Effect of Soil Management and Training System on Negroamaro Wine Aroma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Leaf Area
2.3. Plant Water Status (ψstem)
2.4. Yield Components and Fruit Composition
2.5. Winemaking
2.6. Wine Volatile Compounds Extraction and GC/MS Analysis
2.7. Data Processing
2.8. Statistical Analysis
3. Results and Discussion
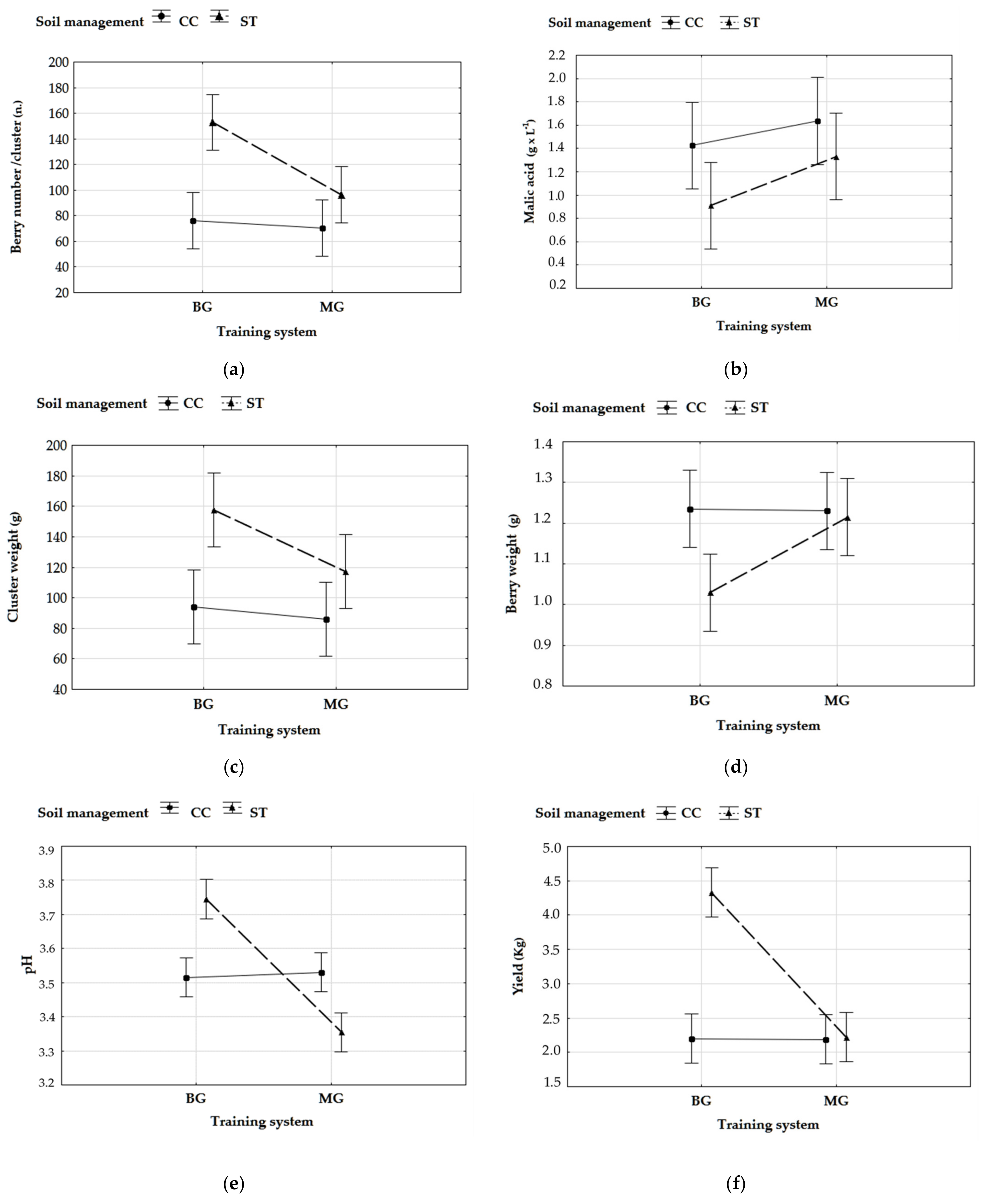
3.1. Yield Parameters and Fruit Composition
3.2. Leaf Area and Leaf Area to Crop Ratio
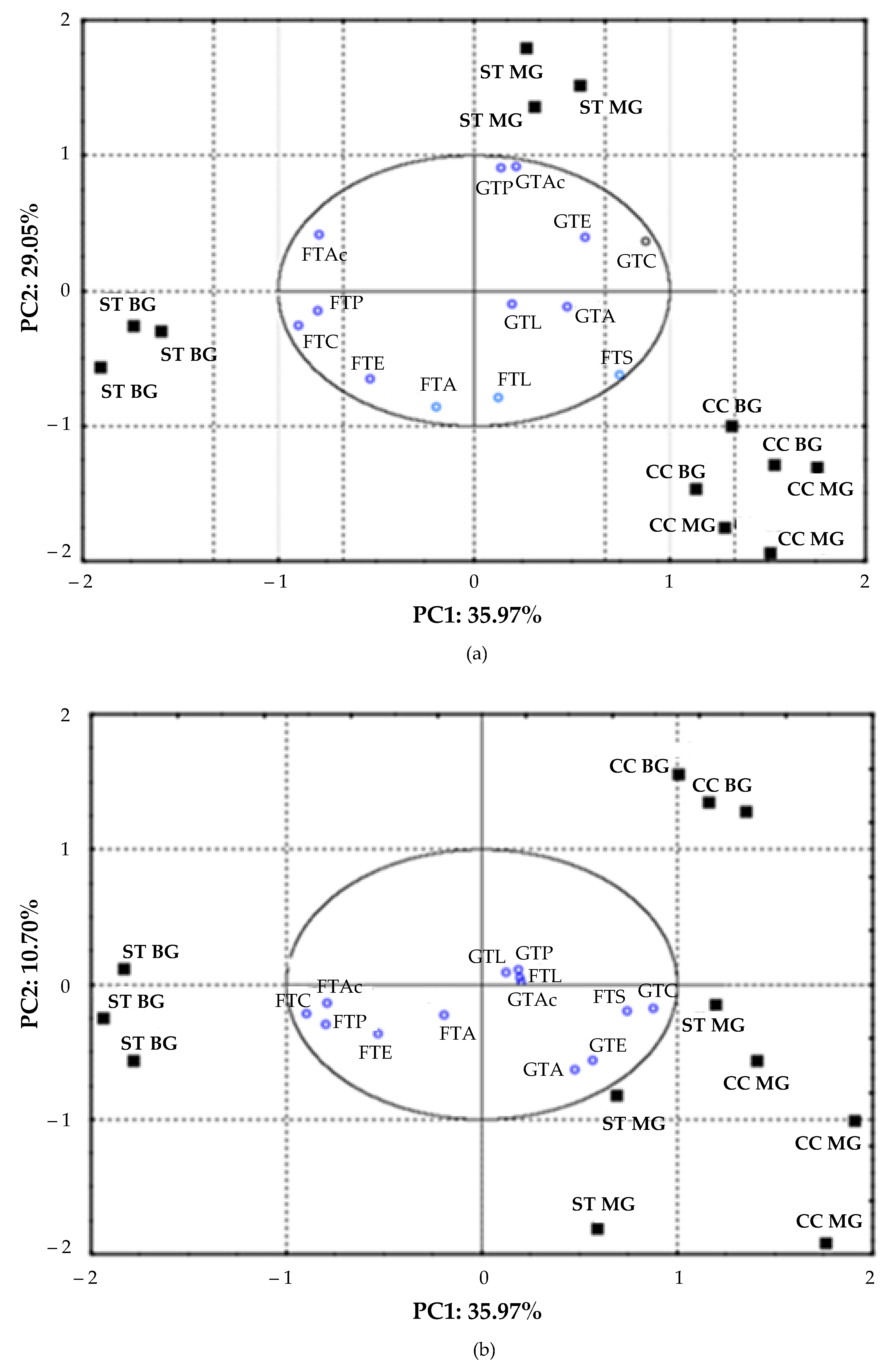
3.3. Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sáenz-Navajas, M.P.; Ferrero-del-Teso, S.; Jeffery, D.W.; Ferreira, V.; Fernández-Zurbano, P. Effect of aroma perception on taste and mouthfeel dimensions of red wines: Correlation of sensory and chemical measurements. Food Res. Int. 2020, 131, 108945. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Coletta, A.; Antonacci, D. Analysis of carotenoids in grapes to predict norisoprenoid varietal aroma of wines from Apulia. J. Agric. Food Chem. 2010, 58, 9647–9656. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Menicatti, M.; De Rosso, M.; Gardiman, M.; Mayr, C.; Pallecchi, M.; Danza, G. Combining liquid chromatography and tandem mass spectrometry approaches to the study of monoterpene glycosides (aroma precursors) in wine grape. J. Mass Spectrom. 2018, 53, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Alegre, Y.; Arias-Pérez, I.; Hernández-Orte, P.; Ferreira, V. Development of a new strategy for studying the aroma potential of winemaking grapes through the accelerated hydrolysis of phenolic and aromatic fractions (PAFs). Food Res. Int. 2020, 127, 108728. [Google Scholar] [CrossRef] [PubMed]
- Ribèreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Trattato di Enologia I—Microbiologia del Vino e Vinificazioni; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G.; Grieco, F. Influence of autochthonous Saccharomyces cerevisiae strains on volatile profile of Negroamaro wines. LWT Food Sci. Technol. 2014, 58, 35–48. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Volatile and sensory profiling of Shiraz wine in response to alcohol management: Comparison of harvest timing versus technological approaches. Food Res. Int. 2018, 109, 561–571. [Google Scholar] [CrossRef]
