Aliphatic-Bridged Early Lanthanide Metal–Organic Frameworks: Topological Polymorphism and Excitation-Dependent Luminescence
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
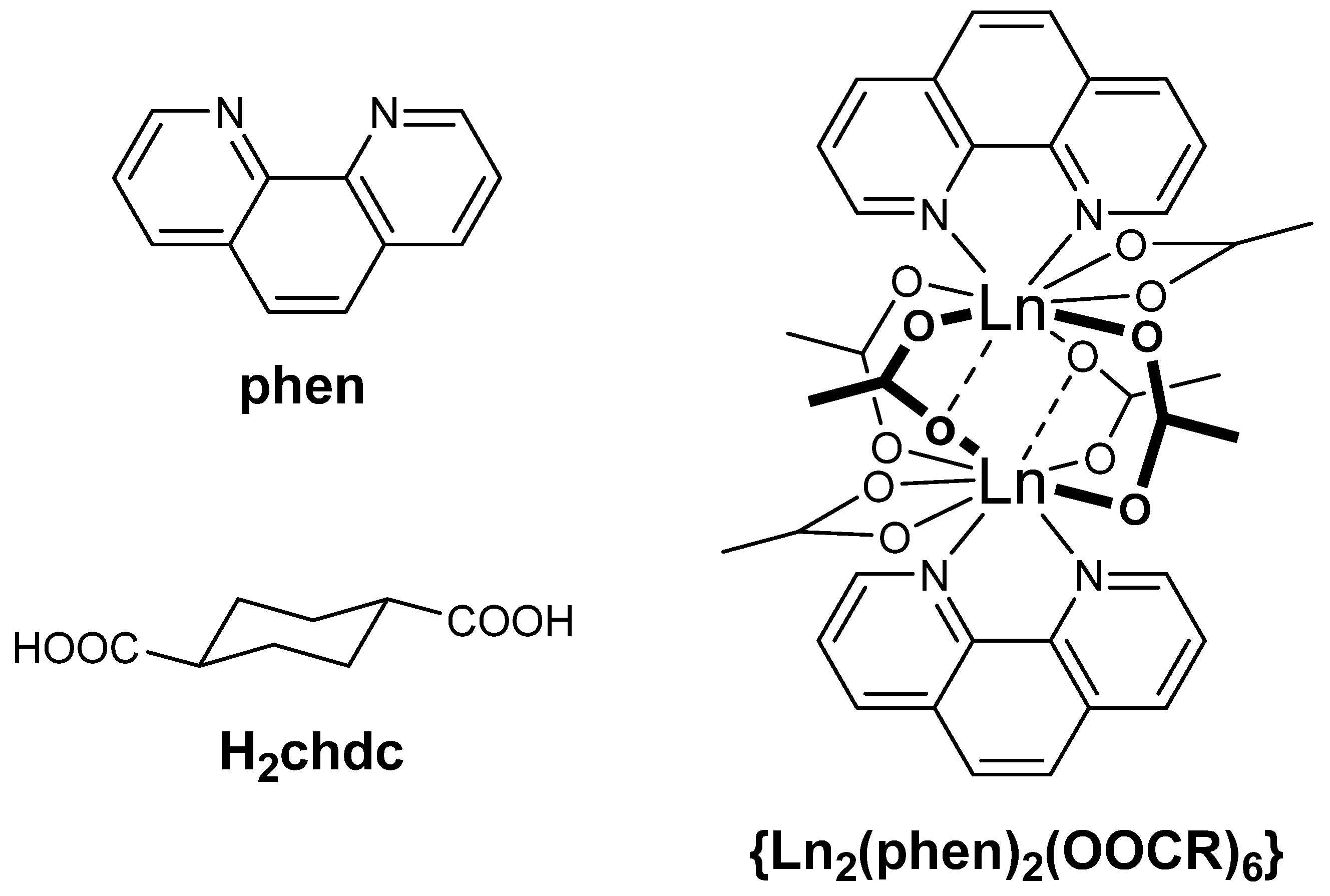
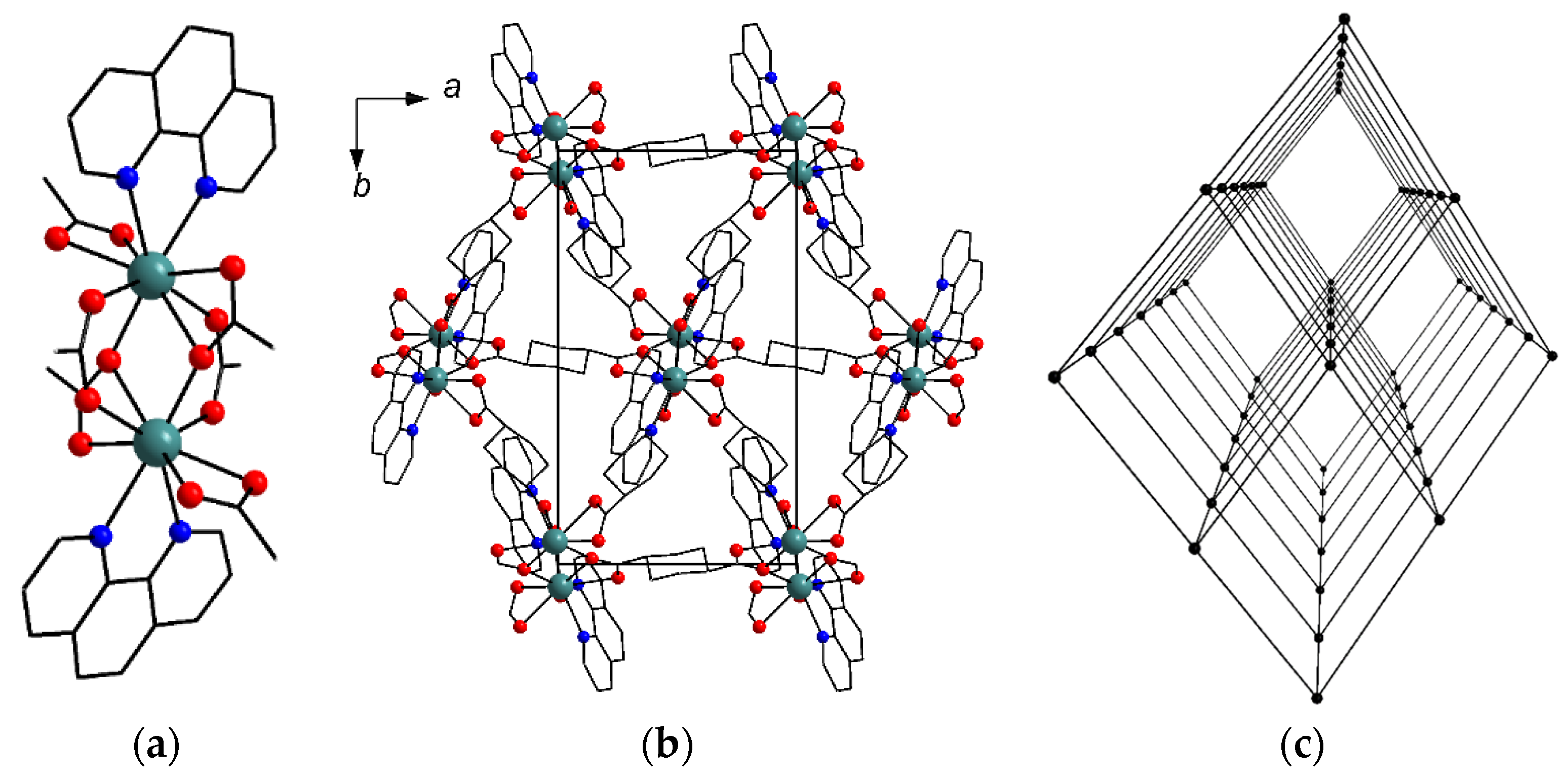
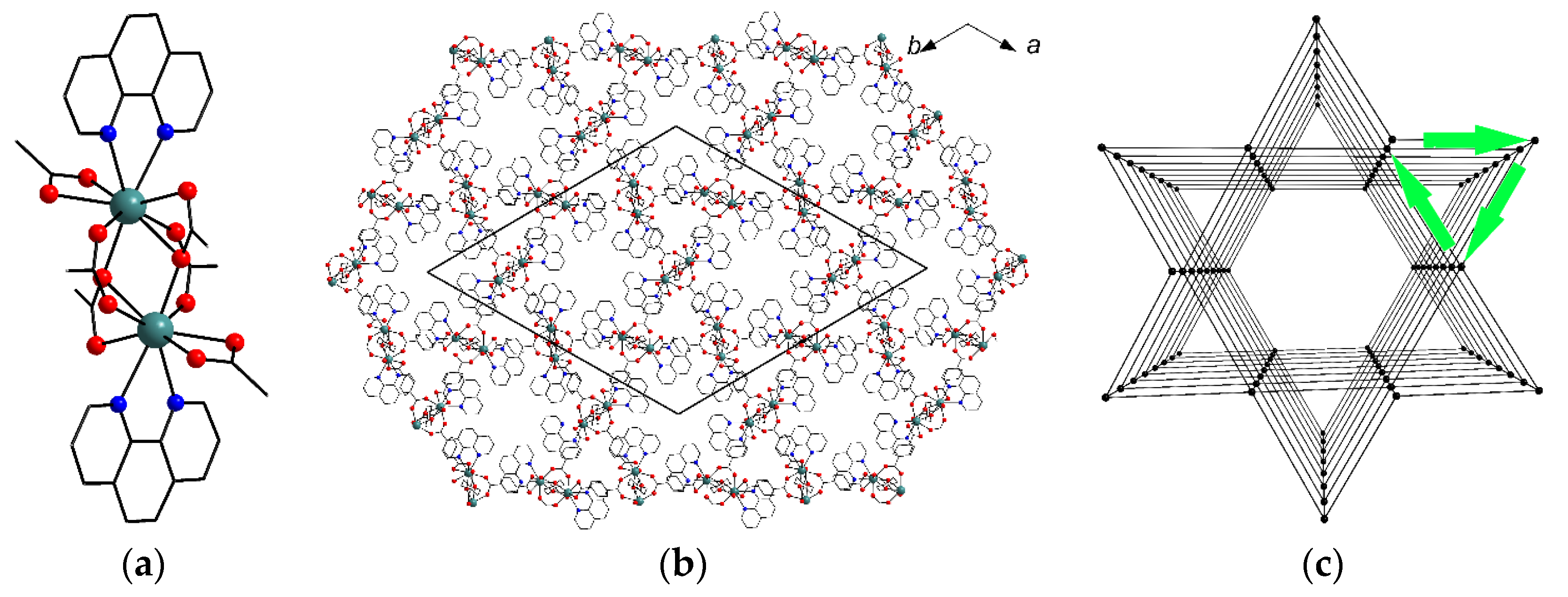
2.2. Crystal Structure Description
2.3. Characterization and Thermal Properties
2.4. Luminescent Properties
3. Experimental
3.1. Materials
3.2. Instruments
3.3. Synthetic Methods
