Synthesis, Structure and Photoluminescence Properties of Cd and Cd-Ln Pentafluorobenzoates with 2,2′:6′,2′-Terpyridine Derivatives
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of the Compounds
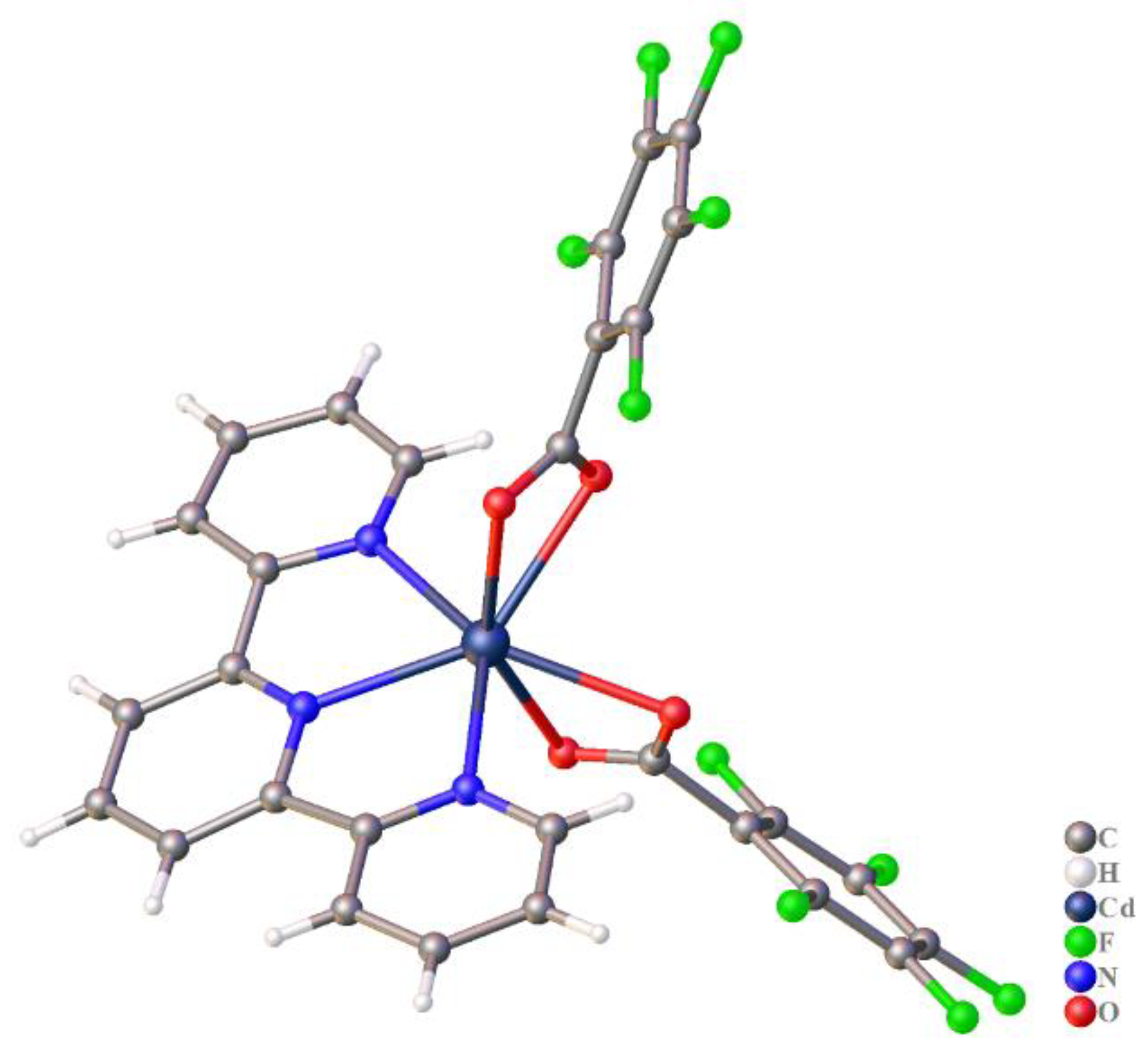
2.2.1. [Cd(tpy)(pfb)2] (1)
2.2.2. [Eu2Cd2(tpy)2(pfb)10] (2Eu)
2.2.3. [Tb2Cd2(tpy)2(pfb)10] (2Tb)
2.2.4. [Eu2Cd2(tbtpy)2(pfb)10]×2MeCN (3Eu)
2.2.5. [Tb2Cd2(tbtpy)2(pfb)10]×2MeCN (3Tb)
2.2.6. [Eu2Cd2(tppz)(pfb)10]n (4)
2.3. X-ray Diffraction Studies
2.4. Photo-Physical Measurements
3. Results
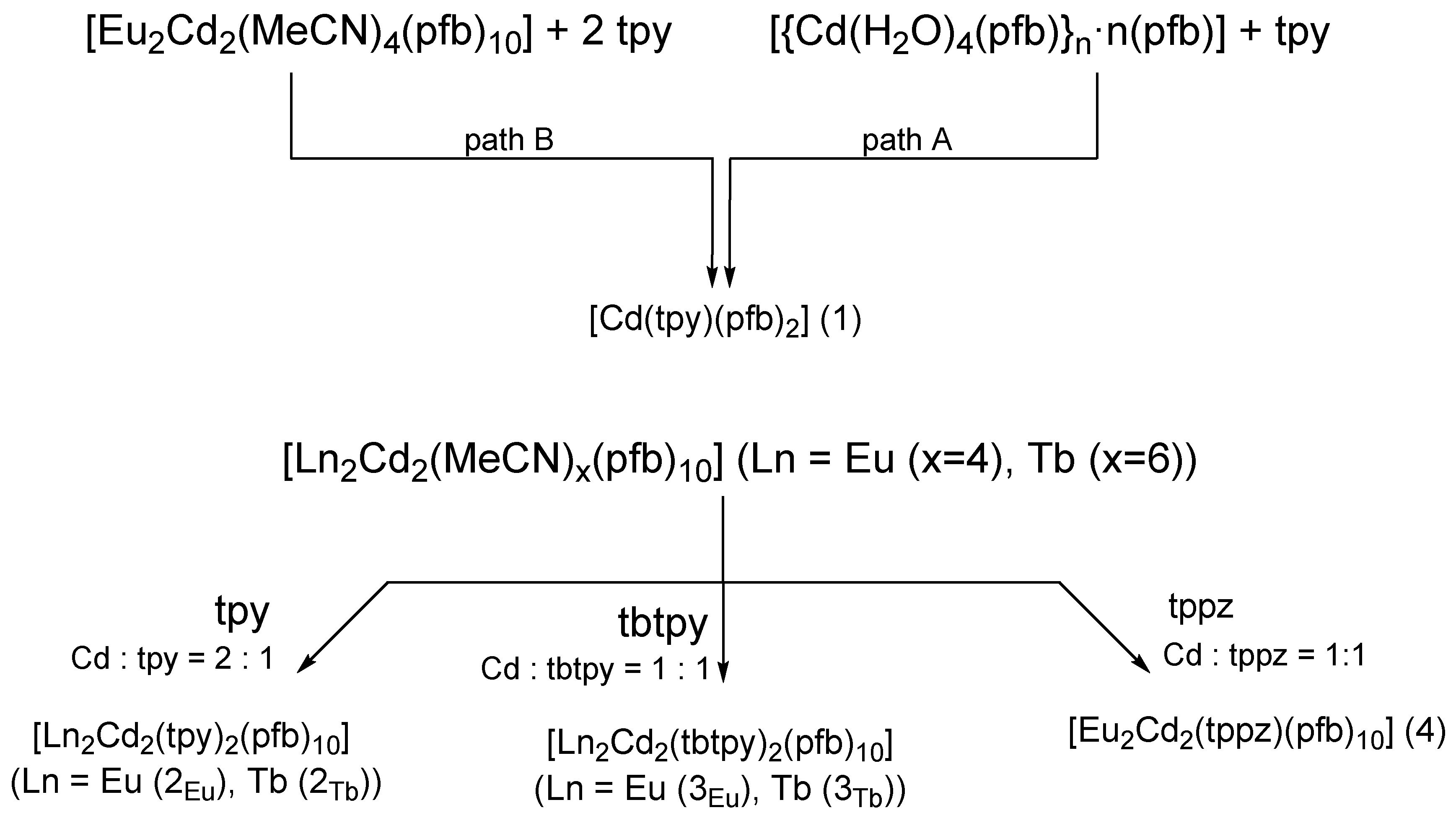
3.1. Synthesis of Complexes
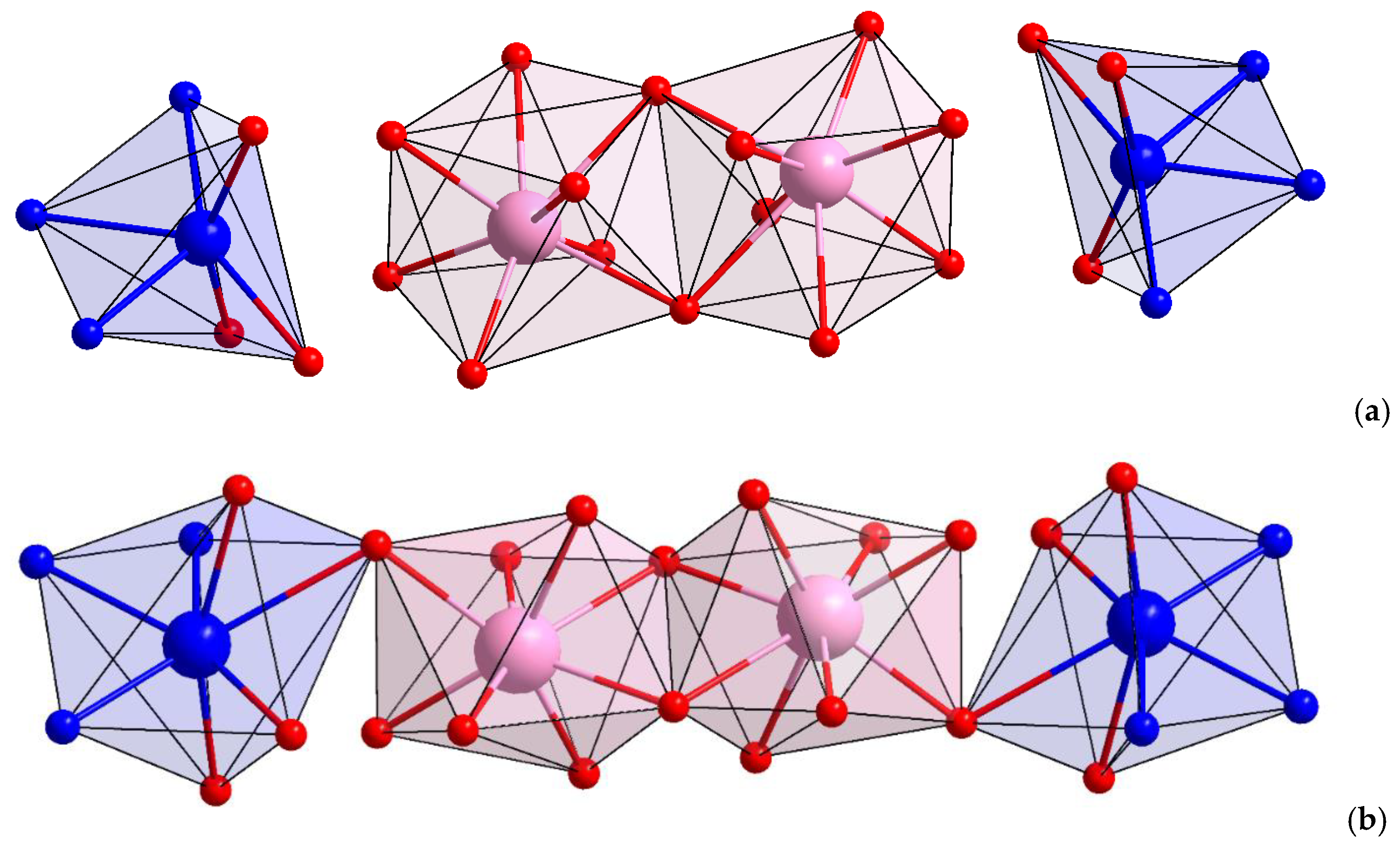
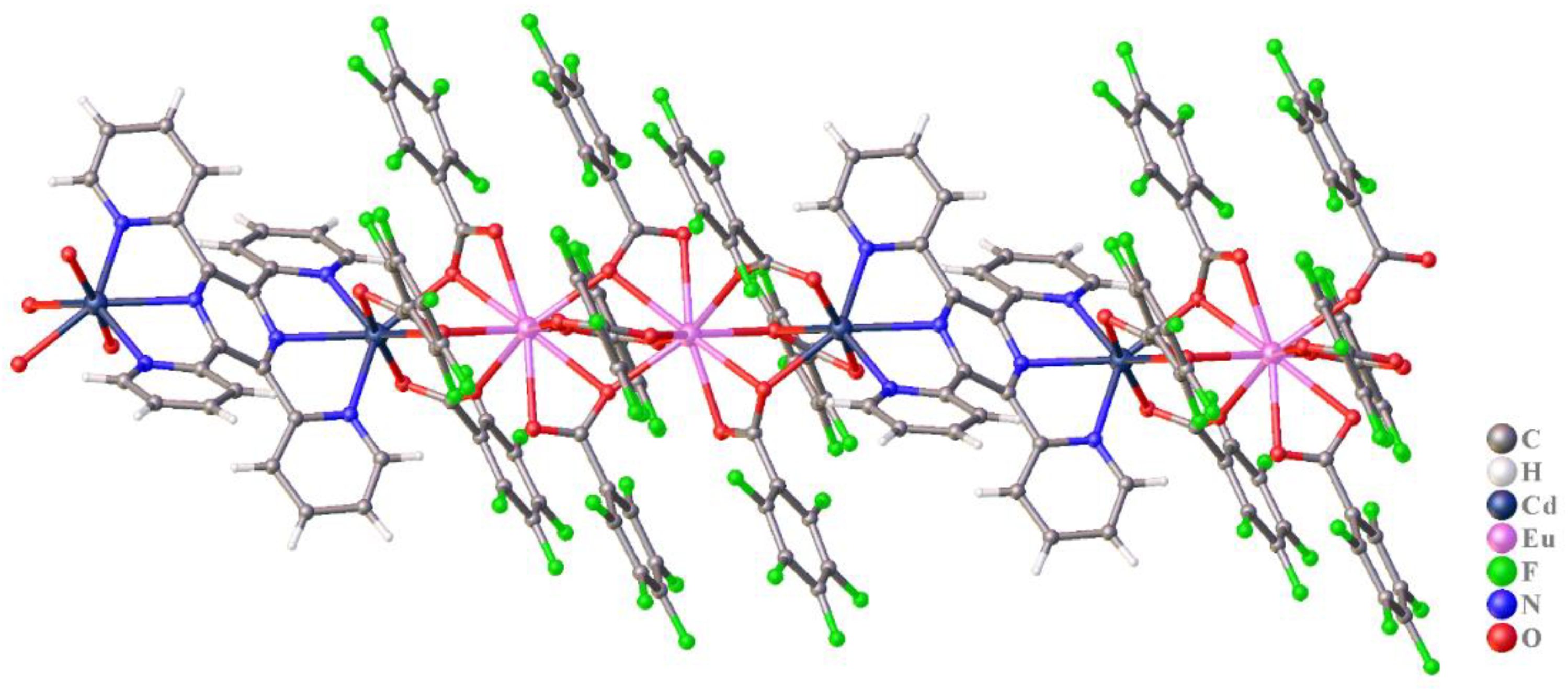
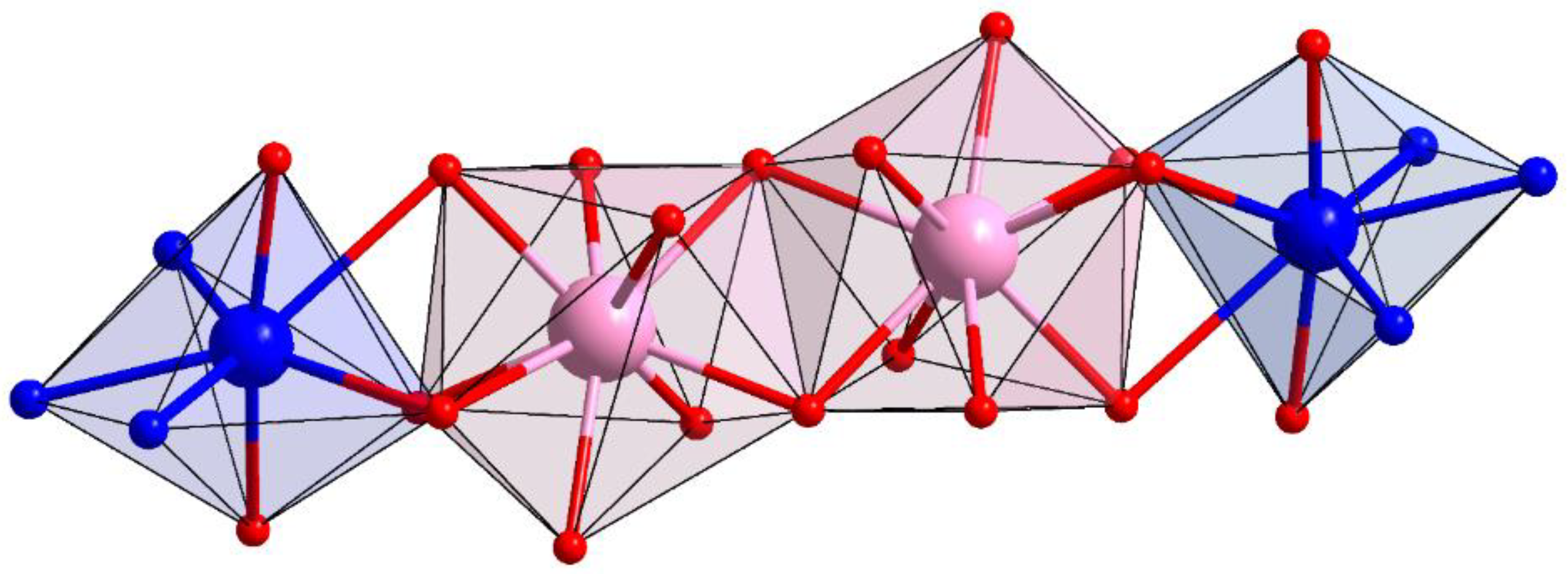
3.2. The Structure of Complexes
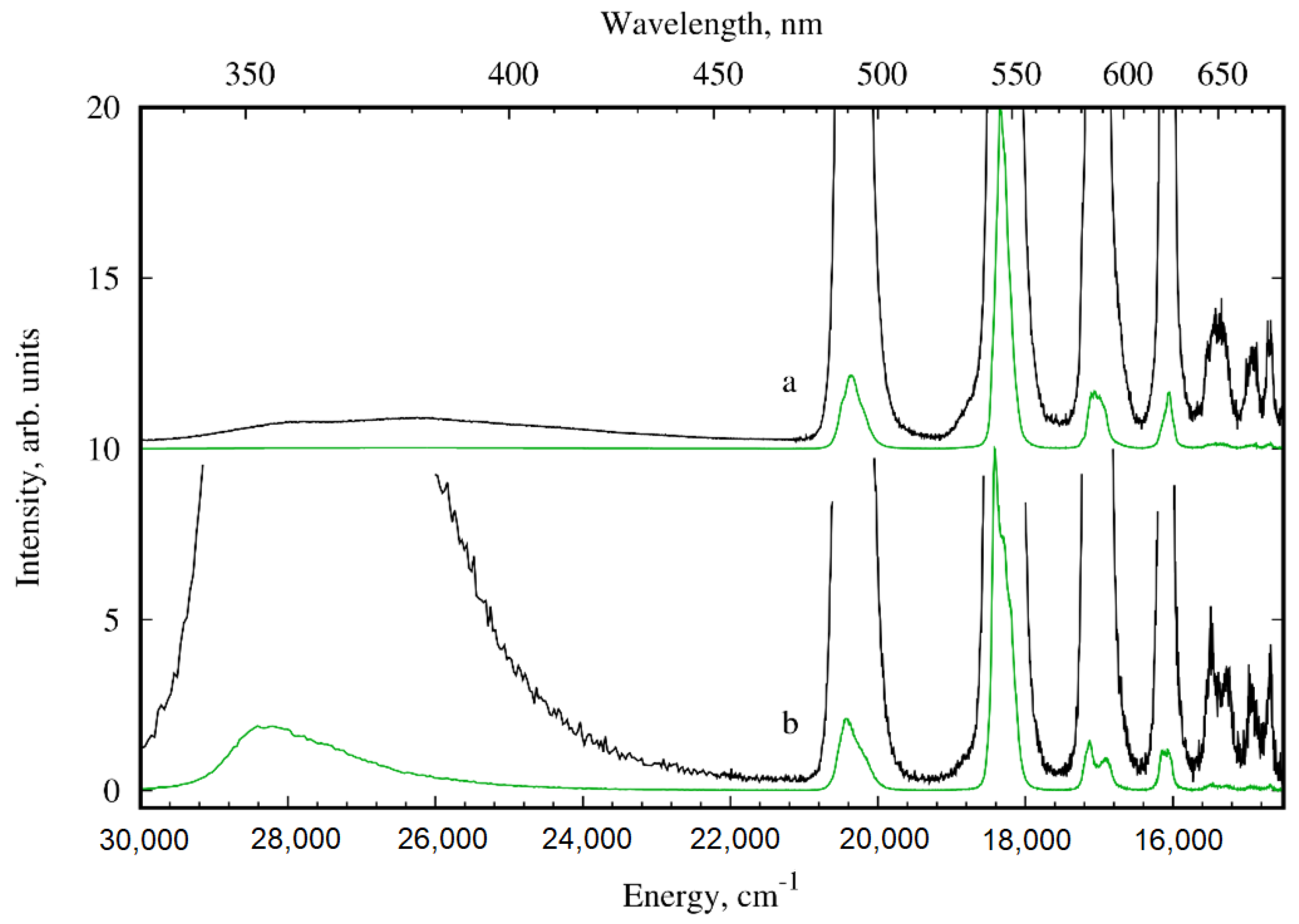
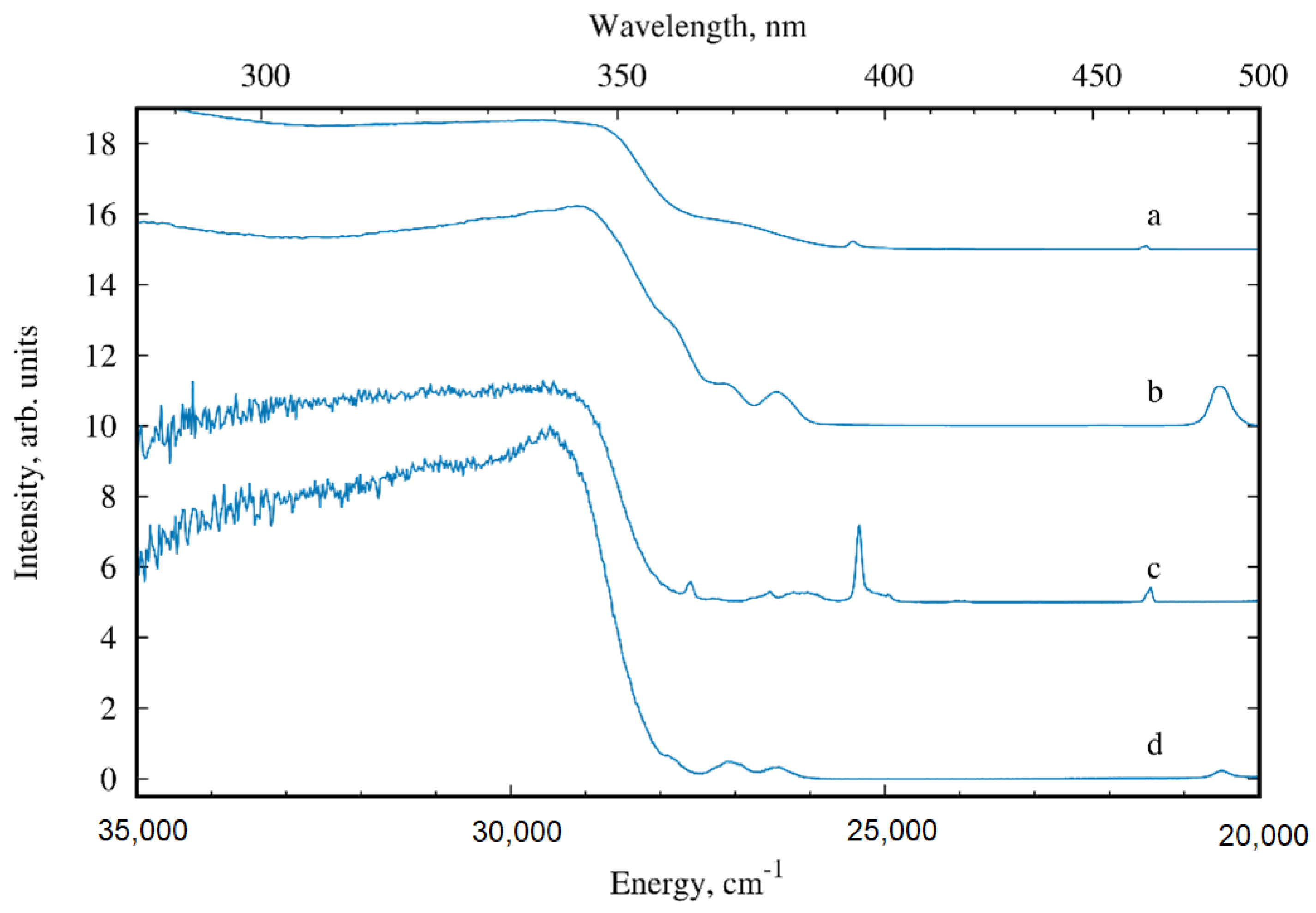
3.3. Photoluminescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, L.-J.; Kong, Y.-J.; Sheng, N.; Jiang, X.-L. A new europium fluorous metal–organic framework with pentafluorobenzoate and 1,10-phenanthroline ligands: Synthesis, structure and luminescent properties. J. Fluor. Chem. 2014, 166, 122–126. [Google Scholar] [CrossRef]
- Efimov, N.N.; Koroteev, P.S.; Gavrikov, A.V.; Ilyukhin, A.B.; Dobrokhotova, Z.V.; Novotortsev, V.M. Magnetic Behavior of Carboxylate and β-Diketonate Lanthanide Complexes Containing Stable Organometallic Moieties in the Core-Forming Ligand. Magnetochemistry 2016, 2, 38. [Google Scholar] [CrossRef] [Green Version]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Wang, L.; Zhao, Z.; Fang, P.; Bian, Z.; Liu, Z. Highly Efficient and Air-Stable Lanthanide EuII Complex: New Emitter in Organic Light Emitting Diodes. Angew. Chem. 2020, 59, 19011–19015. [Google Scholar] [CrossRef]
- Zhao, S.N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Chakraborty, A.; Maji, T.K. Lanthanide–organic frameworks for gas storage and as magneto-luminescent materials. Coord. Chem. Rev. 2014, 273–274, 139–164. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Polunin, R.A.; Gogoleva, N.V.; Evstifeev, I.S.; Vasilyev, P.N.; Dmitriev, A.A.; Varaksina, E.A.; Efimov, N.N.; Taydakov, I.V.; Sidorov, A.A.; et al. Cadmium-inspired self-polymerization of {LnIIICd2} units: Structure, magnetic and photoluminescent properties of novel trimethylacetate 1D-polymers (Ln = Sm, Eu, Tb, Dy, Ho, Er, Yb). Molecules 2021, 26, 4296. