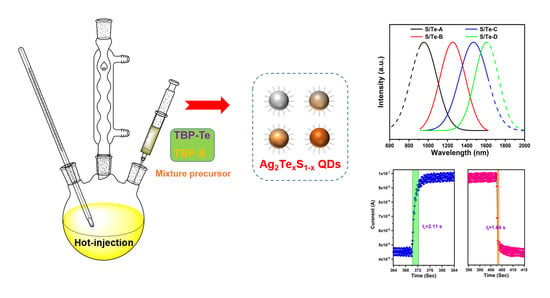
Engineering Band Gap of Ternary Ag2TexS1−x Quantum Dots for Solution-Processed Near-Infrared Photodetectors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of TBP-Te and TBP-S Precursors
2.3. Synthesis of Ag2TexS1−x QDs
2.4. Photodetector Fabrication
2.5. Characterizations
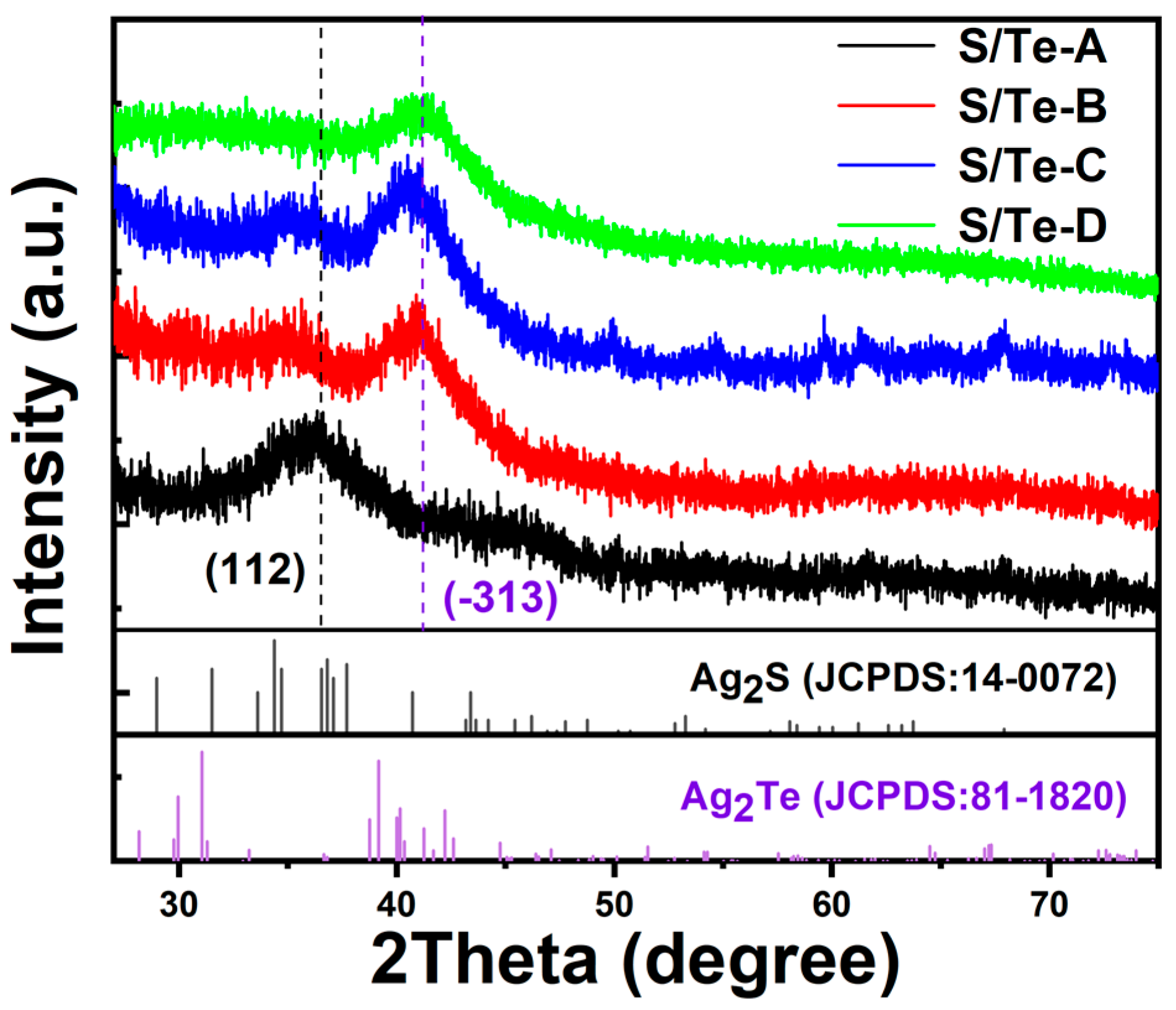
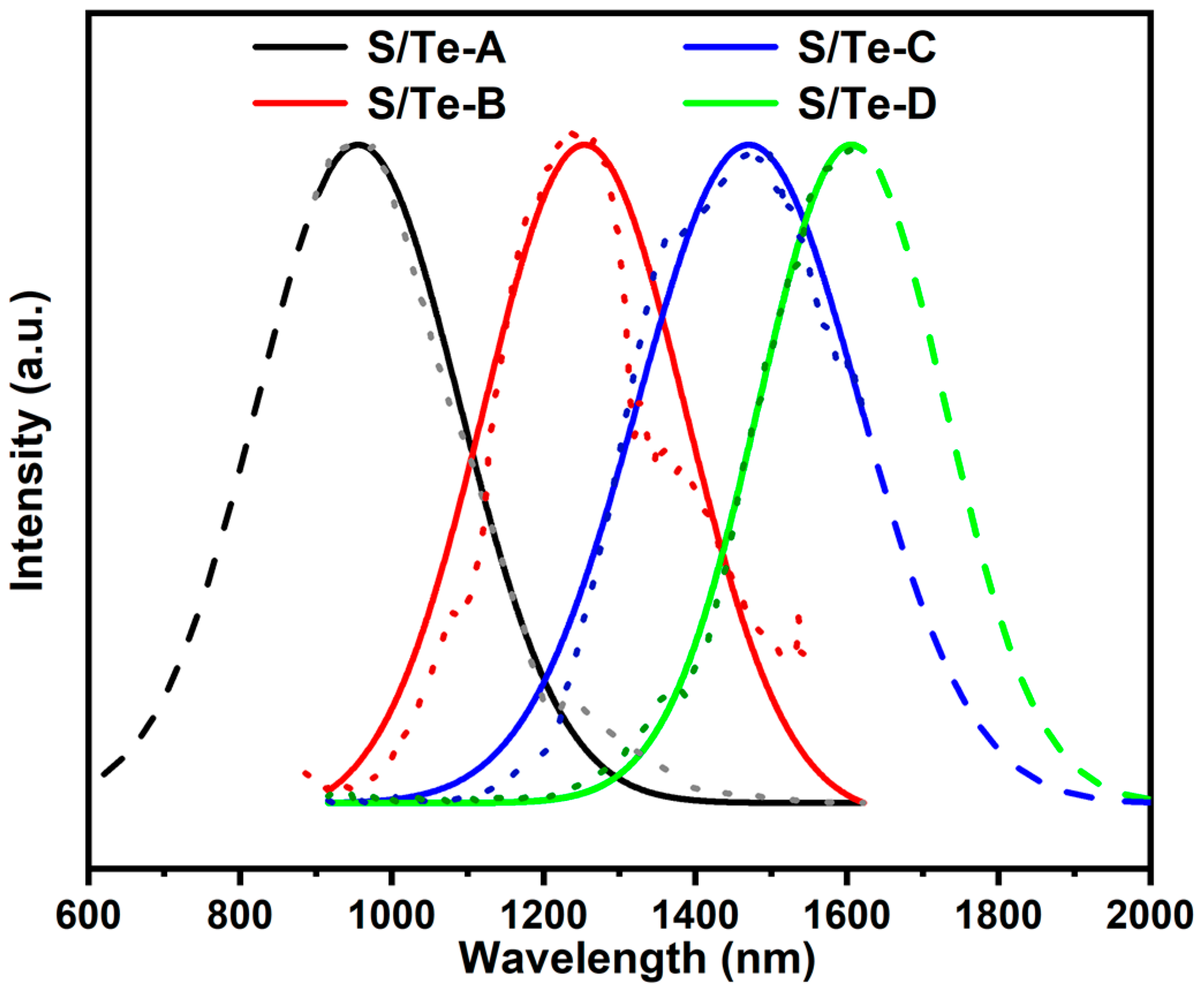
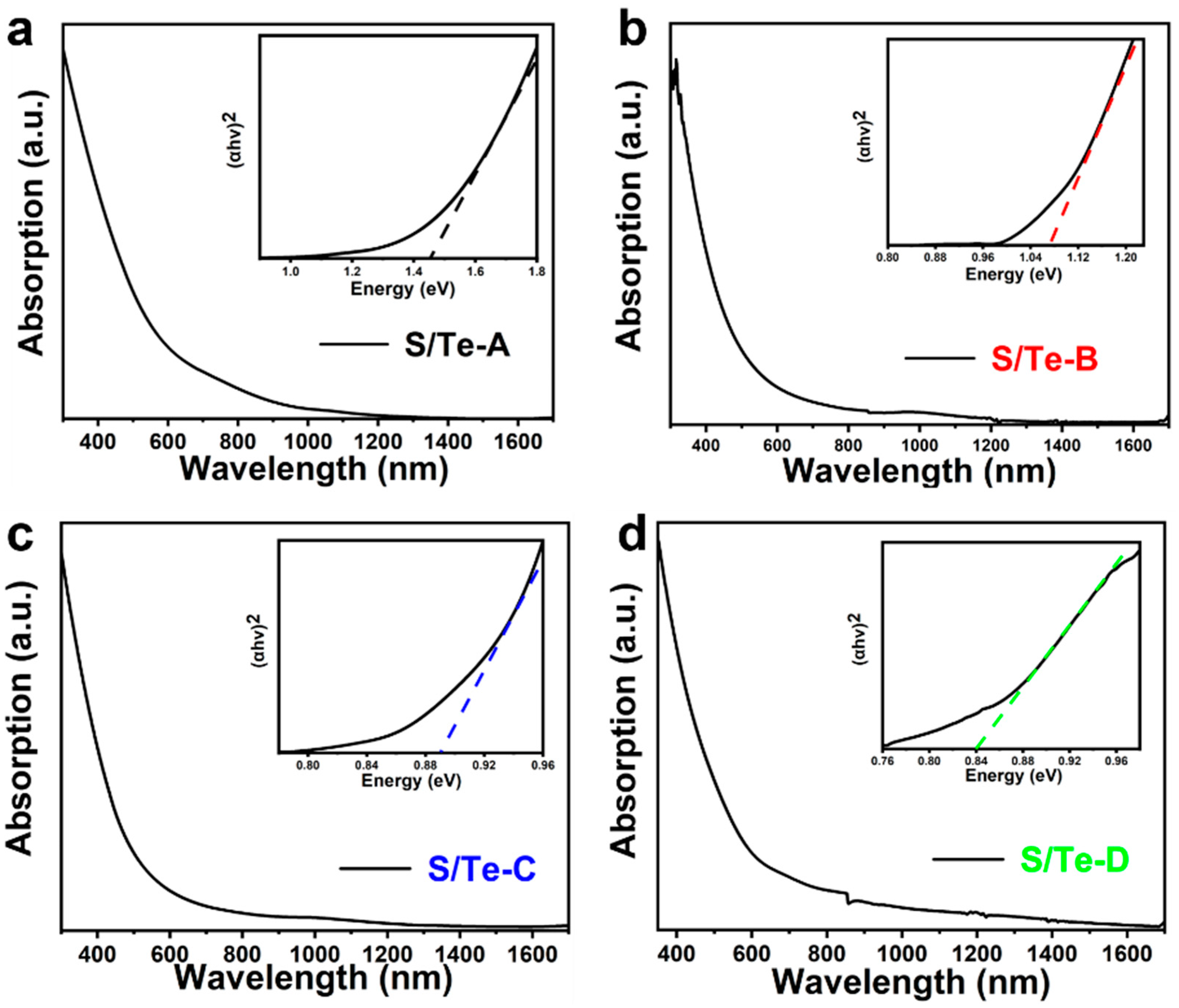
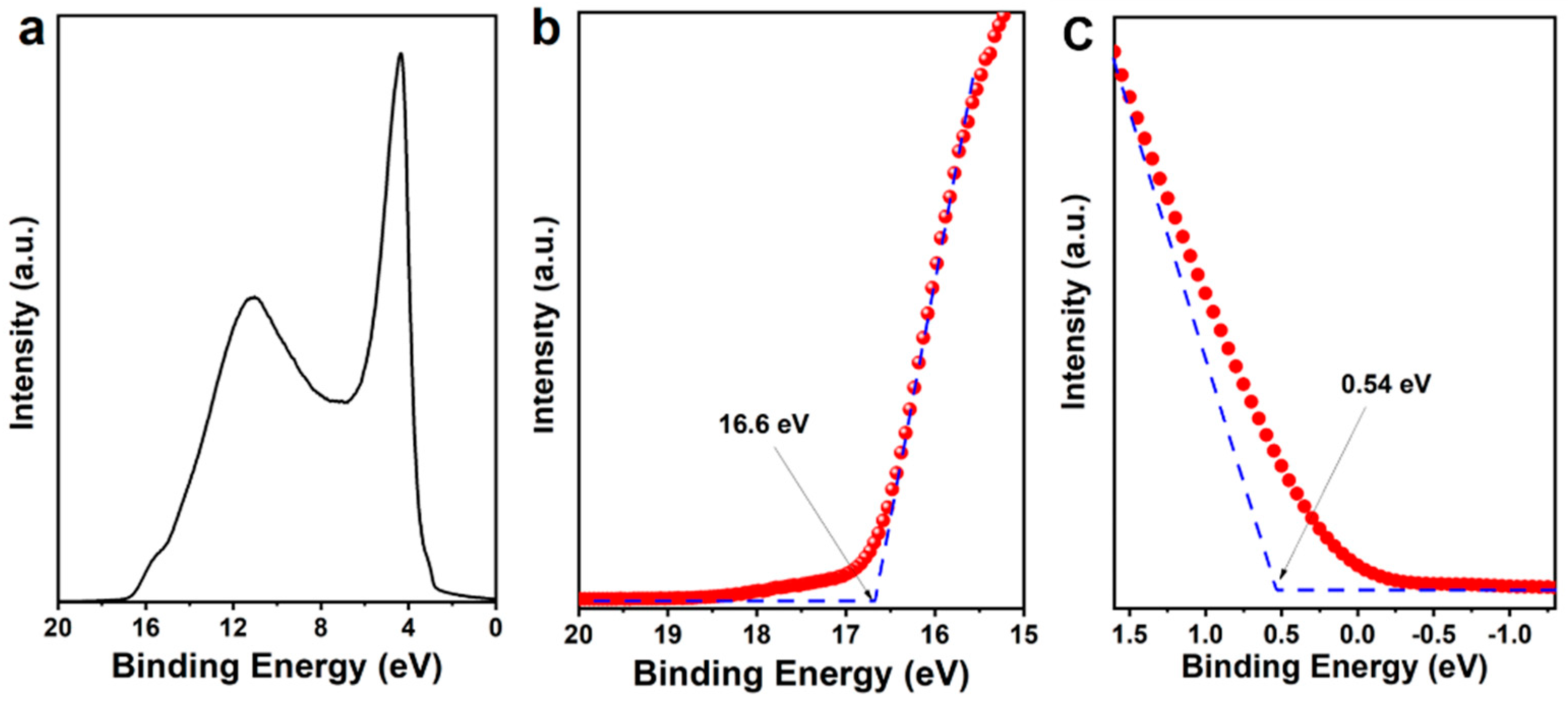
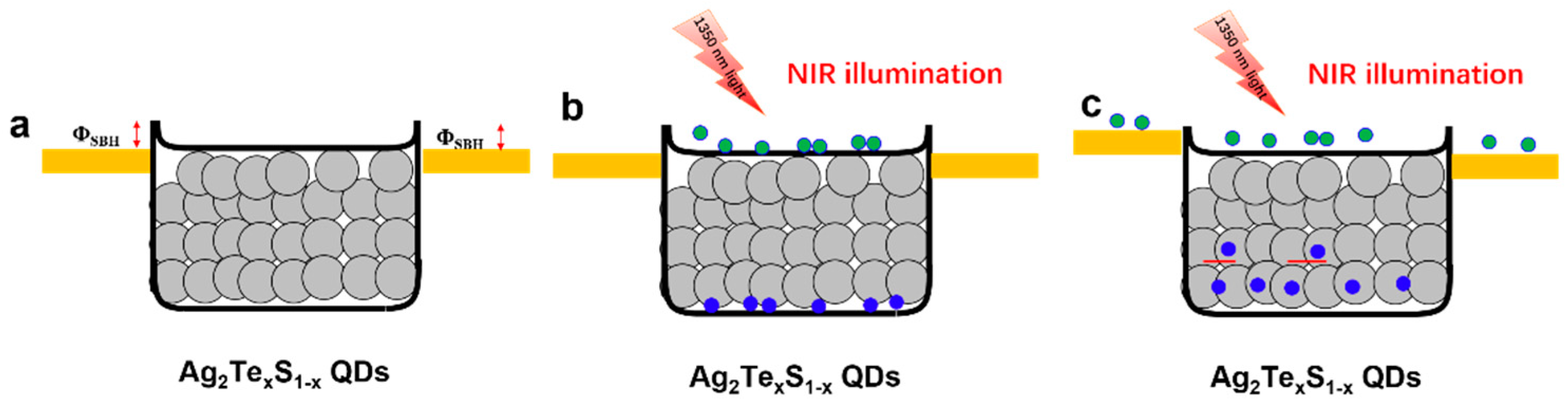
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aiello, R.; Di Sarno, V.; Santi, M.G.D.; De Rosa, M.; Ricciardi, I.; Giusfredi, G.; De Natale, P.; Santamaria, L.; Maddaloni, P. Lamb-dip saturated-absorption cavity ring-down rovibrational molecular spectroscopy in the near-infrared. Photonics Res. 2022, 10, 1803–1809. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Li, Y.; Jiang, C.H.; Sun, W.B.; Tao, J.W.; Lu, L.H. Near-infrared light-enhanced generation of hydroxyl radical for cancer immunotherapy. Adv. Healthc. Mater. 2023, 12, 2301502. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, M.D.; Dong, F.L.; Feng, L.F.; Chu, W.G. Broadband achromatic polarization insensitive metalens over 950 nm bandwidth in the visible and near-infrared. Chin. Opt. Lett. 2022, 20, 013601. [Google Scholar] [CrossRef]
- Tan, L.L.; Fu, Y.Q.; Kang, S.L.; Wondraczek, L.; Lin, C.G.; Yue, Y.Z. Broadband NIR-emitting Te cluster-doped glass for smart light source towards night-vision and NIR spectroscopy applications. Photonics Res. 2022, 10, 1187–1193. [Google Scholar] [CrossRef]