- Bouzas-Cid, Y.; Trigo-Córdoba, E.; Falqué, E.; Orriols, I.; Mirás-Avalos, J.M. Influence of supplementary irrigation on the amino acid and volatile composition of Godello wines from the Ribeiro Designation of Origin. Food Res. Int. 2018, 111, 715–723. [Google Scholar] [CrossRef]
- Wang, J.; Abbey, T.; Kozak, B.; Madilao, L.L.; Tindjau, R.; Del Nin, J.; Castellarin, S.D. Evolution over the growing season of volatile organic compounds in Viognier (Vitis vinifera L.) grapes under three irrigation regimes. Food Res. Int. 2019, 125, 108512. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Cacho, J.; Moncayo, L.; Palma, J.C.; Ferreira, V.; Cullere, L. The impact of grape variety on the aromatic chemical composition of non-aromatic Peruvian pisco. Food Res. Int. 2013, 54, 373–381. [Google Scholar] [CrossRef]
- Toci, A.T.; Crupi, P.; Gambacorta, G.; Dipalmo, T.; Antonacci, D.; Coletta, A. Free and bound aroma compounds characterization by GC-MS of Negroamaro wine as affected by soil management. J. Mass Spectrom. 2012, 47, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Orte, P.; Concejero, B.; Astrain, J.; Lacau, B.; Cacho, J.; Ferreira, V. Influence of viticulture practices on grape aroma precursors and their relation with wine aroma. J. Sci. Food Agric. 2014, 95, 688–701. [Google Scholar]
- Nogueira Cardoso, E.J.; Figueiredo Vasconcellos, R.L.; Bini, D.; Miyauchi Horta, M.Y.; Alcantara dos Santos, C.; Lopes Alves, P.R.; Monteiro de Paula, A.; Nakatani, A.S.; de Moraes Pereira, J.; Nogueira, M.A. Soil health: Looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric. 2013, 70, 274–289. [Google Scholar] [CrossRef] [Green Version]
- Bond, W.; Grundy, A.C. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [Google Scholar] [CrossRef]
- Wheeler, S.J.; Black, A.S.; Pickering, G.J. Vineyard floor management improves wine quality in highly vigorous Vitis vinifera ‘Cabernet Sauvignon’ in New Zealand. N. Z. J. Crop Hortic. Sci. 2005, 33, 317–328. [Google Scholar] [CrossRef]
- Xi, Z.; Tao, Y.; Zhang, L.; Li, H. Impact of cover crops in vineyard on the aroma compounds of Vitis vinifera L. cv Cabernet Sauvignon wine. Food Chem. 2011, 127, 516–522. [Google Scholar] [CrossRef]