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F. Molecular Magnetism. Annu. Rev. Mater. Res. 2022, 52, 79–101. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Sokolov, M.N. Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines. Crystals 2020, 10, 289. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, M.A.; Adonin, S.A.; Novikov, A.S.; Sokolov, M.N.; Fedin, V.P. Supramolecular Bromoantimonate(V) Polybromide (2,6-BrPyH)3[SbBr6]{(Br2)Br} · 2H2O: Specific Features of Halogen···Halogen Contacts in the Crystal Structure. Russ. J. Coord. Chem. 2020, 46, 302. [Google Scholar] [CrossRef]
- Adonin, S.A.; Novikov, A.S.; Fedin, V.P. Crystal Structure of the Heteroligand Complex [(2-Br-5-MePy)2CoCl2] · (2-Br-5-MePy): Formation of Supramolecular Associates due to the Halogen Bond. Russ. J. Coord. Chem. 2020, 46, 37. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Chistyakov, A.S.; Razgonyaeva, G.A.; Kovalev, V.V.; Voronina, J.K.; Dolgushin, F.M.; Gogoleva, N.V.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Effect of non-covalent interactions on the 2,4-and 3,5-dinitrobenzoate Eu-Cd complex structures. Crystals 2022, 12, 508. [Google Scholar] [CrossRef]
- Zhou, W.-L.; Chen, Y.; Lin, W.; Liu, Y. Luminescent lanthanide–macrocycle supramolecular assembly. Chem. Commun. 2021, 57, 11443–11456. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-covalent intramolecular interactions through ligand-design promoting efficient photoluminescence from transition metal complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Itoh, T.; Kondo, M.; Kanaike, M.; Masaoka, S. Arene–perfluoroarene interactions for crystal engineering of metal complexes: Controlled self-assembly of paddle-wheel dimers. CrystEngComm 2013, 15, 6122–6126. [Google Scholar] [CrossRef]
- Kusakawa, T.; Sakai, S.; Nakajima, K.; Yuge, H.; Rzeznicka, I.; Hori, A. Synthesis, structures and co-crystallizations of perfluorophenyl substituted β-diketone and triketone compounds. Crystals 2019, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Cockcroft, J.K.; Li, J.G.Y.; Williams, J.H. Influence of methyl-substitution on the dynamics of the C–H⋯F–C interaction in binary adducts. CrystEngComm 2019, 21, 5578–5585. [Google Scholar] [CrossRef] [Green Version]
- Ikumura, Y.; Habuka, Y.; Sakai, S.; Shinohara, T.; Yuge, H.; Rzeznicka, I.I.; Hori, A. Enhanced and Heteromolecular Guest Encapsulation in Nonporous Crystals of a Perfluorinated Triketonato Dinuclear Copper Complex. Chem.-Eur. J. 2020, 26, 5051. [Google Scholar] [CrossRef]
