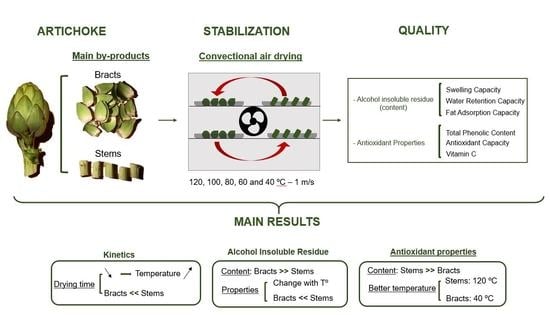
Artichoke by Products as a Source of Antioxidant and Fiber: How It Can Be Affected by Drying Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Drying
2.3. Modeling of Drying Kinetics
2.4. Dried Product Characterization
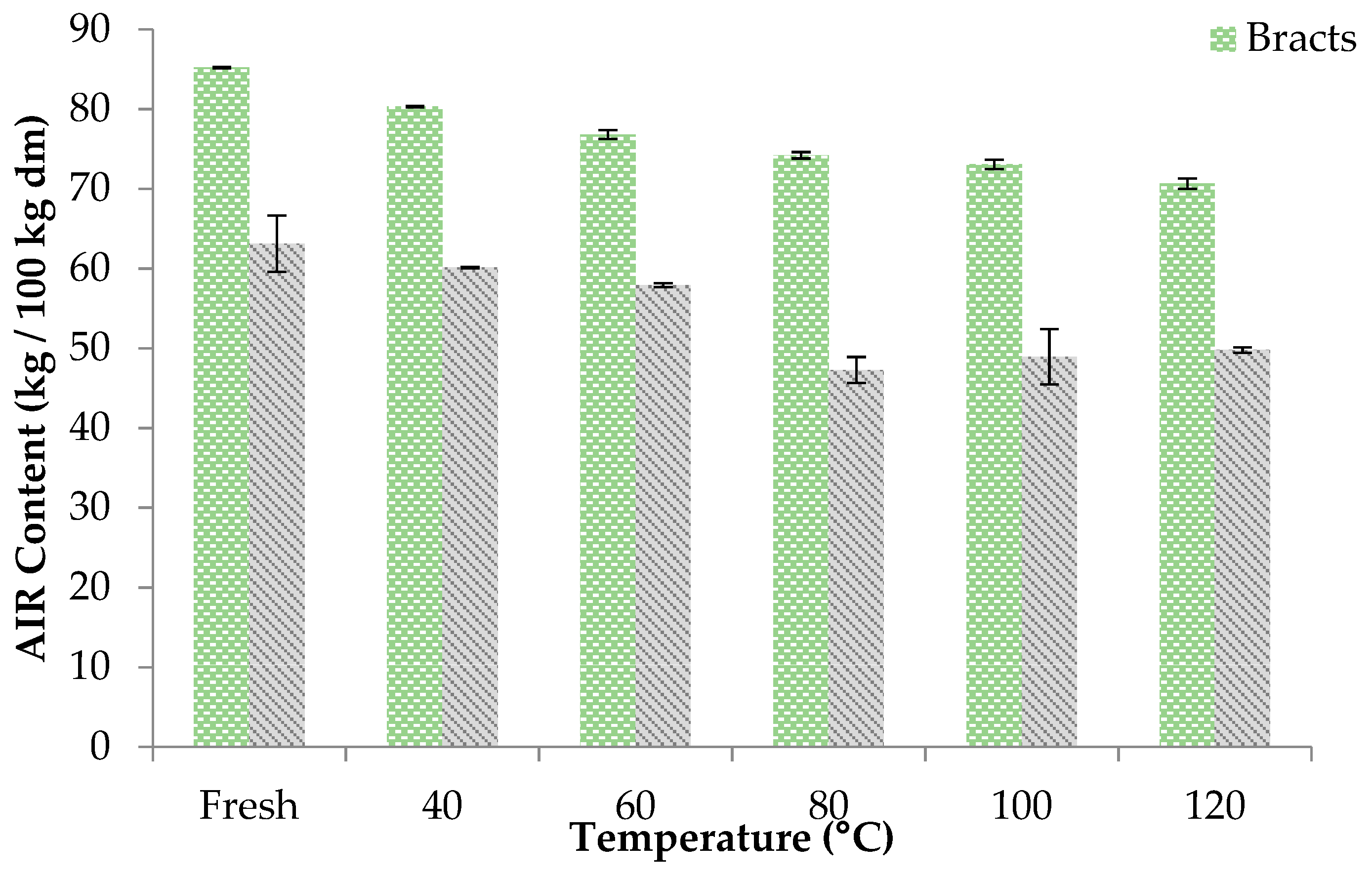
2.4.1. Alcohol Insoluble Residue (AIR)
2.4.2. Antioxidant Properties
- Total Phenolic Content (TPC): The TPC was determined following the method described by Gao et al. [22] using the Folin-Ciocalteu reagent. Each trial was performed in triplicate. An aliquot of 0.1 mL of extract was homogenized with 0.2 mL of Folin-Ciocalteu reagent and 2 mL of distilled water. This mixture was kept at room temperature (20 ± 1 °C) for 3 min. Then, 1 mL of 20% Na2CO3 (w/v) was added, homogenized and maintained in the dark for one hour at room temperature. Finally, the absorbance of the samples at 765 nm was measured in a spectrophotometer (Helios Gamma, Thermo Spectronic, Cambridge, UK). The TPC was determined using a calibration curve built with a known concentration of gallic acid. The results were expressed as milligrams of gallic acid equivalent per gram of dry matter (mg GAE/g d.m.).
- Antioxidant Capacity (AC): The AC was measured by the FRAP (Ferric Reducing Antioxidant Power) method [23]. This method is based on the power of an antioxidant substance to reduce the 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ) ferric complex, which is colorless, to a ferrous complex, which is blue in color. This difference is measured from the determination of maximum absorbance at 595 nm. The FRAP method requires a previous preparation of 