‘Autoreconstruction’ of the Mandible—Report of a Case
Abstract
:1. Introduction
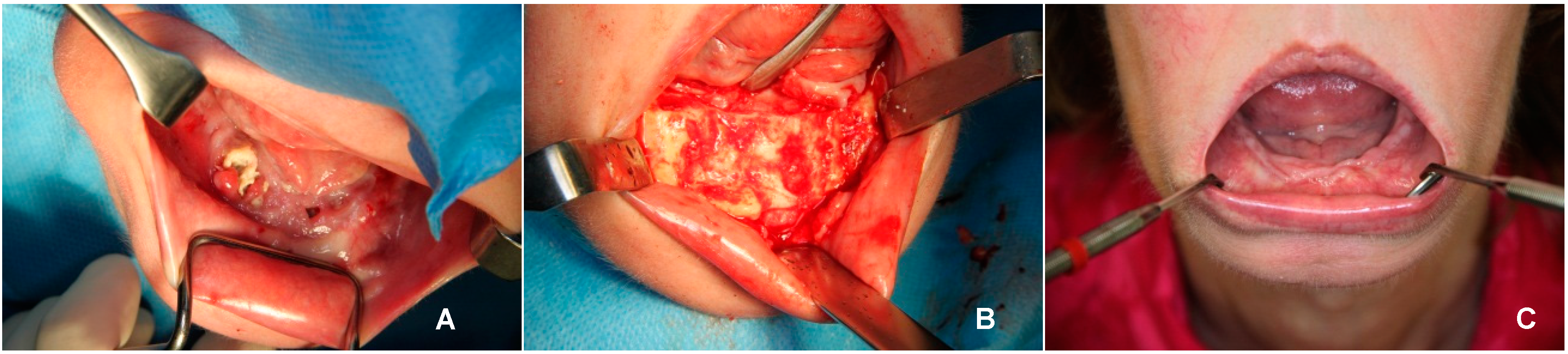
