity of digested peptides Dderived from salmon myofibrillar protein conjugated with a small quantity of alginate oligosaccharide. Biosci. Biotechnol. Biochem. 2015, 79, 1518–1527. [Google Scholar] [CrossRef]
- Sugihara, K.; Nishizawa-Higashi, M.; Joe, G.H.; Onishi, Y.; Shimizu, Y.; Saeki, H. Improvement of anti-inflammatory activity of salmon muscle peptides by conjugation with alginate oligosaccharide and recovery of the active fraction using ampholyte-free isoelectric focusing. Fish. Sci. 2021, 87, 569–577. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.F.M. Tilapia Culture, 2nd ed.; El-Sayed, A.F.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128165416. [Google Scholar]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine collagen peptides from the skin of nile tilapia (Oreochromis Niloticus): Characterization and wound healing evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, Y.; Yang, Z.; Jin, H. Physicochemical properties of collagen from Acaudina molpadioides and its protective effects against H2O2-induced injury in RAW264.7 cells. Mar. Drugs 2020, 18, 370. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of collagen from skin, scale and bone of carp (Cyprinus Carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Huang, Z.; Jia, S.; Zhang, Y.; Shi, J.; Hong, H.; Feng, L.; Luo, Y. Modification of gelatin hydrolysates from grass carp (Ctenopharyngodon Idellus) scales by Maillard reaction: Antioxidant activity and volatile compounds. Food Chem. 2019, 295, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, Q.; Hong, H.; Feng, L.; Liu, J.; Luo, Y. Physicochemical and functional properties of Maillard reaction products derived from cod (Gadus Morhua L.) skin collagen peptides and xylose. Food Chem. 2020, 333, 127489. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, M.; Hu, J.; Zeng, S.; Bai, X. Correspondence analysis of antioxidant activity and UV-vis absorbance of Maillard reaction products as related to reactants. LWT-Food Sci. Technol. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Chen, X.M.; Kitts, D.D. Correlating changes that occur in chemical properties with the generation of antioxidant capacity in different sugar-amino acid Maillard reaction models. J. Food Sci. 2011, 76, C831–C837. [Google Scholar] [CrossRef]
- Lertittikul, W.; Benjakul, S.; Tanaka, M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein-glucose model system as influenced by pH. Food Chem. 2007, 100, 669–677. [Google Scholar] [CrossRef]
- Ajandouz, E.H.; Tchiakpe, L.S.; Dalle Ore, F.; Benajiba, A.; Puigserver, A. Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Hernandez, M.J.M.; Alvarez-Coque, M.C.G. Available lysine in protein, assay using O-phthalaldehyde/ N-Acetyl-L-cysteine spectrophotometric method. J. Food Sci. 1992, 57, 503–505. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sajithlal, G.B.; Chithra, P.; Chandrakasan, G. An in vitro study on the role of metal catalyzed oxidation in glycation and crosslinking of collagen. Mol. Cell. Biochem. 1999, 194, 257–263. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kobayashi, T.; Meng, D.W.; Miyamoto, N.; Tsutsumi, N.; Ura, K.; Takagi, Y. Free radical scavenging activity of type II collagen peptides and chondroitin sulfate oligosaccharides from by-products of mottled skate processing. Food Biosci. 2021, 41, 100991. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, N.; Zhang, Z.; Jing, P. Effects of dietary cyanidin-3-diglucoside-5-glucoside complexes with rutin/Mg(II) against H2O2-induced cellular oxidative stress. Food Res. Int. 2019, 126, 108591. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis Niloticus). LWT-Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Q.; Zhang, J.; Zhao, P.; Ding, Y. Characterization of silver carp (Hypophthalmichthys Molitrix) myosin protein glycated with konjac oligo-glucomannan. Food Hydrocoll. 2016, 57, 114–121. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2011, 11, 364–373. [Google Scholar] [CrossRef]