- Wang, Y.H.; Bai, S.C.; Sun, J.; Liang, H.; Li, C.; Tan, T.Y.; Yang, G.; Wang, J.W. Highly efficient visible and near-infrared luminescence of Sb3+, Tm3+ co-doped Cs2NaYCl6 lead-free double perovskite and light emitting diodes. J. Alloys Compd. 2023, 947, 169602. [Google Scholar] [CrossRef]
- Kufer, D.; Nikitskiy, I.; Lasanta, T.; Navickaite, G.; Koppens, F.H.L.; Konstantatos, G. Hybrid 2D-0D MoS2-PbS quantum dot photodetectors. Adv. Mater. 2015, 27, 176–180. [Google Scholar] [CrossRef]
- Zhang, H.; Kumar, S.; Sua, Y.M.; Zhu, S.Y.; Huang, Y.P. Near-infrared 3D imaging with upconversion detection. Photonics Res. 2022, 10, 2760–2767. [Google Scholar] [CrossRef]
- Zeng, X.K.; Wang, C.Y.; Cai, Y.; Lin, Q.G.; Lu, X.W.; Lin, J.H.; Yuan, X.M.; Cao, W.H.; Ai, Y.X.; Xu, S.X. High spatial-resolution biological tissue imaging in the second near-infrared region via optical parametric amplification pumped by an ultrafast vortex pulse. Chin. Opt. Lett. 2022, 20, 100003. [Google Scholar] [CrossRef]
- Konstantatos, G.; Badioli, M.; Gaudreau, L.; Osmond, J.; Bernechea, M.; Garcia de Arquer, F.P.; Gatti, F.; Koppens, F.H.L. Hybrid graphene-quantum dot phototransistors with ultrahigh gain. Nat. Nanotechnol. 2012, 7, 363–368. [Google Scholar] [CrossRef]
