Change Regularity of Taste and the Performance of Endogenous Proteases in Shrimp (Penaens vannamei) Head during Autolysis
Abstract
:1. Introduction
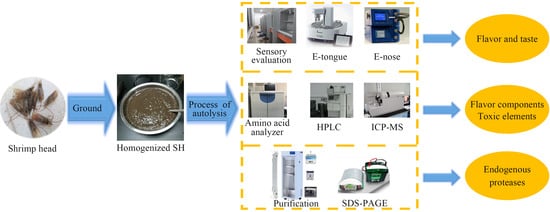
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Determination of Pb, Cd, Hg, As, and Proximate Composition
2.3. Autolysis of SH
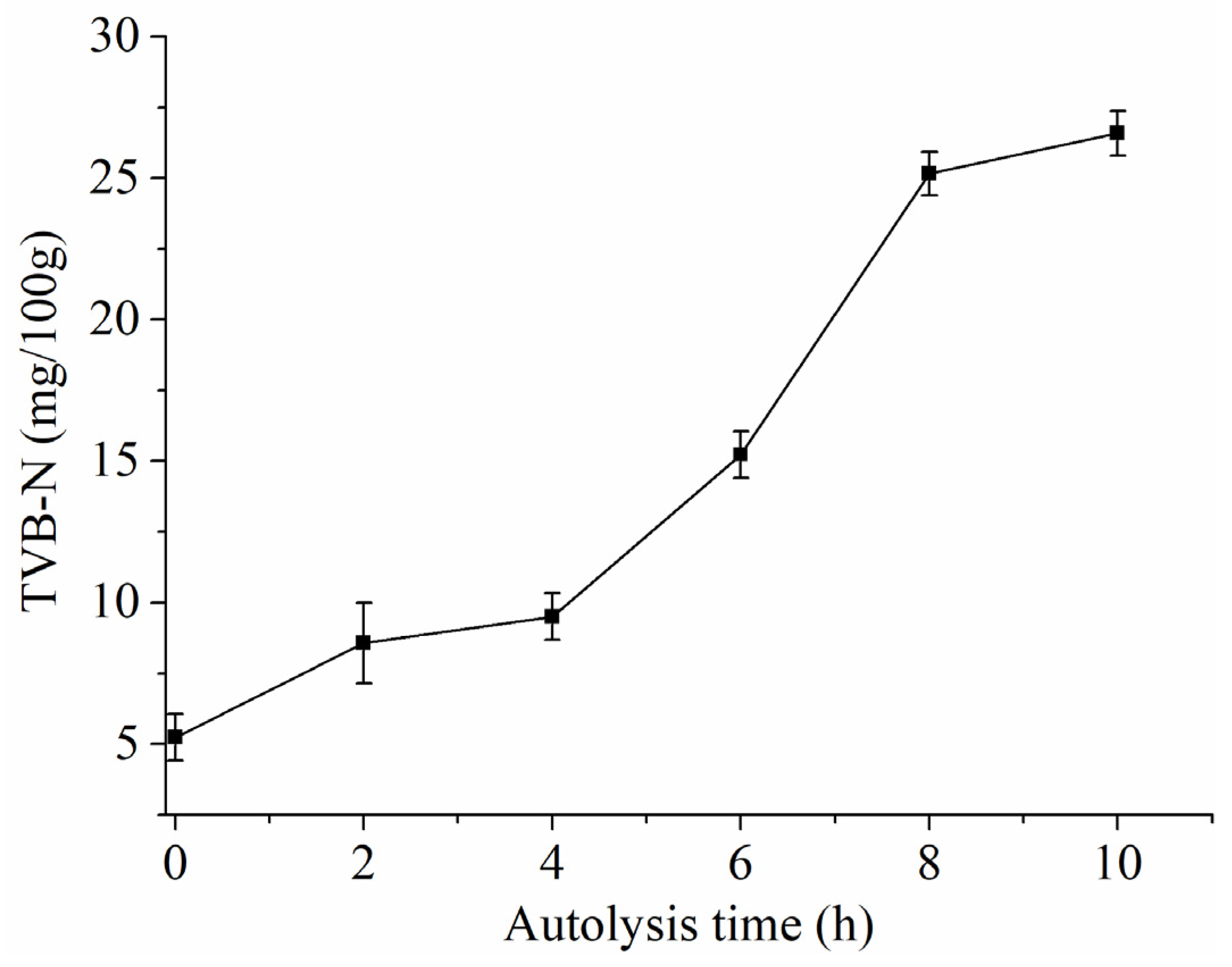
2.4. Determination of TVB-N
2.5. Determination of AAs
2.6. Determination of Nucleotide-Related Compounds
2.7. Sensory Evaluation
2.8. E-tongue Analysis
2.9. E-nose analysis
2.10. Determination of pH Value
2.11. Determination of Endogenous Enzyme Activity
2.12. Separation of Enzyme
2.13. SDS–PAGE
2.14. Statistical Analysis
3. Results
3.1. Food Safety and Proximate Composition of SH
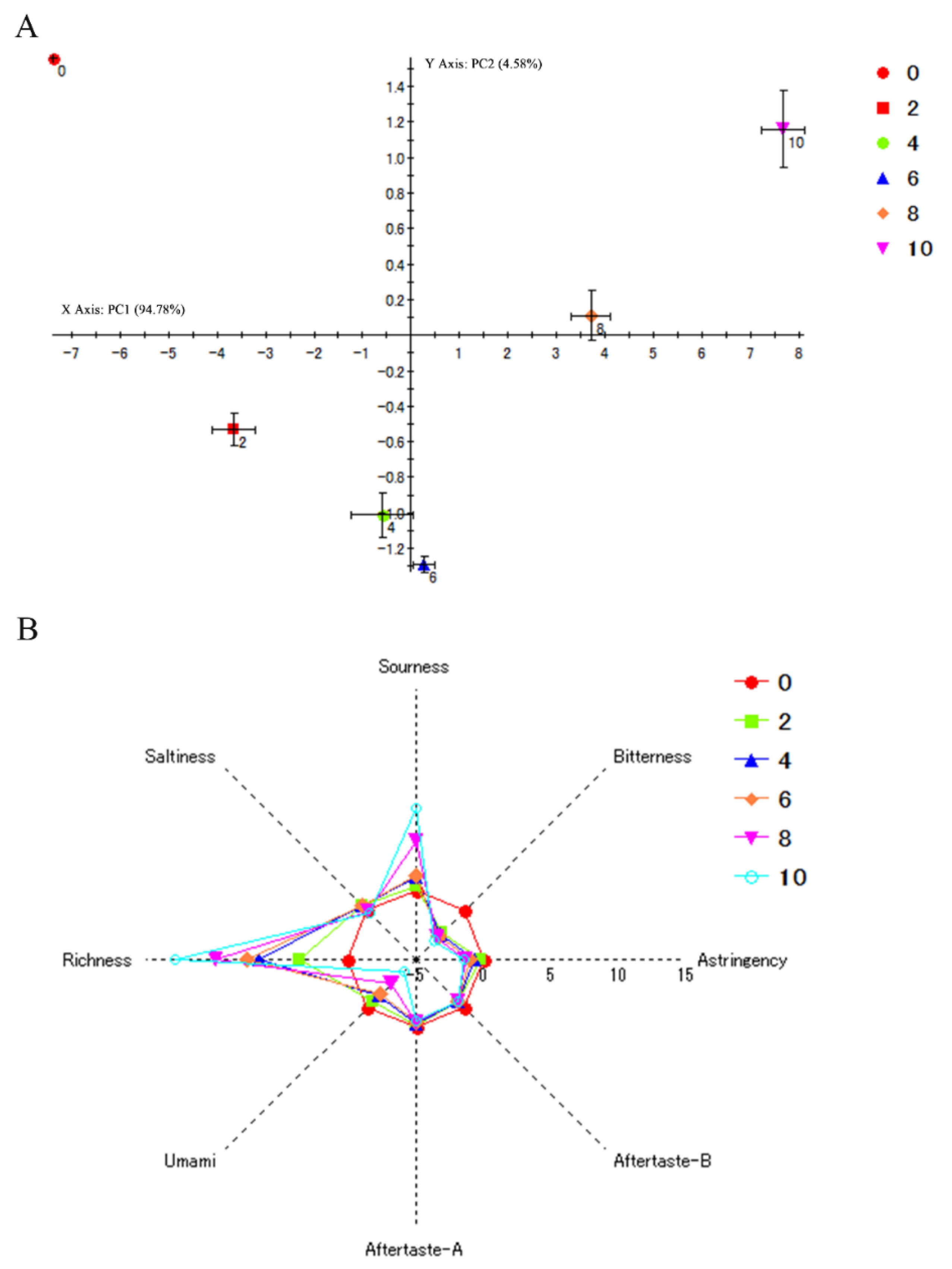
3.2. Change Regularity of Taste in SH during Autolysis
3.3. E-Tongue and E-Nose Response Signal of SH during Autolysis
3.4. Change Regularity of pH and Enzymatic Activity in SH during Autolysis
3.5. Characteristics of Endogenous Protease of SH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cui, H.; Xiao, L. Current situation of China shrimp industry in 2011–2012 and its prospect. China Fish. 2012, 39, 85–87. [Google Scholar]
- Cao, W.; Zhang, C.; Hong, P.; Ji, H.; Hao, J.; Zhang, J. Autolysis of shrimp head by gradual temperature and nutritional quality of the resulting hydrolysate. LWT 2009, 42, 244–249. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, C.; Hong, P.; Ji, H. Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 2008, 109, 176–183. [Google Scholar] [CrossRef]