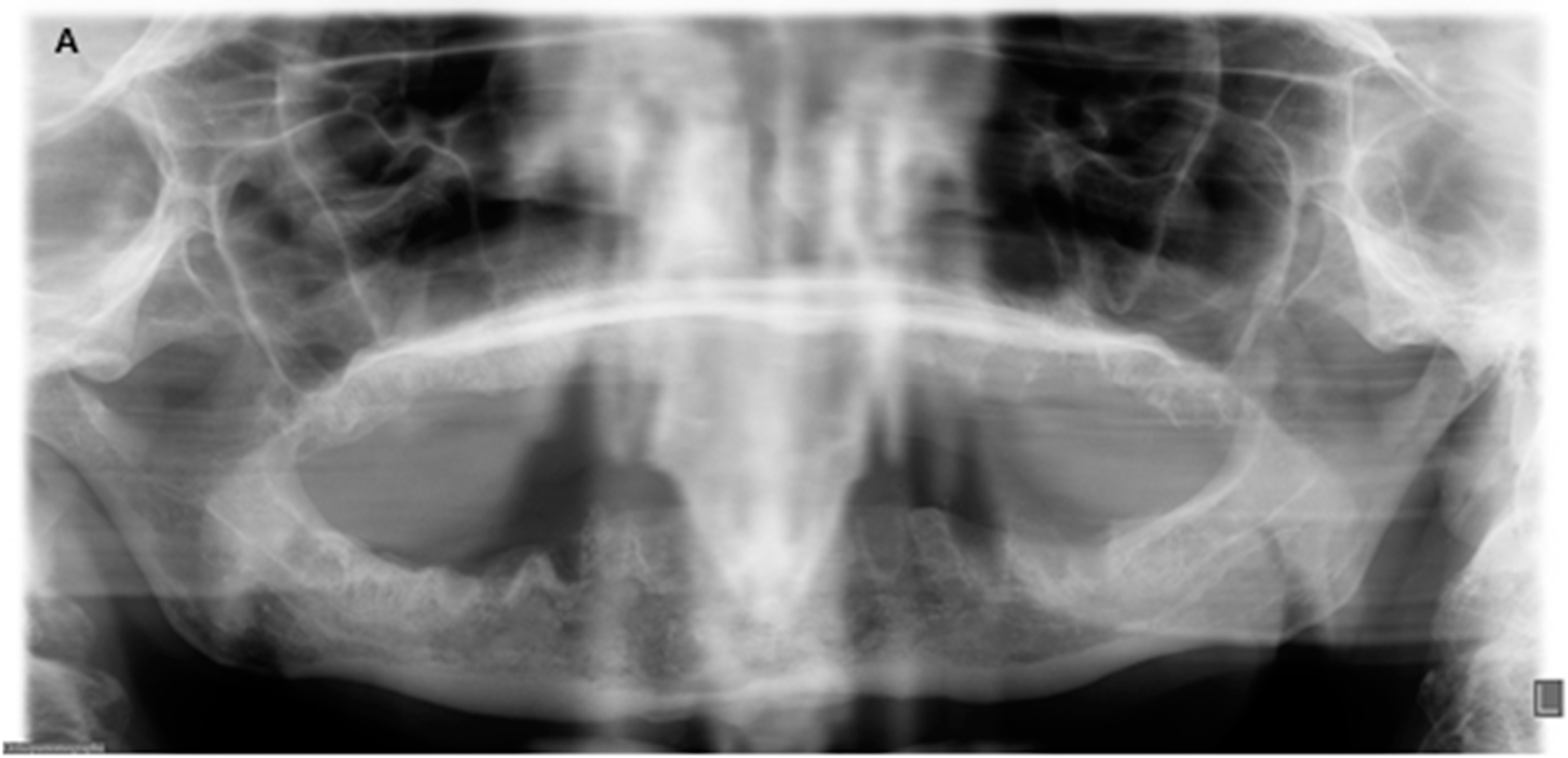
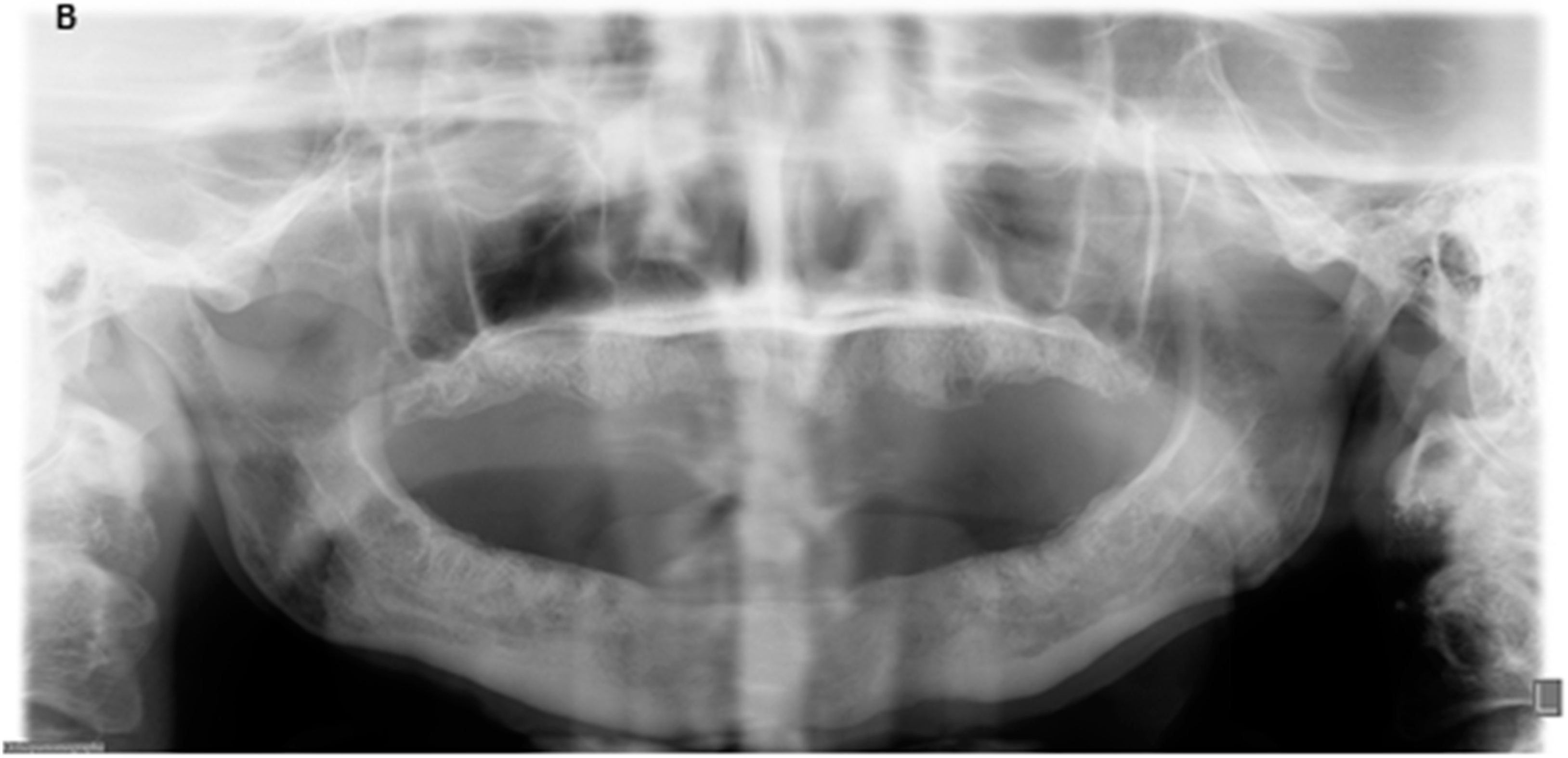
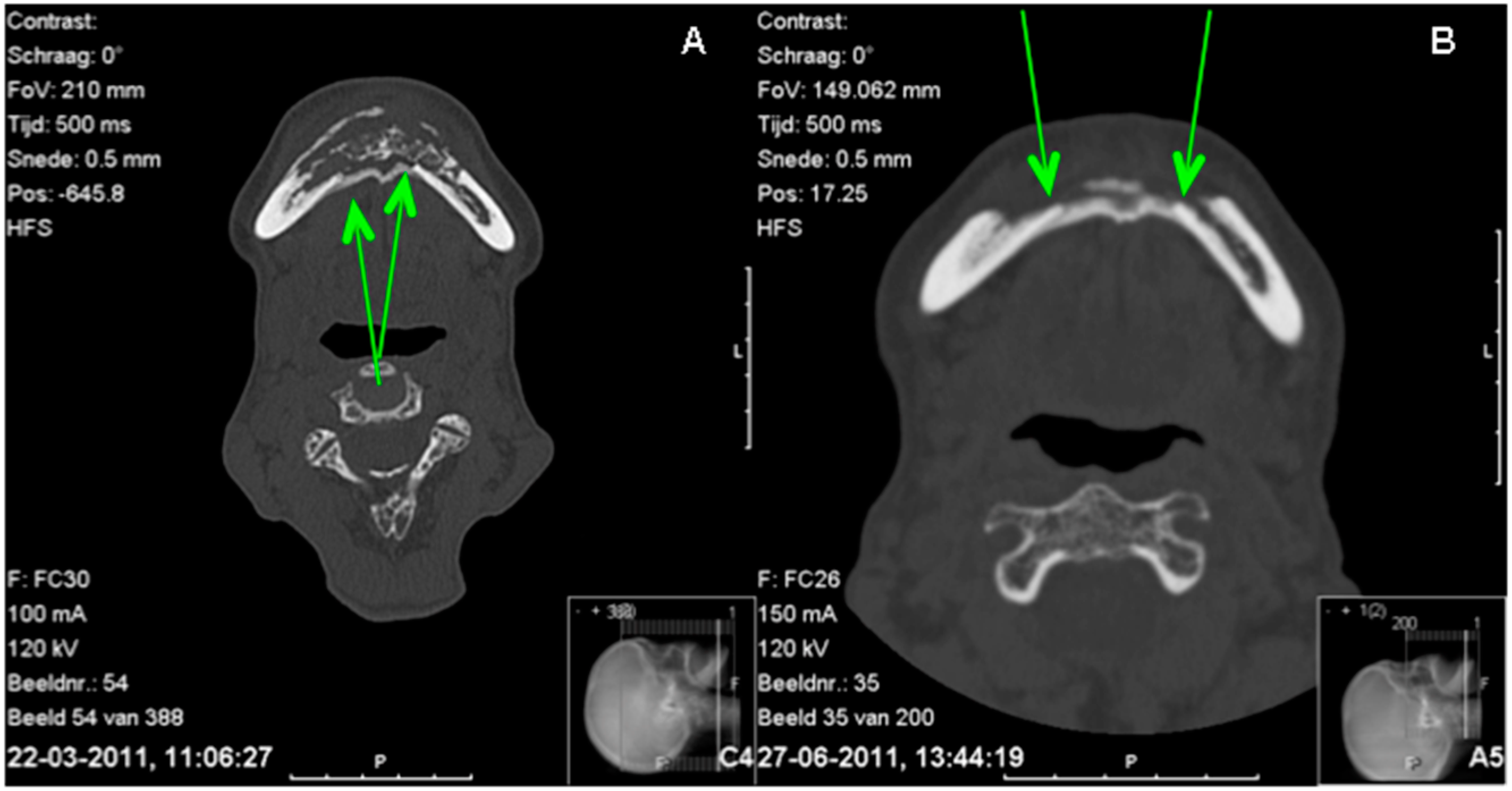
2. Case Report
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons (AAOMS). American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376.