- Utochnikova, V.V.; Kuzmina, N.P. Photoluminescence of lanthanide aromatic carboxylates. Russ. J. Coord. Chem. 2016, 42, 679. [Google Scholar] [CrossRef]
- Zeng, Y.; Niu, Y.; Peng, Q.; Zheng, X. Origin of Nonmonotonical Variation of Luminescence Efficiency under Pressure in Organic Molecule. J. Phys. Chem. A 2022, 126, 4147–4155. [Google Scholar] [CrossRef] [PubMed]
- Oguadinma, P.O.; Rodrigue-Witchel, A.; Reber, C.; Schaper, F. Intramolecular π-stacking in copper(I) diketiminate phenanthroline complexes. Dalton Trans. 2010, 39, 8759–8768. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Kiskin, M.; Shalygina, O.; Kozyukhin, S.; Dobrokhotova, Z.; Nikolaevskii, S.; Sidorov, A.; Sokolov, S.; Timoshenko, V.; Goloveshkin, A.; et al. Tetranuclear heterometallic {Zn2Eu2} complexes with 1-naphthoate anions: Synthesis, structure and photoluminescence properties. Asian J. Chem. 2016, 11, 604. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Kiskin, M.A.; Voronina, J.K.; Babeshkin, K.A.; Efimov, N.N.; Varaksina, E.A.; Korshunov, V.M.; Taydakov, I.V.; Gogoleva, N.V.; Sidorov, A.A.; et al. Molecular and Polymer Ln2M2 (Ln = Eu, Gd, Tb, Dy; M = Zn, Cd) Complexes with Pentafluorobenzoate Anions: The Role of Temperature and Stacking Effects in the Structure; Magnetic and Luminescent Properties. Materials 2020, 13, 5689. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Kuznetsova, G.N.; Dolgushin, F.M.; Voronina Yu, K.; Gogoleva, N.V.; Kiskin, M.A.; Ivanov, V.K.; Sidorov, A.A.; Eremenko, I.L. Influence of the Fluorinated Aromatic Fragments on the Structures of the Cadmium and Zinc Carboxylate Complexes Using Pentafluorobenzoates and 2,3,4,5-Tetrafluorobenzoates as Examples. Russ. J. Coord. Chem. 2021, 47, 127. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Kuznetsova, G.N.; Kiskin, M.A.; Voronina, Y.K.; Yakushev, I.A.; Ivanova, T.M.; Nelyubina, Y.V.; Sidorov, A.A.; Eremenko, I.L. Cd(II) and Cd(II)–Eu(III) Complexes with Pentafluorobenzoic Acid Anions and N-Donor Ligands: Synthesis and Structures. Russ. J. Coord. Chem. 2020, 46, 557–572. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Voronina Yu, K.; Gogoleva, N.V.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. Synthesis and Crystal Structure of {Eu2IIICd2}, {Tb2IIICd2} and {Eu2IIIZn2} Complexes with Pentafluorobenzoic Acid Anions and Acetonitrile. Russ. J. Coord. Chem. 2022, 48, 224–232. [Google Scholar] [CrossRef]