- Cao, W.; Tan, C.; Zhan, X.; Li, H.; Zhang, C. Ultraviolet irradiation and gradient temperature assisted autolysis for protein re-covery from shrimp head waste. Food Chem. 2014, 164, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.P.; Meireles, M.; Ângela, A.; Lopes, B.L.F.; Cabral, F.A. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Food Eng. 2011, 102, 87–93. [Google Scholar] [CrossRef]
- Fanimo, A.; Oduguwa, O.; Onifade, A.; Olutunde, T. Protein quality of shrimp-waste meal. Bioresour. Technol. 2000, 72, 185–188. [Google Scholar] [CrossRef]
- Carlos Sainz, J.; García-Carreño, F.L.; Hernández-Cortés, P. Penaeus vannamei isotrypsins: Purification and characterization. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 138, 155–162. [Google Scholar] [CrossRef]
- Sriket, C.; Benjakul, S.; Visessanguan, W. Characterisation of proteolytic enzymes from muscle and hepatopancreas of fresh water prawn (Macrobrachium rosenbergii). J. Sci. Food Agric. 2010, 91, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties de-termination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar]
- Bekhit, A.E.-D.A.; Holman, B.W.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Chan, S.T.; Yao, M.W.Y.; Wong, Y.C.; Wong, T.; Mok, C.S.; Sin, D.W.M. Evaluation of chemical indicators for monitoring freshness of food and determination of volatile amines in fish by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Eur. Food Res. Technol. 2006, 224, 67–74. [Google Scholar] [CrossRef]