- ALjahdali, N.; Carbonero, F. Impact of Maillard reaction products on nutrition and health: Current knowledge and need to understand their fate in the human digestive system. Crit. Rev. Food Sci. Nutr. 2019, 59, 474–487. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanism of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef] [Green Version]
- Mehta, B.M.; Deeth, H.C. Blocked lysine in dairy products: Formation, occurrence, analysis, and nutritional implications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 206–218. [Google Scholar] [CrossRef]
- Bohlender, J.M.; Franke, S.; Stein, G.; Wolf, G. Advanced glycation end products and the kidney. Am. J. Physiol.-Ren. Physiol. 2005, 289, F645–F659. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.S.; Liu, L.J.; OuYang, X.K.; Qu, Y.L.; Chen, Y.; Ding, G.F. Protective effect of polysaccharides from Sargassum horneri against oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2014, 68, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhao, Y.Q.; Zhao, G.X.; Chi, C.F.; Wang, B. Antioxidant peptides from collagen hydrolysate of redlip croaker (Pseudosciaena Polyactis) scales: Preparation, characterization, and cytoprotective effects on H2O2-damaged HepG2 cells. Mar. Drugs 2020, 18, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Xu, B.; Yuan, F.; Gong, J.; Yang, Z. Collagen peptides from swim bladders of giant croaker (Nibea japonica) and their protective effects against H2O2-induced oxidative damage toward human umbilical vein endothelial cells. Mar. Drugs 2020, 18, 430. [Google Scholar] [CrossRef] [PubMed]

 ), 10–5 kDa (
), 10–5 kDa (  ), 5–1 kDa (
), 5–1 kDa (  ), and <1 kDa (
), and <1 kDa (  ).
).
 ), 10–5 kDa (
), 10–5 kDa (  ), 5–1 kDa (
), 5–1 kDa (  ), and <1 kDa (
), and <1 kDa (  ).
).
 ), C-Glu (
), C-Glu (  ), C-Sor (
), C-Sor (  ). Different capital letters A–C indicate significant differences among the three collagen groups at the same reaction time. Different lowercase letters a–d indicate significant differences among the different reaction times in the same collagen group.
). Different capital letters A–C indicate significant differences among the three collagen groups at the same reaction time. Different lowercase letters a–d indicate significant differences among the different reaction times in the same collagen group.
 ), C-Glu (
), C-Glu (  ), C-Sor (
), C-Sor (  ). Different capital letters A–C indicate significant differences among the three collagen groups at the same reaction time. Different lowercase letters a–d indicate significant differences among the different reaction times in the same collagen group.
). Different capital letters A–C indicate significant differences among the three collagen groups at the same reaction time. Different lowercase letters a–d indicate significant differences among the different reaction times in the same collagen group.



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Joe, G.-H.; Li, W.; Shimizu, Y.; Saeki, H. Comparison of Maillard-Type Glycated Collagen with Alginate Oligosaccharide and Glucose: Its Characterization, Antioxidant Activity, and Cytoprotective Activity on H2O2-Induced Cell Oxidative Damage. Foods 2022, 11, 2374. https://doi.org/10.3390/foods11152374
Yang B, Joe G-H, Li W, Shimizu Y, Saeki H. Comparison of Maillard-Type Glycated Collagen with Alginate Oligosaccharide and Glucose: Its Characterization, Antioxidant Activity, and Cytoprotective Activity on H2O2-Induced Cell Oxidative Damage. Foods. 2022; 11(15):2374. https://doi.org/10.3390/foods11152374
Chicago/Turabian StyleYang, Boxue, Ga-Hyun Joe, Wenzhao Li, Yutaka Shimizu, and Hiroki Saeki. 2022. "Comparison of Maillard-Type Glycated Collagen with Alginate Oligosaccharide and Glucose: Its Characterization, Antioxidant Activity, and Cytoprotective Activity on H2O2-Induced Cell Oxidative Damage" Foods 11, no. 15: 2374. https://doi.org/10.3390/foods11152374