- Koppens, F.H.L.; Mueller, T.; Avouris, P.; Ferrari, A.C.; Vitiello, M.S.; Polini, M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat. Nanotechnol. 2014, 9, 780–793. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.B.; Huang, F.F.; Hua, Y.J.; Tian, Y.; Zhang, X.H.; Xu, S.Q. Multifunctional optical materials based on transparent inorganic glasses embedded with PbS QDs. J. Alloys Compd. 2023, 942, 169040. [Google Scholar] [CrossRef]
- Semonin, O.E.; Luther, J.M.; Choi, S.; Chen, H.Y.; Gao, J.B.; Nozik, A.J.; Beard, M.C. Peak external photocurrent quantum efficiency exceeding 100% via MEG in a quantum dot solar cell. Science 2011, 334, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Lu, H.P.; Abdelazim, N.M.; Zhu, Y.; Wang, Z.; Ren, W.; Kershaw, S.V.; Rogach, A.L.; Zhao, N. Mercury telluride quantum dot based phototransistor enabling high-sensitivity room-temperature photodetection at 2000 nm. ACS Nano 2017, 11, 5614–5622. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.M.; Zhou, L.; Zhang, F. Epitaxial seeded growth of rare-earth nanocrystals with efficient 800 nm near-infrared to 1525 nm short-wavelength infrared downconversion photoluminescence for in vivo bioimaging. Angew. Chem. Int. Ed. 2014, 53, 12086–12090. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Dong, B.H.; Li, C.Y.; Chen, G.C.; Zhang, Y.J.; Zhang, Y.; Deng, M.J.; Wang, Q.B. Facile synthesis of highly photoluminescent Ag2Se quantum dots as a new fluorescent probe in the second near-infrared window for in vivo imaging. Chem. Mater. 2013, 25, 2503–2509. [Google Scholar] [CrossRef]