- Bouzas-Cid, Y.; Trigo-Córdoba, E.; Orriols, I.; Falqué, E.; Mirás-Avalos, J.M. Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region. Beverages 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Zoecklein, B.W.; Wolf, T.K.; Pélanne, L.; Miller, M.K.; Birkenmaier, S.S. Effect of vertical shoot-positioned, Smart-Dyson, and Geneva double-curtain training systems on Viognier grape and wine composition. Am. J. Enol. Vitic. 2008, 59, 11–21. [Google Scholar]
- Xu, X.Q.; Cheng, G.; Duan, L.L.; Jiang, R.; Pan, Q.H.; Duan, C.Q.; Wang, J. Effect of training systems on fatty acids and their derived volatiles in Cabernet-Sauvignon grapes and wines of the north foot of Mt. Tianshan. Food Chem. 2015, 181, 198–206. [Google Scholar] [CrossRef]
- Lopes, C.M.; Pinto, P.A. Easy and accurate estimation of grapevine leaf area with simple mathematical models. Vitis 2005, 44, 55–61. [Google Scholar]
- Office International de la Vigne et du Vin (OIV). Recueil des Methodes Internationales d’Analyse des Vins et des Mouts; Office International de la Vigne et du Vin: Paris, France, 2019. [Google Scholar]
- Monteiro, A.; Lopes, C.M. Influence of cover crop on water use and performance of vineyard in Mediterranean Portugal. Agric. Ecosyst. Environ. 2007, 121, 336–342. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Wardle, D.A. Impact of Training System and Vine Spacing on Vine Performance and Berry Composition of Seyval blanc. Am. J. Enol. Vitic. 1994, 45, 444–451. [Google Scholar]
- Vilanova, M.; Genisheva, Z.; Tubio, M.; Álvarez, K.; Lissarague, J.R.; Oliveira, J.M. Effect of vertical shoot-positioned, Scott-Henry, Geneva double-curtain, Arch-Cane, and Parral training system on the volatile composition of Albariño wines. Molecules 2017, 22, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, R.F.; Cantos-Villar, E.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Moreno-Rojas, J.M. Influence of vertical training systems on warm climate red winemaking: Wine parameters, polyphenols, volatile composition, and sensory analysis. OENO One 2019, 3, 471–486. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.M.; Monteiro, A.; Rückert, F.E.; Gruber, B.; Steinberg, B.; Schultz, H.R. Transpiration of grapevines and co-habitating cover crop and weed species in a vineyard. A “snapshot” at diurnal trends. Vitis 2004, 43, 111–117. [Google Scholar]