- Albuquerque, F.E.A.; Minervino, A.H.H.; Miranda, M.; Herrero-Latorre, C.; Barrêto Júnior, R.A.; Oliveira, F.L.C.; Dias, S.R.; Ortolani, E.L.; López-Alonso, M. Toxic and es-sential trace element concentrations in the freshwater shrimp Macrobrachium amazonicum in the Lower Amazon, Brazil. J. Food Compos. Anal. 2020, 86, 103361. [Google Scholar] [CrossRef]
- The Hygiene Ministry of China. Determination of volatile base nitrogen in foodstuffs. In National Food Safety Standard; The Hygiene Ministry of China: Beijing, China, 2003. [Google Scholar]
- Dabadé, D.S.; Jacxsens, L.; Miclotte, L.; Abatih, E.; Devlieghere, F.; De Meulenaer, B. Survey of multiple biogenic amines and corre-lation to microbiological quality and free amino acids in foods. Food Control 2021, 120, 107497. [Google Scholar] [CrossRef]
- Zhang, R.; Qiu, W.; Zhang, M.; Row, K.H.; Cheng, Y.; Jin, Y. Effects of different heating methods on the contents of nucleotides and related compounds in minced Pacific white shrimp and Antarctic krill. LWT 2018, 87, 142–150. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, H.; Guo, R.; Yang, B.; You, G.; Zhao, M.; Liu, X. Taste, umami-enhance effect and amino acid sequence of peptides sepa-rated from silkworm pupa hydrolysate. Food Res. Int. 2018, 108, 144–150. [Google Scholar] [CrossRef] [PubMed]
- The Standardization Administration of China. Sensory Analysis-Methodology-Method of Investigating Sensitivity of Taste, MOD; The Standardization Administration of China: Beijing, China, 2012.
- The Standardization Administration of China. Sensory Analysis-Methodology-Ranking; The Standardization Ad-ministration of China: Beijing, China, 2008.