- SMART (Control) and SAINT (Integration) Software, Version 5.0; Bruker AXS Inc.: Madison, WI, USA, 1997.
- Sheldrick, G.M. SADABS-2004/1, Program for Scaling and Correction of Area Detector Data; Göttingen University: Göttinngen, Germany, 2004. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Gogoleva, N.V.; Kuznetsova, G.N.; Shmelev, M.A.; Lyssenko, K.A.; Kayumova, D.B.; Malkerova, I.P.; Alikhanyan, A.S.; Starikova, A.A.; Barabanova, E.D.; Kiskin, M.A.; et al. Polynuclear architectures with cadmium and lithium ions based on the {Li2Cd2(O2CCMe3)6} fragment. J. Solid State Chem. 2021, 294, 121842. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Sidorov, A.A.; Kiskin, M.A.; Voronina, J.K.; Nelyubina, Y.V.; Varaksina, E.A.; Korshunov, V.M.; Taydakov, I.V.; Eremenko, I.L. Coordination polymers based on 3,5-di-tert-butylbenzoate {Cd2Eu} moieties. Inorg. Chim. Acta. 2021, 51524, 120050. [Google Scholar] [CrossRef]
- Sidorov, A.A.; Gogoleva, N.V.; Bazhina, E.S.; Nikolaevskii, S.A.; Shmelev, M.A.; Zorina-Tikhonova, E.N.; Starikov, A.G.; Kiskin, M.A.; Eremenko, I.L. Some aspects of the formation and structural features of low nuclearity heterometallic carboxylates. Pure Appl. Chem. 2020, 92, 1093–1110. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Sidorov, A.A.; Nelyubina, Y.A.; Dolgushin, F.M.; Voronina, Y.K.; Kiskin, M.A.; Aleksandrov, G.G.; Varaksina, E.A.; Taydakov, I.V.; et al. Chemical Assembling of Heterometallic {Cd–M} (M=Li, Mg, Eu, Tb) Molecules with 3,5-Di-tert-butylbenzoate Bridges and N-Donor Ligands. ChemistrySelect 2020, 5, 8475–8482. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Voronina, Y.K.; Gogoleva, N.V.; Sidorov, A.A.; Kiskin, M.A.; Dolgushin, F.M.; Nelyubina, Y.V.; Aleksandrov, G.G.; Varaksina, E.A.; Taydakov, I.V.; et al. Influence of the steric properties of pyridine ligands on the structure of complexes containing the {LnCd2(bzo)7} fragment. Russ. Chem. Bull. 2020, 69, 1544–1560. [Google Scholar] [CrossRef]
- Subhapriya, G.; Kalyanaraman, S.; Surumbarkuzhali, N.; Vijayalakshmi, S.; Krishnakumar, V. Investigation of intermolecular hydrogen bonding in 2,3,4,5,6 pentafluorobenzoic acid through molecular structure and vibrational analysis—A DFT approach. J. Mol. Struct. 2015, 1083, 48–56. [Google Scholar] [CrossRef]