0.3 M anhydrous sodium acetate buffer pH 3.6; FeCl3 6H2O 20 mM; and TPTZ 10 mM in HCL 40 mM. Subsequently, the FRAP reagent was prepared by mixing 10 mL of the buffer, 10 mL of the TPTZ solution and 10 mL of the FeCl3 solution and leaving it for 30 min in a bath (Tecton 200, P-Selecta, Barcelona, Spain) at 37 °C. Then, 30 µL of distilled water was added to a disposable cell. Next, 30 μL of bract extract (or ethanol in the case of the blank) was added. In the case of the stems, it was necessary to use a different proportion: 7.5 μL of sample and 22.5 μL of ethanol (1:4 dilution). Finally, 900 μL of the FRAP reagent was added and cells were placed into a 37 °C bath for 30 min. Finally, the absorbance was measured on a spectrophotometer (Helios Gamma, Thermo Spectronic, Cambridge, UK) at 595 nm. The results were expressed in μmol TROLOX/g d.m.
- Vitamin C (VC): The VC was estimated by determining the content of ascorbic acid using the method proposed by Dani and Jagota [24] with slight modifications. For this purpose, 0.5 mL of sample extract was mixed with 0.5 mL of a 7.5% solution of trichloroacetic acid. The solution was homogenized, maintained for 5 min at 4 °C and filtered. Then, 0.2 mL of the prepared extract, 2 mL of distilled water and 0.2 mL of a diluted solution (1:10 v/v) of the Folin-Ciocalteu reagent were placed into a spectrophotometric cell. After 10 min at room temperature, the absorbance at 760 nm was measured. A calibration curve was prepared with ethanolic solutions of known concentrations of ascorbic acid. The results were expressed as milligrams of ascorbic acid per gram of dry matter (mg VC/g d.m.).