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar] [PubMed]
- Alons, K.; Kuijpers, S.C.; de Jong, E.; van Merkesteyn, J.P. Treating low- and medium-potency bisphosphonate-related osteonecrosis of the jaws with a protocol for the treatment of chronic suppurative osteomyelitis: Report of 7 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.A. Surgical management of bisphosphonate induced osteonecrosis of the jaws. Int. J. Oral Maxillofac. Surg. 2010, 39, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Heufelder, M.; Winter, K.; Hendricks, J.; Frerich, B.; Schramm, A.; Hemprich, A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 111, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.C.; Ng, I.O.; Yeung, K.M. Osteomyelitis with proliferative periostitis: an unusual case. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e14–e19. [Google Scholar] [CrossRef] [PubMed]
- Belli, E.; Matteini, C.; Andreano, T. Sclerosing osteomyelitis of Garre periostitis ossificans. J. Craniofac. Surg. 2002, 13, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.E.; Nortje, C.J.; Grotepass, F.; Schmidt, S.; Harris, A.M. Periostitis ossificans versus Garre's osteomyelitis. Part I. What did Garre really say? Oral Surg Oral Med Oral Pathol Endod. 1988, 65, 773–777. [Google Scholar] [CrossRef]
- Wood, N.K.; Goaz, P.W. Differential Diagnosis of Oral & Maxillofacial Lesions, 5th ed.; Mosby Elsevier Health Sciences: Maryland Heights, MO, USA, 1997; pp. 488–492. [Google Scholar]
- Savory, W.S. A Case of Necrosis of the Jaw and other bones from fumes of phosphorus. Med. Chir. Trans. 1874, 57, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.W.; Baron, R.; Buckland, D.H.; Cooke, M.A.; Craig, J.D.; Duffield, D.P.; Grosart, A.W.; Parkes, P.W.J.; Porter, A. Phosphorus necrosis of the jaw: A present-day study. Brit. J. industr. Med. 1962, 19, 83–99. [Google Scholar] [PubMed]
- Marx, R.E. Uncovering the Cause of “Phossy Jaw” Circa 1858–1906: Oral and Maxillofacial Surgery Closed Case Files-Case Closed. J. Oral. Maxillofac. Surg. 2008, 66, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- Hellstein, J.W.; Marek, C.L. Bisphosphonate Osteochemonecrosis (Bis-Phossy Jaw): Is this Phossy Ja of the 21st Century? J. Oral Maxillofac. Surg. 2005, 63, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Abu-Id, M.H.; Warnke, P.H.; Gottschalk, J.; Springer, I.; Wiltfang, J.; Acil, Y.; Russo, P.A.J.; Kreusch, T. “Bis-phossy jaws”—High and low risk factors for bisphosphonate-induced osteonecrosis of the jaw. J. Craniomaxillofac. Surg. 2008, 36, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Whalen, J.P.; O’Donohue, N.; Krook, L.; Nunez, E.A. Pathogenesis of Abnormal Remodeling of Bones: Effects of Yellow Phosphorus in the Growing Rat. Anat. Rec. 1973, 177, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.C. Case of Salivation and diseased jaw from the fumes of phosphorus. Medical Times 1846, 15, 224–225. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pichardo, S.E.C.; De Roos, P.; Van Merkesteyn, J.P.R. ‘Autoreconstruction’ of the Mandible—Report of a Case. Dent. J. 2016, 4, 9. https://doi.org/10.3390/dj4020009
Pichardo SEC, De Roos P, Van Merkesteyn JPR. ‘Autoreconstruction’ of the Mandible—Report of a Case. Dentistry Journal. 2016; 4(2):9. https://doi.org/10.3390/dj4020009
Chicago/Turabian StylePichardo, Sarina E.C., Pieter De Roos, and J.P. Richard Van Merkesteyn. 2016. "‘Autoreconstruction’ of the Mandible—Report of a Case" Dentistry Journal 4, no. 2: 9. https://doi.org/10.3390/dj4020009