- Morsali, A. Syntheses and characterization of two new lead(II) acetate complexes, Pb(L)(CH3COO)2, L = 2,2′:6′,2″-terpyridine (tpy) and 2,4,6-tris(2-pyridyl)-1,3,5-triazine (trz), crystal structure of Pb(tpy)(CH3COO)2. Z. für Nat. B 2004, 59, 1039. [Google Scholar] [CrossRef]
- Grdenic, D.; Popovic, Z.; Bruvo, M.; Korpar-Colig, B. Terpyridine complexes of Hg(OCOCF3)2 and Hg(BF4)2. Crystal and molecular structure of mercuric trifluoroacetato-2,2′:6′,2″-terpyridine. Inorg. Chim. Acta 1991, 190, 169. [Google Scholar] [CrossRef]
- Carter, K.P.; Pope, S.J.A.; Cahill, C.L. A series of Ln-p-chlorobenzoic acid–terpyridine complexes: Lanthanide contraction effects, supramolecular interactions and luminescent behavior. CrystEngComm 2014, 16, 1873. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Ren, N.; Zhang, J.-J. Supramolecular of lanthanide-2,6-dimethylbenzoic acid-2,2′:6′,2″-terpyridine materials: Crystal structures, luminescent property, and thermochemical behaviour. Polyhedron 2020, 194, 114892. [Google Scholar] [CrossRef]
- Casanovas, B.; Porcar, O.; Speed, S.; Vicente, R.; Font-Bardia, M.; El Fallah, M.S. Field-Induced SMM and Vis/NIR Luminescence on Mononuclear Lanthanide Complexes with 9-Anthracenecarboxylate and 2,2′:6,2″-Terpyridine. Magnetochem 2021, 7, 124. [Google Scholar] [CrossRef]
- Carter, K.P.; Cahill, C.L. Combining coordination and supramolecular chemistry to explore uranyl assembly in the solid state. Inorg. Chem. Front 2015, 2, 141. [Google Scholar] [CrossRef]
- Geng, S.; Ren, N.; He, S.-M.; Zhang, J.-J. Synthesis and structural characterization of lanthanide metal complexes by 2-fluorobenzoic acid with 2,2′:6′,2″-terpyridine, and their fluorescence properties. J. Mol. Struct. 2022, 1252, 132165. [Google Scholar] [CrossRef]
- Geng, S.; Ren, N.; Zhang, Y.-Y.; Tang, K.; Zhang, J.-J. Studies on preparation, crystal structure, thermal properties and fluorescence properties of rare earth complexes composed of 2-chloro-4-fluorobenzoic acid and 2,2′:6′,2″-terpyridine. J. Solid State Chem. 2022, 305, 122633. [Google Scholar] [CrossRef]
- Cairns, K.R.; Levason, W.; Reid, G.; Zhang, W. Heterocyclic nitrogen donor complexes of aluminium, gallium and indium with weakly coordinating triflate anions. Polyhedron 2021, 207, 115367. [Google Scholar] [CrossRef]