2.5. Statistical Analysis
3. Results
3.1. Drying Kinetics and Modeling
3.1.1. Influence of Drying Temperature
3.1.2. Modeling
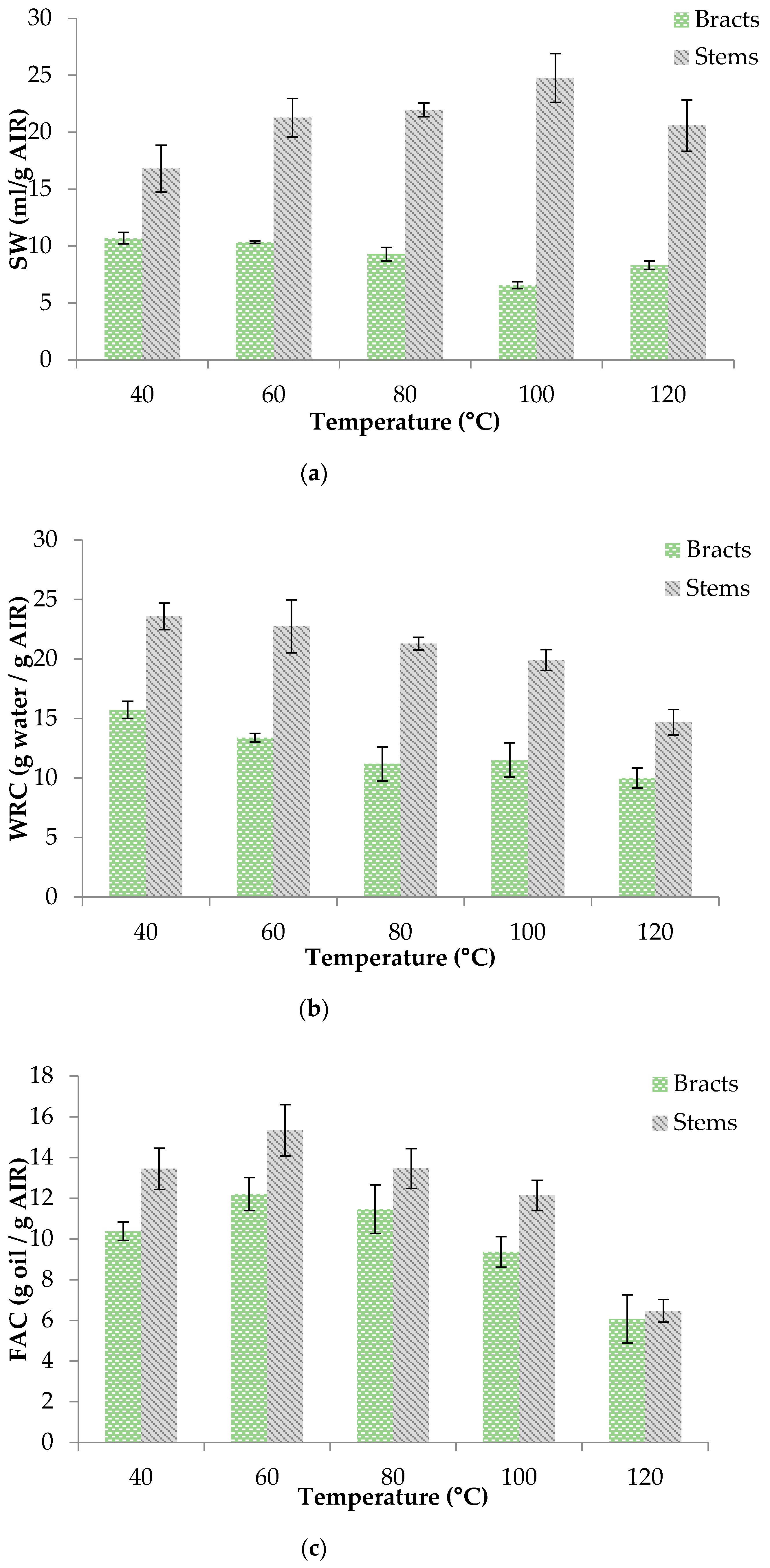
3.2. Influence of Drying Conditions on Product Characteristics
3.2.1. Alcohol Insoluble Residue (AIR)
3.2.2. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [Green Version]
- Salem, M.B.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, K.M. Pharmacological Studies of Artichoke Leaf Extract and Their Health Benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef]
- Guida, V.; Ferrari, G.; Pataro, G.; Chambery, A.; Di Maro, A.; Parente, A. The effects of ohmic and conventional blanching on the nutritional, bioactive compounds and quality parameters of artichoke heads. LWT Food Sci. Technol. 2013, 53, 569–579. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke biorefinery: from food to advanced technological applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT Food Sci. Technol. 2020, 132, 109883. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- Domingo, C.S.; Rojas, A.M.; Fissore, E.N.; Gerschenson, L.N. Rheological behavior of soluble dietary fiber fractions isolated from artichoke residues. Eur. Food Res. Technol. 2019, 245, 1239–1249. [Google Scholar] [CrossRef]
- Boubaker, M.; Omri, A.E.L.; Blecker, C.; Bouzouita, N. Fibre concentrate from artichoke (Cynara scolymus L.) stem by-products: Characterization and application as a bakery product ingredient. Food Sci. Technol. Int. 2016, 22, 759–768. [Google Scholar] [CrossRef]
- Dabbou, S.; Dabbou, S.; Flamini, G.; Pandino, G.; Gasco, L.; Helal, A.N. Phytochemical Compounds from the Crop Byproducts of Tunisian Globe Artichoke Cultivars. Chem. Biodivers. 2016, 13, 1475–1483. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Reuse potential of artichoke (Cynara scolimus L.) waste for the recovery of phenolic compounds and bioenergy. J. Clean. Prod. 2016, 111, 279–284. [Google Scholar] [CrossRef]