- Sadras, V.O.; Stevens, R.M.; Pech, J.M.; Taylor, E.J.; Nicholas, P.R.; Mccarthy, M.G. Quantifying phenotypic plasticity of berry traits using an allometric-type approach: A case study on anthocyanins and sugars in berries of Cabernet Sauvignon. Aust. J. Grape Wine Res. 2007, 13, 72–80. [Google Scholar] [CrossRef]
- Keller, M.; Mills, L.J.; Wample, R.L.; Spayd, S.E. Cluster thinning effects on three deficit-irrigated Vitis vinifera cultivars. Am. J. Enol. Vitic. 2005, 56, 91–103. [Google Scholar]
- Reynolds, A.G.; Vanden Heuvel, J.E. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf Area/Crop Weight Ratios of Grapevines: Influence on Fruit Composition and Wine Quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Howell, G.S. Sustainable grape productivity and the growth yield relationship: A review. Am. J. Enol. Vitic. 2001, 52, 165–174. [Google Scholar]
- Fragasso, M.; Antonacci, D.; Pati, S.; Tufariello, M.; Baiano, A.; Forleo, M.R.; Caputo, A.R.; La Notte, E. Influence of training system on volatile and sensory profiles of Primitivo grapes and wines. Am. J. Enol. Vitic. 2012, 63, 477–485. [Google Scholar] [CrossRef]
- Ugliano, M.; Genovese, A.; Moio, L. Hydrolysis of wine aroma precursors during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. J. Agric. Food Chem. 2003, 51, 5073–5078. [Google Scholar] [CrossRef]
- Darriet, P.; Thibon, C.; Doubardieu, D. Aroma and aroma precursors in grape berry. In The Biochemistry of the Grape Berry; Gerós, H., Chaves, M.M., Delrot, S., Eds.; Bentham Sciences Publishers: Sharjah, United Arab Emirates, 2012; pp. 111–136. [Google Scholar]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical characterization of the aroma of Tinta Negra Mole red wine: Identification of the main odorants compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Bubola, M.; Rusjan, D.; Lukić, I. Crop level vs. leaf removal: Effects on Istrian Malvasia wine aroma and phenolic acids composition. Food Chem. 2020, 312, 126046. [Google Scholar] [CrossRef]
- Moreno, D.; Valdés, E.; Uriarte, D.; Gamero, E.; Talaverano, I.; Vilanova, M. Early leaf removal applied in warm climatic conditions: Impact on Tempranillo wine volatiles. Food Res. Int. 2017, 98, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T. Factors affecting the formation of volatile fatty acids during grape must fermentation. Agr. Biol. Chem. 1986, 50, 3197–3199. [Google Scholar]