- Zhu, W.; Luan, H.; Bu, Y.; Li, X.; Li, J.; Ji, G. Flavor characteristics of shrimp sauces with different fermentation and storage time. LWT 2019, 110, 142–151. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and growth tracking of fungi contamination in peaches using electronic nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef]
- Shi, C.; Cui, J.; Luo, Y.; Zhu, S.; Zhou, Z. Post-Mortem Changes of Silver Carp (Hypophthalmichthys Molitrix) Stored at 0 °C Assessed by Electrical Conductivity. Int. J. Food Prop. 2015, 18, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Hang, Y.J.; Li, X.Y. Properties of Autolysis Enzymes from Antarctic Krill. Food Sci. 2011, 13, 198–200. [Google Scholar]
- Cahú, T.B.; Santos, S.D.; Mendes, A.; Córdula, C.R.; Chavante, S.F.; Carvalho, L.B.; Nader, H.B.; Bezerra, R.S. Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. Process. Biochem. 2012, 47, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Beloborodov, S.S.; Bao, J.; Krylova, S.M.; Shala-Lawrence, A.; Johnson, P.E.; Krylov, S.N. Aptamer facilitated purification of func-tional proteins. J. Chromatogr. B 2018, 1073, 201–206. [Google Scholar] [CrossRef]
- Soultani, G.; Sele, V.; Rasmussen, R.R.; Pasias, I.; Stathopoulou, E.; Thomaidis, N.S.; Sinanoglou, V.J.; Sloth, J.J. Elements of toxicological concern and the arsenolipids’ profile in the giant-red Mediterranean shrimp, Aristaeomorpha foliacea. J. Food Compos. Anal. 2021, 97, 103786. [Google Scholar] [CrossRef]
- The Hygiene Ministry of China. Hygienic Standard for Fresh and Frozen Marine Products of Animal Origin; The Hygiene Ministry of China: Beijing, China, 2015.
- FAO/WHO 1973. Energy and Protein Requirements: Report of a Joint FAO/WHO Ad Hoc Expert Committee; FAO: Rome, Italy; WHO: Geneva, Switzerland, 1971. [Google Scholar]
- Köhler, R.; Kariuki, L.; Lambert, C.; Biesalski, H. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia-Pacific Èntomol. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- Cheung, I.W.; Li-Chan, E.C. Application of taste sensing system for characterisation of enzymatic hydrolysates from shrimp processing by-products. Food Chem. 2014, 145, 1076–1085. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, H.; Wu, W.; Chen, H.; Fang, X.; Han, Y.; Mu, H. Effects of fermentation with different microbial species on the umami taste of Shiitake mushroom (Lentinus edodes). LWT 2021, 141, 110889. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Liu, Y. Characterization and evaluation of umami taste: A review. TrAC Trends Anal. Chem. 2020, 127, 115876. [Google Scholar] [CrossRef]
- Sabikun, N.; Bakhsh, A.; Rahman, M.S.; Hwang, Y.-H.; Joo, S.-T. Volatile and nonvolatile taste compounds and their correlation with umami and flavor characteristics of chicken nuggets added with milkfat and potato mash. Food Chem. 2021, 343, 128499. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.-Q.; Chen, S.-S.; Xie, J.; Qu, Y.-H.; Song, X. Analysis of 10 nucleotides and related compounds in Litopenaeus vannamei during chilled storage by HPLC-DAD. LWT 2016, 67, 187–193. [Google Scholar] [CrossRef]
- Kuda, T.; Fujita, M.; Goto, H.; Yano, T. Effects of retort conditions on ATP-related compounds in pouched fish muscle. LWT 2008, 41, 469–473. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of Inosine Monophosphate and Its Degradation Products during Aging of Pork of Different Qualities in Relation to Basic Taste and Retronasal Flavor Percep-tion of the Meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste Enhancements Between Various Amino Acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- Valentová, H.; Panovská, Z. SENSORY EVALUATION | Taste. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5180–5187. [Google Scholar]