- Icier, F. Ohmic blanching effects on drying of vegetable byproduct. J. Food Process Eng. 2010, 33, 661–683. [Google Scholar] [CrossRef]
- Senadeera, W.; Adiletta, G.; Önal, B.; Di Matteo, M.; Russo, P. Influence of different hot air drying temperatures on drying kinetics, shrinkage, and colour of persimmon slices. Foods 2020, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel Drying Techniques for the Food Industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Vaquiro, H.A. Contribución al Estudio y Optimización del Secado Intermitente: Aplicación al Secado de Mango (Mangifera indica L. var. Tommy Atkins). Ph.D. Thesis, Universitat Politècnica de València, València, Spain, 2009. [Google Scholar]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina, Á.A.; Figiel, A. Comparison of traditional and novel drying techniques and its effect on quality of fruits, vegetables and aromatic herbs. Foods 2020, 9, 1261. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; Varo, M.Á.; Mérida, J.; Serratosa, M.P. Influence of drying processes on anthocyanin profiles, total phenolic compounds and antioxidant activities of blueberry (Vaccinium corymbosum). LWT Food Sci. Technol. 2019, 120, 108931. [Google Scholar] [CrossRef]
- Femenia, A.; Robertson, J.A.; Waldron, K.W.; Selvendran, R.R. Cauliflower (Brassica oleracea L), globe artichoke (Cynara scolymus) and chicory witloof (Cichorium intybus) processing by-products as sources of dietary fibre. J. Sci. Food Agric. 1998, 77, 511–518. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Noriega-Rodríguez, D.; Soto-Maldonado, C.; Torres-Alarcón, C.; Pastrana-Castro, L.; Weinstein-Oppenheimer, C.; Zúñiga-Hansen, M.E. Valorization of globe artichoke (Cynara scolymus) agro-industrial discards, obtaining an extract with a selective eect on viability of cancer cell lines. Processes 2020, 8, 715. [Google Scholar] [CrossRef]
- Sabater, C.; Corzo, N.; Olano, A.; Montilla, A. Enzymatic extraction of pectin from artichoke (Cynara scolymus L.) by-products using Celluclast®1.5L. Carbohydr. Polym. 2017, 190, 43–49. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol. Technol. 2009, 51, 242–249. [Google Scholar] [CrossRef]