- Gu, Y.P.; Cui, R.; Zhang, Z.L.; Xie, Z.X.; Pang, D.W. Ultrasmall near-infrared Ag2Se quantum dots with tunable fluorescence for in vivo imaging. J. Am. Chem. Soc. 2012, 134, 79–82. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, Y.J.; Wang, M.; Zhang, Y.; Chen, G.C.; Li, L.; Wu, D.M.; Wang, Q.B. In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. Biomaterials 2014, 35, 393–400. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, C.L.; Wei, R.; Zou, Y.T.; Kong, W.C.; Huang, T.; Yu, Z.; Yang, J.J.; Li, F.; Han, Y.; et al. Laser-assisted synthesis of Ag2S-quantum-dot-in-perovskite matrix and its application in broadband photodetectors. Adv. Opt. Mater. 2022, 10, 2101535. [Google Scholar] [CrossRef]
- Martin-Garcia, B.; Spirito, D.; Krahne, R.; Moreels, I. Solution-processed silver sulphide nanocrystal film for resistive switching memories. J. Mater. Chem. C 2018, 6, 13128–13135. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, C.; Lu, H.Y.; Zou, T.T.; Singh, S.C.; Yu, Z.; Yao, C.M.; Zheng, X.; Xing, J.; Zou, Y.T.; et al. SERS study on the synergistic effects of electric field enhancement and charge transfer in an Ag2S quantum dots/plasmonic bowtie nanoantenna composite system. Photonics Res. 2020, 8, 548–563. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, G.S.; Zhang, Y.J.; Chen, G.C.; Li, F.; Dai, H.J.; Wang, Q.B. Ag2S quantum dot: A bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano 2012, 6, 3695–3702. [Google Scholar] [CrossRef]