- Verzera, A.; Tripodi, G.; Dima, G.; Condurso, C.; Scacco, A.; Cincotta, F.; Giglio, D.M.L.; Santangelo, T.; Sparacio, A. Leaf removal and wine composition of Vitis vinifera L. cv. Nero d’Avola: The volatile aroma constituents. J. Sci. Food Agric. 2016, 96, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Šuklje, K.; Antalick, G.; Coetzee, Z.; Schmidtke, L.M.; Baša Česnik, H.; Brandt, J.; du Toit, W.J.; Lisjak, K.; Deloire, A. Effect of leaf removal and ultraviolet radiation on the composition and sensory perception of Vitis vinifera L. cv. Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2014, 20, 223–233. [Google Scholar]
- Feng, H.; Skinkis, P.A.; Qian, M.C. Pinot noir wine volatile and anthocyanin composition under different levels of vine fruit zone leaf removal. Food Chem. 2017, 214, 736–744. [Google Scholar] [CrossRef]
- Song, J.Q.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef]
| Variables | Factors | |||||
|---|---|---|---|---|---|---|
| Soil Management | Training System | |||||
| ST | CC | S | MG | BG | S | |
| Yield (kg/vine) | 3.3 ± 1.2 | 2.2 ± 0.7 | ** | 2.3 ± 0.7 | 3.2 ± 1.2 | ** |
| Cluster weight (g) | 140 ± 20 | 90 ± 14 | ** | 100 ± 20 | 130 ± 40 | n.s. |
| Cluster number | 23 ± 5 | 25 ± 4 | n.s. | 20 ± 5 | 28 ± 2 | * |
| Berry weight (g) | 1.12 ± 0.11 | 1.23 ± 0.09 | n.s. | 1.26 ± 0.05 | 1.09 ± 0.11 | * |
| Number of berries | 120 ± 30 | 77 ± 13 | ** | 83 ± 18 | 110 ± 30 | ** |
| TSS (Brix) | 21.2 ± 0.5 | 22.2 ± 0.9 | n.s. | 21.3 ± 0.9 | 22.1 ± 0.4 | n.s. |
| TA (g/L) | 5.4 ± 0.4 | 5.3 ± 0.3 | n.s. | 5.2 ± 0.4 | 5.60 ± 0.15 | n.s. |
| Citric acid (g/L) | 0.23 ± 0.08 | 0.20 ± 0.09 | n.s. | 0.19 ± 0.06 | 0.14 ± 0.05 | n.s. |
| Malic acid (g/L) | 1.1 ± 0.3 | 1.53 ± 0.14 | * | 1.5 ± 0.3 | 1.1 ± 0.2 | * |
| Tartaric acid (g/L) | 5.1 ± 0.7 | 5.2 ± 0.3 | n.s. | 5.1 ± 0.6 | 5.2 ± 0.5 | n.s. |
| pH | 3.55 ± 0.15 | 3.52 ± 0.06 | n.s. | 3.34 ± 0.10 | 3.63 ± 0.14 | * |
| Factors | Growth Stage | ||
|---|---|---|---|
| Berry-set | Harvesting | ||
| Total Leaf Area Per Vine | Total Leaf Area Per Vine | Leaf Area to Fruit Ratio | |
| Soil management | |||
| ST | 4.3 ± 1.2 | 5.8 ± 1.7 | 18 ± 6 |