- Gao, X.; Bjork, L.; Trajkovski, V.; Uggla, M. Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. J. Sci. Food Agric. 2000, 80, 2021–2027. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Dani, H.M.; Jagota, S.K. A New Calorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Martins, M.P.; Cortés, E.J.; Eim, V.; Mulet, A.; Cárcel, J.A. Stabilization of apple peel by drying. Influence of temperature and ultrasound application on drying kinetics and product quality. Dry. Technol. 2019, 37, 559–568. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Silva, W.P.; Queiroz, A.J.; Figueirêdo, R.M.; Gomes, J.P.; Melo, B.A.; Santos, D.C.; Lima, T.L.B.; Branco, R.R.C.; Hamawand, I.; et al. Description of Cumbeba (Tacinga inamoena) Waste Drying at Different Temperatures Using Diffusion Models. Foods 2020, 9, 1818. [Google Scholar] [CrossRef]
- Muñoz, M.; Amaya, I.; Correa, R. Estimating Drying Curves and Diffusion Coefficients in Coffee Drying (Castilla variety) through Global Optimization Strategies. Indian J. Sci. Technol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Tzempelikos, D.A.; Vouros, A.P.; Bardakas, A.V.; Filios, A.E.; Margaris, D.P. Experimental study on convective drying of quince slices and evaluation of thin-layer drying models. Eng. Agric. Environ. Food 2015, 8, 169–177. [Google Scholar] [CrossRef]
- Xiao, H.W.; Le Pang, C.; Wang, L.H.; Bai, J.W.; Yang, W.X.; Gao, Z.J. Drying kinetics and quality of Monukka seedless grapes dried in an air-impingement jet dryer. Biosyst. Eng. 2010, 105, 233–240. [Google Scholar] [CrossRef]
- Domingo, C.S.; Soria, M.; Rojas, A.M.; Fissore, E.N.; Gerschenson, L.N. Protease and hemicellulase assisted extraction of dietary fiber from wastes of Cynara cardunculus. Int. J. Mol. Sci. 2015, 16, 6057–6075. [Google Scholar] [CrossRef] [Green Version]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of solvent type and extraction conditions on the recovery of Phenolic compounds from artichoke waste. Chem. Eng. Trans. 2014, 39, 463–468. [Google Scholar] [CrossRef]