- Ji, C.Y.; Zhang, Y.; Zhang, X.Y.; Wang, P.; Shen, H.Z.; Gao, W.Z.; Wang, Y.D.; Yu, W.W. Synthesis and characterization of Ag2SxSe1-x nanocrystals and their photoelectrochemical property. Nanotechnology 2017, 28, 065602. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.E.; Nie, S.M. Alloyed semiconductor quantum dots: Tuning the optical properties without changing the particle size. J. Am. Chem. Soc. 2003, 125, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Luther, J.M.; Semonin, O.E.; Nozik, A.J.; Beard, M.C. Tuning the synthesis of ternary lead chalcogenide quantum dots by balancing precursor reactivity. ACS Nano 2011, 5, 183–190. [Google Scholar] [CrossRef]
- Kim, T.; Kim, K.H.; Kim, S.; Choi, S.M.; Jang, H.; Seo, H.K.; Lee, H.; Chung, D.Y.; Jang, E. Efficient and stable blue quantum dot light-emitting diode. Nature 2020, 586, 385–389. [Google Scholar] [CrossRef]
- Qian, H.; Dong, C.; Peng, J.; Qiu, X.; Xu, Y.; Ren, J. High-quality and water-soluble near-infrared photoluminescent CdHgTe/CdS quantum dots prepared by adjusting size and composition. J. Phys. Chem. C 2007, 111, 16852–16857. [Google Scholar] [CrossRef]
- Nakazawa, T.; Kim, D.; Oshima, Y.; Sato, H.; Park, J.; Kim, H. Synthesis and application of AgBiS2 and Ag2S nanoinks for the production of IR photodetectors. ACS Omega 2021, 6, 20710–20718. [Google Scholar] [CrossRef]
- Grotevent, M.J.; Hail, C.U.; Yakunin, S.; Bachmann, D.; Kara, G.; Dirin, D.N.; Calame, M.; Poulikakos, D.; Kovalenko, M.V.; Shorubalko, I. Temperature-dependent charge carrier transfer in colloidal quantum dot/graphene infrared photodetectors. ACS Appl. Mater. Interfaces 2021, 13, 848–856. [Google Scholar] [CrossRef]