- Martinez-Vargas, S.; Dorazco-Gonzalez, A.; Hernandez-Ortega, S.; Toscano, R.A.; Barquera-Lozada, J.E.; Valdes-Martinez, J. Interaction between aromatic rings as organizing tools and semi-coordination in Cu(II) compounds. CrystEngComm 2017, 19, 4595. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Chadjistamatis, I.; Terzis, A.; Perlepes, S.P.; Raptopoulou, C.P. Complexes derived from the copper(II)/succinamic acid/N,N′,N″-chelate tertiary reaction systems: Synthesis, structural and spectroscopic studies. Polyhedron 2010, 29, 1870–1879. [Google Scholar] [CrossRef]
- Buyukeksi, S.I.; Erkisa, M.; Sengul, A.; Ulukaya, E.; Oral, A.Y. Structural studies and cytotoxic activity of a new dinuclear coordination compound of palladium(II)–2,2′:6′,2″-terpyridine with rigid dianionic 1,2,4-triazole-3-sulfonate linker. Appl. Organomet. Chem. 2018, 32, e4406. [Google Scholar] [CrossRef]
- Lee Ayscue, R., III; Vallet, V.; Bertke, J.A.; Real, F.; Knope, K.E. Structure–Property Relationships in Photoluminescent Bismuth Halide Organic Hybrid Materials. Inorg. Chem. 2021, 60, 9727–9744. [Google Scholar] [CrossRef] [PubMed]
- Herder, J.A.; Walusiak, B.W.; Cahill, C.L. RE-Halobenzoic Acid-Terpyridine Complexes, Part V: Synthesis and Supramolecular Assembly of Rare-Earth-3,5-Dihalobenzoic Acid-Terpyridine Materials and Subsequent Comparison of Non-covalent Interactions. J. Chem. Cryst. 2021, 51, 317. [Google Scholar] [CrossRef]
- Baffert, C.; Collomb, M.-N.; Deronzier, A.; Pécaut, J.; Limburg, J.; Crabtree, R.H.; Brudvig, G.W. Two New Terpyridine Dimanganese Complexes: A Manganese(III,III) Complex with a Single Unsupported Oxo Bridge and a Manganese(III,IV) Complex with a Dioxo Bridge. Synthesis, Structure, and Redox Properties. Inorg. Chem. 2002, 41, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Pruchnik, F.P.; Robert, F.; Jeannin, Y.; Jeannin, S. New Rhodium(II) Complexes with 2,2′:6′,2″-Terpyridine. Inorg. Chem. 1996, 35, 4261–4263. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Ren, N.; Zhang, J.-J. Lanthanide complexes with 2,6-dimethylbenzoic acid and 2,2′:6′,2″ -terpyridine: Crystal structures, thermochemical property and luminescent behavior. Thermochim. Acta. 2021, 699, 178915. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Edwards, A.J.; Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Intermolecular interactions in molecular crystals: What’s in a name? Faraday Discuss. 2017, 203, 93–112. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Cai, S.-L.; Zhang, K.; Li, C.-J.; Feng, Y.; Fan, J.; Zheng, S.-R.; Zhang, W.-G. Anion- and temperature-dependent assembly, crystal structures and luminescence properties of six new Cd(II) coordination polymers based on 2,3,5,6-tetrakis(2-pyridyl)pyrazine. CrystEngComm 2016, 18, 5164. [Google Scholar] [CrossRef]