| CC | 2.4 ± 0.8 | 4.5 ± 1.3 | 20 ± 7 |
| S | ** | * | * |
| Training systems | |||
| MG | 3.4 ± 1.2 | 5.3 ± 1.9 | 23 ± 9 |
| BG | 4.3 ± 1.1 | 5.0 ± 1.4 | 16 ± 5 |
| S | * | n.s. | ** |
| Interaction | n.s. | n.s. | n.s. |
| Growth Cycle | ||||
|---|---|---|---|---|
| pF-H | pF-B | Bs-V | V-H | |
| Soil management a | ||||
| ST | −1.18 ± 0,08 | −1.08 ± 0.01 | −1.25 ± 0.08 | −1.21 ± 0.14 |
| CC | −1.09 ± 0.10 | −1.18 ± 0.02 | −1.03 ± 0.11 | −1.06 ± 0.09 |
| S | n.s. | * | * | ** |
| Training system b | ||||
| MG | −1.14 ± 0,08 | −1.19 ± 0.01 | −1.09 ± 0.08 | −1.14 ± 0.19 |
| BG | −1.13 ± 0.10 | −1.07 ± 0.01 | −1.19 ± 0.11 | −1.13 ± 0.18 |
| S | n.s. | * | * | n.s. |
| Interaction | n.s. | n.s. | n.s. | n.s. |
| Compounds | Factors | ||||||
|---|---|---|---|---|---|---|---|
| Soil Management | Training System | Interaction | |||||
| ST | CC | S | MG | BG | S | S | |
| Esters | |||||||
| Ethylbutanoate | 280 ± 110 | 370 ± 180 | ** | 340 ± 190 | 310 ± 100 | n.s. | ** |
| Ethyl 3-methylbutanoate (isoamyl acetate) | 380 ± 60 | 250 ± 120 | * | 310 ± 110 | 320 ± 130 | n.s. | n.s. |
| Ethyl hexanoate (ethyl caproate) | 140 ± 30 | 110 ± 60 | n.s. | 130 ± 40 | 110 ± 50 | n.s. | ** |
| Ethyl 2-hydroxypropanoate | 220 ± 60 | 230 ± 50 | n.s. | 240 ± 60 | 230 ± 60 | n.s. | ** |
| Ethyl 3-hydroxybutanoate | n.d. | 6.3 ± 1.1 | *** | 6.3 ± 1.1 | n.d. | *** | *** |
| Diethyl butanedioate (diethyl succinate) | 570 ± 80 | 530 ± 80 | n.s. | 550 ± 60 | 550 ± 100 | n.s. | *** |
| Ethyl 4,4-ethoxyhydroxybutanoate | 3200 ± 1700 | 3300 ± 900 | n.s. | 2800 ± 1300 | 3600 ± 1300 | * | *** |
| Total | 5000 ± 2000 | 4800 ± 1400 | n.s. | 4500 ± 1700 | 5200 ± 1700 | n.s. | *** |
| Carboxylic Acids | |||||||
| Acetic acid | 670 ± 140 | 200 ± 30 | *** | 230 ± 40 | 630 ± 180 | *** | *** |
| Propanoic acid | 4.3 ± 0.4 | 2.6 ± 0.3 | ** | 13 ± 4 | 44 ± 7 | *** | ** |
| 2-methyl propanoic acid (isobutyric acid) | 110 ± 40 | 121 ± 16 | ** | 100 ± 40 | 130 ± 20 | *** | *** |
| Butanoic acid | 70 ± 40 | 58 ± 18 | n.s. | 82 ± 5 | 48 ± 8 | ** | ** |
| 3-methyl-butanoic acid (isovaleric acid) | 280 ± 80 | 380 ± 40 | *** | 320 ± 120 | 350 ± 10 | * | *** |
| Pentanoic acid (valeric acid) | 82 ± 5 | 53 ± 3 | *** | 73 ± 5 | 55 ± 7 | ** | *** |
| Hexanoic acid (caproic acid) | 1300 ± 200 | 1280 ± 80 | n.s. | 1240 ± 100 | 1380 ± 160 | ** | *** |