- Bhatt, V.; Kumar, M.; Kim, E.C.; Chung, H.J.; Yun, J.H. Wafer-scale, thickness-controlled p-CuInSe2/n-Si heterojunction for self-biased, highly sensitive, and broadband photodetectors. ACS Appl. Electron. 2022, 4, 6284–6299. [Google Scholar] [CrossRef]
- Hu, H.; Wu, C.; He, C.; Shen, J.; Cheng, Y.; Wu, F.; Wang, S.; Guo, D. Improved photoelectric performance with self-powered characteristics through TiO2 surface passivation in an α- Ga2O3 nanorod array deep ultraviolet photodetector. ACS Appl. Electron. Mater. 2022, 4, 3801–3806. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yang, H.C.; An, X.Y.; Wang, Z.; Yang, X.H.; Yu, M.X.; Zhang, R.; Sun, Z.Q.; Wang, Q.B. Controlled synthesis of Ag2Te@Ag2S core-shell quantum dots with enhanced and tunable fluorescence in the second near-infrared window. Small 2020, 16, 2001003. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.H.; Gu, Y.J.; Hu, Y.G.; Wu, W.P. Solution-processed self-powered near-infrared photodetectors of toxic heavy metal-free AgAuSe colloidal quantum dots. J. Mater. Chem. C 2022, 10, 1097–1104. [Google Scholar] [CrossRef]
- Kim, G.; Choi, D.; Eom, S.Y.; Song, H.; Jeong, K.S. Extended short-wavelength infrared photoluminescence and photocurrent of nonstoichiometric silver telluride colloidal nanocrystals. Nano Lett. 2021, 21, 8073–8079. [Google Scholar] [CrossRef]
- Yan, S.G.; Deng, D.Y.; Li, L.; Chen, Y.C.; Song, H.J.; Lv, Y. Glutathione modified Ag2Te nanoparticles as a resonance Rayleigh scattering sensor for highly sensitive and selective determination of cytochrome C. Sens. Actuators B Chem. 2016, 228, 458–464. [Google Scholar] [CrossRef]