- Kundu, T.; Sarkar, B.; Mondal, T.K.; Fiedler, J.; Mobin, S.M.; Kaim WLahiri, G.K. Carboxylate Tolerance of the Redox-Active Platform [Ru(μ-tppz)Ru]n, where tppz = 2,3,5,6-Tetrakis(2-pyridyl)pyrazine, in the Electron-Transfer Series [(L)ClRu(μ-tppz)RuCl(L)]n, n = 2+, +, 0, −, 2−, with 2-Picolinato, Quinaldato, and 8-Quinolinecarboxylato Ligands (L−). Inorg. Chem. 2010, 49, 6565–6574. [Google Scholar]
- Thangavelu, S.G.; Butcher, R.J.; Cahill, C.L. Role of N-Donor Sterics on the Coordination Environment and Dimensionality of Uranyl Thiophenedicarboxylate Coordination Polymers. Cryst. Growth Des. 2015, 15, 3481–3492. [Google Scholar] [CrossRef]
- Mariano Ldos, S.; Rosa, I.M.L.; De Campos, N.R.; Doriguetto, A.C.; Dias, D.F.; do Pim, W.D.; Valdo, A.K.S.M.; Martins, F.T.; Ribeiro, M.A.; De Paula, E.E.B.; et al. Polymorphic Derivatives of NiII and CoII Mesocates with 3D Networks and “Brick and Mortar” Structures: Preparation, Structural Characterization, and Cryomagnetic Investigation of New Single-Molecule Magnets. Cryst. Growth Des. 2020, 20, 2462–2476. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Gogoleva, N.V.; Makarov, D.A.; Kiskin, M.A.; Yakushev, I.A.; Dolgushin, F.M.; Aleksandrov, G.G.; Varaksina, E.A.; Taidakov, I.V.; Aleksandrov, E.V.; et al. Synthesis of Coordination Polymers from the Heterometallic Carboxylate Complexes with Chelating N-Donor Ligands. Russ. J. Coord. Chem. 2020, 46, 1–14. [Google Scholar] [CrossRef]
- Thomas Tuerk, T.; Resch, U.; Fox, M.A.; Vogler, A. Spectroscopic studies of zinc benzenethiolate complexes: Electron transfer to methyl viologen. Inorg. Chem. 1992, 31, 1854–1857. [Google Scholar] [CrossRef] [Green Version]
- Puntus, L.N.; Lyssenko, K.A.; Antipin, M.Y.; Bünzli, J.C.G. Role of Inner- and Outer-Sphere Bonding in the Sensitization of EuIII-Luminescence Deciphered by Combined Analysis of Experimental Electron Density Distribution Function and Photophysical Data. Inorg. Chem. 2008, 47, 11095–11107. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Pekareva, I.S.; Bünzli, J.-C.G. Intermolecular Interactions as Actors in Energy-Transfer Processes in Lanthanide Complexes with 2,2′-Bipyridine. J. Phys. Chem. B 2009, 113, 9265–9277. [Google Scholar] [CrossRef] [PubMed]