| Octanoic acid (capric acid) | 1700 ± 300 | 1500 ± 300 | n.s. | 1500 ± 200 | 1700 ± 400 | n.s. | ** |
| Benzoic acid | 250 ± 80 | 290 ± 150 | n.s. | 260 ± 170 | 280 ± 50 | n.s. | n.s. |
| Benzeneacetic acid | 290 ± 60 | 130 ± 30 | *** | 200 ± 50 | 220 ± 140 | n.s. | *** |
| Total | 4800 ± 1000 | 4000 ± 700 | * | 4100 ± 700 | 5200 ± 1000 | ** | *** |
| Alcohols | |||||||
| 2-methyl-propanol | 1100 ± 110 | 560 ± 50 | ** | 550 ± 50 | 1100 ± 110 | *** | *** |
| 3-methyl-butanol (isoamyl alcohol) | 20,000 ± 2700 | 23,000 ± 1800 | ** | 20,000 ± 4000 | 22,000 ± 1000 | n.s. | ** |
| 3-methyl-1-pentanol | 27 ± 4 | 71 ± 19 | *** | 50 ± 30 | 43 ± 18 | n.s. | n.s. |
| 1-hexanol | 470 ± 80 | 420 ± 40 | * | 450 ± 50 | 450 ± 100 | n.s. | *** |
| 3-ethoxy-1-propanol | n.d. | 0.27 ± 0.08 | *** | 0.17 ± 0.09 | 0.10 ± 0.02 | ** | ** |
| 2-phenyl-ethanol (phenylethyl alcohol) | 28,000 ± 3000 | 24,000 ± 2000 | * | 24,600 ± 1100 | 27,000 ± 5000 | n.s. | * |
| Total | 50,000 ± 6000 | 48,000 ± 4000 | * | 46,000 ± 5000 | 51,000 ± 6000 | n.s. | * |
| Phenolics | |||||||
| 2,6-dimethoxy-phenol (syringol) | 104 ± 9 | 84 ± 5 | ** | 98 ± 13 | 90 ± 12 | n.s. | n.s. |
| Acetamides | |||||||
| N-(2-phenylethyl)-acetamide | 1030 ± 140 | 960 ± 200 | n.s. | 890 ± 110 | 1130 ± 150 | * | n.s. |
| N-(3-methylbutyl)-acetamide | 1090 ± 140 | 490 ± 140 | *** | 800 ± 300 | 800 ± 300 | n.s. | * |
| Total | 2100 ± 300 | 1500 ± 300 | *** | 1700 ± 400 | 1900 ± 400 | n.s. | n.s. |
| Sulfurs | |||||||
| Dihydro-2-methyl-3-(2H)-thiphenone | n.d. | 10 ± 3 | *** | 4.0 ± 1.8 | n.s. | n.s. | |
| 3-methylthio-1-propanol | 11.7 ± 1.0 | 120 ± 40 | *** | 38 ± 4 | *** | *** | |
| Total | 11.7 ± 1.0 | 130 ± 40 | *** | 42 ± 6 | *** | *** | |
| Ketones Lactones Aldehydes | |||||||
| 2-octanone | 154 ± 19 | 230 ± 30 | *** | 210 ± 50 | n.s. | n.s. | |
| Butyrolactone | 40 ± 6 | 36 ± 5 | n.s. | 48 ± 7 | *** | *** | |
| Benzaldehyde | 19 ± 4 | 23 ± 6 | n.s. | 21 ± 7 | n.s. | n.s. | |
| Total | 210 ± 30 | 290 ± 40 | *** | 280 ± 60 | n.s. | * | |
| Compounds | Factors | ||||||
|---|---|---|---|---|---|---|---|
| Soil management | Training system | Interaction | |||||
| ST | CC | S | MG | BG | S | S | |
| Esters | |||||||