- Chang, P.J.; Cheng, H.Y.; Zhao, F.Y. Photocatalytic reduction of aromatic nitro compounds with Ag/AgxS composites under visible light irradiation. J. Phys. Chem. C 2021, 125, 26021–26030. [Google Scholar] [CrossRef]
- Dinda, D.; Ahmed, M.E.; Mandal, S.; Mondal, B.; Saha, S.K. Amorphous molybdenum sulfide quantum dots: An efficient hydrogen evolution electrocatalyst in neutral medium. J. Mater. Chem. A 2016, 4, 15486–15493. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. VEGARD LAW. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, S.V.; Susha, A.S.; Rogach, A.L. Narrow bandgap colloidal metal chalcogenide quantum dots: Synthetic methods, heterostructures, assemblies, electronic and infrared optical properties. Chem. Soc. Rev. 2013, 42, 3033–3087. [Google Scholar] [CrossRef]
- Du, Y.P.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q.B. Near-infrared photoluminescent Ag2S quantum dots from a single source precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.J.; Hong, G.S.; He, W.; Zhou, K.; Yang, K.; Li, F.; Chen, G.C.; Liu, Z.; Dai, H.J.; et al. Biodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in mice. Biomaterials 2013, 34, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; He, X.W.; Gao, L.; Ma, N. Cation exchange-based facile aqueous synthesis of small, stable, and nontoxic near-infrared Ag2Te/ZnS core/shell quantum dots emitting in the second biological window. ACS Appl. Mater. Interfaces 2013, 5, 1149–1155. [Google Scholar] [CrossRef]