- Djendoubi Mrad, N.; Boudhrioua, N.; Kechaou, N.; Courtois, F.; Bonazzi, C. Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod. Process. 2012, 90, 433–441. [Google Scholar] [CrossRef]
- Fratianni, A.; Adiletta, G.; Di Matteo, M.; Panfili, G.; Niro, S.; Gentile, C.; Farina, V.; Cinquanta, L.; Corona, O. Evolution of Carotenoid Content, Antioxidant Activity and Volatiles Compounds in Dried Mango Fruits (Mangifera Indica L.). Foods 2020, 9, 1424. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Ó.; Santacatalina, J.V.; Simal, S.; Garcia-Perez, J.V.; Femenia, A.; Rosselló, C. Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. J. Food Eng. 2014, 129, 21–29. [Google Scholar] [CrossRef]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of antioxidant high dietary fiber powder from carrot peels. LWT Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Di Scala, K.; Crapiste, G. Drying kinetics and quality changes during drying of red pepper. LWT Food Sci. Technol. 2008, 41, 789–795. [Google Scholar] [CrossRef]
- Solval, K.M.; Sundararajan, S.; Alfaro, L.; Sathivel, S. Development of cantaloupe (Cucumis melo) juice powders using spray drying technology. LWT Food Sci. Technol. 2012, 46, 287–293. [Google Scholar] [CrossRef]
- Jin, X.; Oliviero, T.; van der Sman, R.G.M.; Verkerk, R.; Dekker, M.; van Boxtel, A.J.B. Impact of different drying trajectories on degradation of nutritional compounds in broccoli (Brassica oleracea var. italica). LWT Food Sci. Technol. 2014, 59, 189–195. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
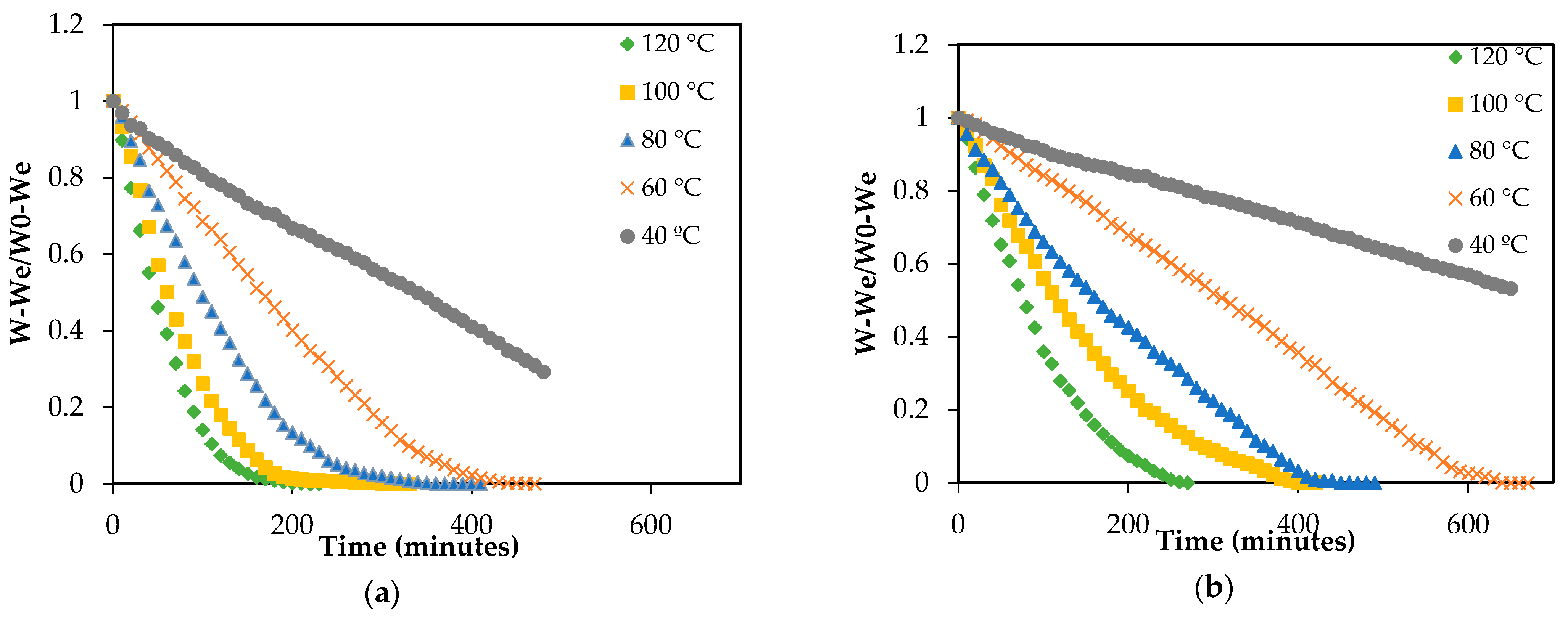
| Drying Temperature (°C) | Drying Time (h) | Deff (10−10 m2/s) | k (10−4 m2/s) | % VAR | |
|---|---|---|---|---|---|
| Bracts | 40 | 12.0 ± 0.3 a | 5.0 ± 0.4 a | 4.1 ± 0.2 a | 99.5 ± 0.8 |
| 60 | 6.00 ± 0.17 b | 8.8 ± 0.3 b | 4.3 ± 0.1 b | 99.5 ± 0.2 | |
| 80 | 5.00 ± 0.15 c | 15 ± 5 c | 13.6 ± 0.6 c | 99.8 ± 0.3 | |