| Diethyl butanedioate (diethyl succinate) | 64 ± 9 | 145 ± 14 | *** | 132 ± 11 | 77 ± 9 | *** | *** |
| Ethyl 4,4-ethoxyhydroxybutanoate | 290 ± 60 | 210 ± 130 | n.s. | 310 ± 90 | 190 ± 70 | * | n.s. |
| Total | 350 ± 70 | 360 ± 140 | n.s. | 440 ± 100 | 270 ± 80 | ** | n.s. |
| Carboxylic Acids | |||||||
| Acetic acid | 72 ± 9 | 80 ± 30 | n.s. | 149 ± 10 | n.d. | *** | n.s. |
| Butanoic acid | 58 ± 14 | n.d. | *** | 56 ± 12 | n.d. | *** | *** |
| Pentanoic acid (valeric acid) | 61 ± 17 | n.d. | *** | 59 ± 14 | n.d. | *** | *** |
| Hexanoic acid (caproic acid) | 250 ± 30 | 80 ± 20 | *** | 180 ± 20 | 150 ± 20 | n.s. | *** |
| Octanoic acid (capric acid) | 510 ± 50 | 850 ± 80 | *** | 640 ± 40 | 720 ± 60 | * | ** |
| Benzoic acid | 260 ± 40 | 360 ± 60 | ** | 340 ± 70 | 280 ± 50 | * | n.s. |
| Benzeneacetic acid | 97 ± 12 | 158 ± 11 | *** | 260 ± 70 | n.d. | *** | *** |
| Total | 1310 ± 170 | 1500 ± 200 | ** | 1700 ± 200 | 1150 ± 130 | *** | ** |
| Alcohols | |||||||
| 3-methyl-butanol (isoamyl alcohol) | 90 ± 20 | 139 ± 120 | *** | 210 ± 80 | 230 ± 50 | *** | *** |
| 2-phenyl-ethanol (phenylethyl alcohol) | 150 ± 50 | 70 ± 20 | *** | 132 ± 17 | 90 ± 20 | * | *** |
| Total | 240 ± 70 | 210 ± 30 | n.s. | 340 ± 90 | 110 ± 30 | ** | * |
| Phenolics | |||||||
| 2,6-dimethoxy-phenol (syringol) | 15 ± 2 | 36.3 ± 1.1 | ** | 28 ± 16 | 24 ± 11 | n.s. | n.s. |
| Acetamides | |||||||
| N-(2-phenylethyl)-acetamide | 3.9 ± 1.4 | n.d. | *** | 4.9 ± 1.2 | n.d. | *** | *** |
| Ketones | |||||||
| 2-octanone | 132 ± 11 | 130 ± 20 | n.s. | 142 ± 14 | 123 ± 14 | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coletta, A.; Toci, A.T.; Pati, S.; Ferrara, G.; Grieco, F.; Tufariello, M.; Crupi, P. Effect of Soil Management and Training System on Negroamaro Wine Aroma. Foods 2021, 10, 454. https://doi.org/10.3390/foods10020454
Coletta A, Toci AT, Pati S, Ferrara G, Grieco F, Tufariello M, Crupi P. Effect of Soil Management and Training System on Negroamaro Wine Aroma. Foods. 2021; 10(2):454. https://doi.org/10.3390/foods10020454
Chicago/Turabian StyleColetta, Antonio, Aline Theodoro Toci, Sandra Pati, Giuseppe Ferrara, Francesco Grieco, Maria Tufariello, and Pasquale Crupi. 2021. "Effect of Soil Management and Training System on Negroamaro Wine Aroma" Foods 10, no. 2: 454. https://doi.org/10.3390/foods10020454