- Jeon, J.W.; Jeon, D.W.; Sahoo, T.; Kim, M.; Baek, J.H.; Hoffman, J.L.; Kim, N.S.; Lee, I.H. Effect of annealing temperature on optical band-gap of amorphous indium zinc oxide film. J. Alloys Compd. 2011, 509, 10062–10065. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.J.; Li, X.M.; Liu, Y.; Liu, F.H.; Wu, W.P. Recent progress of quantum dot infrared photodetectors. Adv. Opt. Mater. 2023, 11, 2300970. [Google Scholar] [CrossRef]
- Guo, D.Y.; Wu, Z.P.; An, Y.H.; Guo, X.C.; Chu, X.L.; Sun, C.L.; Li, L.H.; Li, P.G.; Tang, W.H. Oxygen vacancy tuned Ohmic-Schottky conversion for enhanced performance in β-Ga2O3 solar-blind ultraviolet photodetectors. Appl. Phys. Lett. 2014, 105, 023507. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gu, Y.; Aleksandrov, D.; Liu, F.; He, H.; Wu, W. Engineering Band Gap of Ternary Ag2TexS1−x Quantum Dots for Solution-Processed Near-Infrared Photodetectors. Inorganics 2024, 12, 1. https://doi.org/10.3390/inorganics12010001
Wang Z, Gu Y, Aleksandrov D, Liu F, He H, Wu W. Engineering Band Gap of Ternary Ag2TexS1−x Quantum Dots for Solution-Processed Near-Infrared Photodetectors. Inorganics. 2024; 12(1):1. https://doi.org/10.3390/inorganics12010001
Chicago/Turabian StyleWang, Zan, Yunjiao Gu, Daniil Aleksandrov, Fenghua Liu, Hongbo He, and Weiping Wu. 2024. "Engineering Band Gap of Ternary Ag2TexS1−x Quantum Dots for Solution-Processed Near-Infrared Photodetectors" Inorganics 12, no. 1: 1. https://doi.org/10.3390/inorganics12010001