| 100 | 4.0 ± 0.2 d | 25 ± 4 d | 26 ± 5 d | 99.8 ± 0.4 | |
| 120 | 3.0 ± 0.2 e | 34 ± 4 e | 47 ± 4 e | 99.6 ± 0.2 | |
| Stems | 40 | 18.0 ± 0.6 j | 3.6 ± 0.3 f | 1.3 ± 0.8 f | 99.6 ± 0.2 |
| 60 | 10.0 ± 0.4 g | 4.1 ± 0.5 fg | 2.2 ± 0.3 fg | 99.1 ± 0.1 | |
| 80 | 7.0 ± 0.3 h | 4.6 ± 0.2 h | 4.3 ± 0.2 gh | 99.2 ± 0.7 | |
| 100 | 6.00 ± 0.14 i | 5.4 ± 0.3 hi | 5 ± 1 h | 99.0 ± 0.1 | |
| 120 | 4.0 ± 0.2 j | 6.1 ± 0.6 i | 7.1 ± 0.2 i | 98.7 ± 0.3 |
| Drying Temperature (°C) | TPC (mg GAE/g dm) | VC (mg VC/g dm) | AC (mg TROLOX/g dm) | |
|---|---|---|---|---|
| Bracts | - | 0.034 ± 0.003 a | 0.46 ± 0.05 a | 0.34 ± 0.03 a |
| 40 | 0.022 ± 0.003 b | 0.13 ± 0.02 b | 0.09 ± 0.01 b | |
| 60 | 0.009 ± 0.003 c | 0.06 ± 0.02 c | 0.05 ± 0.01 c | |
| 80 | 0.009 ± 0.002 c | 0.03 ± 0.01 d | 0.039 ± 0.005 d | |
| 100 | 0.008 ± 0.002 c | 0.04 ± 0.01 d | 0.031 ± 0.003 d | |
| 120 | 0.008 ± 0.002 c | 0.07 ± 0.02 c | 0.032 ± 0.006 d | |
| Stems | - | 0.48 ± 0.04 d | 2.0 ± 0.1 d | 0.37 ± 0.03 e |
| 40 | 0.04 ± 0.01 e | 0.12 ± 0.05 e | 0.08 ± 0.02 f | |
| 60 | 0.04 ± 0.01 e | 0.04 ± 0.02 f | 0.07 ± 0.02 g | |
| 80 | 0.03 ± 0.01 e | 0.08 ± 0.03 df | 0.06 ± 0.02 g | |
| 100 | 0.07 ± 0.01 f | 0.36 ± 0.06 g | 0.21 ± 0.02 h | |
| 120 | 0.09 ± 0.01 g | 0.55 ± 0.06 h | 0.23 ± 0.02 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsini, A.A.; Llavata, B.; Umaña, M.; Cárcel, J.A. Artichoke by Products as a Source of Antioxidant and Fiber: How It Can Be Affected by Drying Temperature. Foods 2021, 10, 459. https://doi.org/10.3390/foods10020459
Borsini AA, Llavata B, Umaña M, Cárcel JA. Artichoke by Products as a Source of Antioxidant and Fiber: How It Can Be Affected by Drying Temperature. Foods. 2021; 10(2):459. https://doi.org/10.3390/foods10020459
Chicago/Turabian StyleBorsini, Ariel A., Beatriz Llavata, Mónica Umaña, and Juan A. Cárcel. 2021. "Artichoke by Products as a Source of Antioxidant and Fiber: How It Can Be Affected by Drying Temperature" Foods 10, no. 2: 459. https://doi.org/10.3390/foods10020459