- Feng, Z.; Li, S.; Hao, W.; Yuan, J.; Liu, M.; Gao, S.; Liu, Y. Effect of pH on the taste of umami flavors. China Food Addit. 2017, 2, 144–148. [Google Scholar]
- Ma, J.; Chen, Y.; Zhu, Y.; Ayed, C.; Fan, Y.; Chen, G.; Liu, Y. Quantitative analyses of the umami characteristics of disodium succinate in aqueous solution. Food Chem. 2020, 316, 126336. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, S.; Ji, H.; Liu, S. Localization of trypsin-like protease in postmortem tissue of white shrimp (Litopenaeus van-namei) and its effect in muscle softening. Food Chem. 2019, 290, 277–285. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Lyu, J.; Kong, C.; Song, S.; Luo, Y. The impact of stunning methods on stress conditions and quality of silver carp (Hypophthalmichthys molitrix) fillets stored at 4 °C during 72 h postmortem. Food Chem. 2017, 216, 130–137. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Choo, W.S.; Dykes, G.A. Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) fresh-ly harvested and stored on ice. LWT 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Khaledian, S.; Basiri, S.; Shekarforoush, S.S. Shelf-life extension of pacific white shrimp using tragacanth gum -based coatings containing Persian lime peel (Citrus latifolia) extract. LWT 2021, 141, 110937. [Google Scholar] [CrossRef]





| Toxic Elements | Limit of ECR | Concentrations in SH |
|---|---|---|
| Pb | ≤0.5 | 0.03 |
| As | Not established | Not detected |
| Cd | ≤0.5 | 0.26 |
| Hg | ≤0.5 | 0.01 |
| Moisture Content | Crude Ash | Protein |
|---|---|---|
| 77.47 ± 0.05 | 4.47 ± 0.01 | 10.32 ± 0.09 |
| Amino Acid | Content |
|---|---|
| Glycine | 7.62 ± 0.08 |
| Alanine | 5.86 ± 0.02 |
| Histidine | 2.32 ± 0.19 |
| Tyrosine | 4.68 ± 0.55 |
| Serine | 3.95 ± 0.05 |
| Cysteine | 0.40 ± 0.05 |
| Aspartic acid | 8.23 ± 0.08 |
| Glutamic acid | 15.46 ± 0.17 |
| Arginine | 8.98 ± 0.11 |
| Proline | 6.75 ± 0.05 |
| Leucine | 6.98 ± 0.08 |
| Isoleucine | 3.77 ± 0.03 |
| Valine | 4.35 ± 0.04 |
| Threonine | 3.77 ± 0.04 |
| Methionine | 5.18 ± 0. 34 |
| Phenylalanine | 5.28 ± 0.51 |
| Lysine | 7.20 ± 0.26 |
| Total amino acids (TAAs) | 100.83 ± 1.68 |
| Essential amino acids (EAAs) | 36.55 ± 0.54 |
| Nonessential amino acids (NEAAs) | 64.27 ± 1.15 |
| Sweet and umami amino acids (SUAAs) | 56.84 ± 0.43 |
| SUAAs/TAAs | 57% |
| EAAs/TAAs | 37% |
| EAAs: NEAAs | 0.57 |
| Autolysis Time (h) | 0 | 2 | 4 | 6 | 8 | 10 |
|---|---|---|---|---|---|---|
| Sum of ranks | 39 c | 40 c | 36 b,c | 15 a | 15 a | 23 a,b |
| Purification | Enzymatic Ratio Activity (U/mg Protein) | Recovery of Enzymatic Activity (%) | Purification Fold |
|---|---|---|---|
| Crude extract | 2.93 | 100 | 1 |
| Crude endogenous protease | 4.16 | 89.13 | 1.42 |
| I | 3.22 | 15.12 | 1.10 |
| II | 6.54 | 60.88 | 2.23 |
| III | Not detected | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhao, M.; Gao, S.; Xu, Y.; Zhao, X.; Liu, M.; Liu, X. Change Regularity of Taste and the Performance of Endogenous Proteases in Shrimp (Penaens vannamei) Head during Autolysis. Foods 2021, 10, 1020. https://doi.org/10.3390/foods10051020
Wu S, Zhao M, Gao S, Xu Y, Zhao X, Liu M, Liu X. Change Regularity of Taste and the Performance of Endogenous Proteases in Shrimp (Penaens vannamei) Head during Autolysis. Foods. 2021; 10(5):1020. https://doi.org/10.3390/foods10051020
Chicago/Turabian StyleWu, Shujian, Mouming Zhao, Shijue Gao, Yue Xu, Xiaoying Zhao, Mingyuan Liu, and Xiaoling Liu. 2021. "Change Regularity of Taste and the Performance of Endogenous Proteases in Shrimp (Penaens vannamei) Head during Autolysis" Foods 10, no. 5: 1020. https://doi.org/10.3390/foods10051020