- Kalugin, A.E.; Minyaev, M.E.; Puntus, L.N.; Taydakov, I.V.; Varaksina, E.A.; Lyssenko, K.A.; Nifant’ev, I.E.; Roitershtein, D.M. Diarylphosphate as a New Route for Design of Highly Luminescent Ln Complexes. Molecules 2020, 25, 3934. [Google Scholar] [CrossRef]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; van der Tol, E.B.; Verhoeven, J.W. New Sensitizer-Modified Calix[4]arenes Enabling Near-UV Excitation of Complexed Luminescent Lanthanide Ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef] [Green Version]
- Mikhalyova, E.A.; Yakovenko, A.V.; Zeller, M.; Gavrilenko, K.S.; Kiskin, M.A.; Smola, S.S.; Dotsenko, V.P.; Eremenko, I.L.; Addison, A.W.; Pavlishchuk, V.V. Crystal structures and intense luminescence of tris(3-(2′-pyridyl)-pyrazolyl)borate Tb3+ and Eu3+ complexes with carboxylate co-ligands. Dalton Trans. 2017, 46, 3457–3469. [Google Scholar] [CrossRef]


| Compound | Arad, s−1 | Anrad, s−1 | τobs, ms | ηsens, % | ||
|---|---|---|---|---|---|---|
| 2Eu | 370 | 300 | 1.49 | 55 | 39 | 71 |
| 2Tb | - | - | 1.35 | - | 11 | - |
| 3Eu | 410 | 380 | 1.27 | 52 | 31 | 60 |
| 3Tb | - | - | 1.83 | - | 24 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmelev, M.A.; Voronina, J.K.; Evtyukhin, M.A.; Dolgushin, F.M.; Varaksina, E.A.; Taydakov, I.V.; Sidorov, A.A.; Eremenko, I.L.; Kiskin, M.A. Synthesis, Structure and Photoluminescence Properties of Cd and Cd-Ln Pentafluorobenzoates with 2,2′:6′,2′-Terpyridine Derivatives. Inorganics 2022, 10, 194. https://doi.org/10.3390/inorganics10110194
Shmelev MA, Voronina JK, Evtyukhin MA, Dolgushin FM, Varaksina EA, Taydakov IV, Sidorov AA, Eremenko IL, Kiskin MA. Synthesis, Structure and Photoluminescence Properties of Cd and Cd-Ln Pentafluorobenzoates with 2,2′:6′,2′-Terpyridine Derivatives. Inorganics. 2022; 10(11):194. https://doi.org/10.3390/inorganics10110194
Chicago/Turabian StyleShmelev, Maxim A., Julia K. Voronina, Maxim A. Evtyukhin, Fedor M. Dolgushin, Evgenia A. Varaksina, Ilya V. Taydakov, Aleksey A. Sidorov, Igor L. Eremenko, and Mikhail A. Kiskin. 2022. "Synthesis, Structure and Photoluminescence Properties of Cd and Cd-Ln Pentafluorobenzoates with 2,2′:6′,2′-Terpyridine Derivatives" Inorganics 10, no. 11: 194. https://doi.org/10.3390/inorganics10110194