3.4. Single-Crystal X-ray Diffraction Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the structure and function of metal–organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Lv, X.-L.; Yan, T.-H.; Zhou, H.-C. Hierarchically porous metal–organic frameworks: Synthetic strategies and applications. Natl. Sci. Rev. 2020, 7, 1743–1758. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, J.; Bai, N.; Zhang, X.; Han, X.; Da Silva, I.; Morris, C.G.; Xu, S.; Wilary, D.M.; Sun, Y.; et al. Refinement of pore size at sub-angstrom precision in robust metal–organic frameworks for separation of xylenes. Nat. Commun. 2020, 11, 4280. [Google Scholar] [CrossRef]
- Yin, W.; Tao, C.; Wang, F.; Huang, J.; Qu, T.; Wang, J. Tuning optical properties of MOF-based thin films by changing the ligands of MOFs. Sci. China Mater. 2018, 61, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Wei, R.; Gaggioli, A.C.; Li, G.; Islamoglu, T.; Zhang, Z.; Yu, P.; Farha, O.K.; Cramer, C.J.; Gagliardi, L.; Yang, D.; et al. Tuning the Properties of Zr6O8 Nodes in the Metal Organic Framework UiO-66 by Selection of Node-Bound Ligands and Linkers. Chem. Mater. 2019, 31, 1655–1663. [Google Scholar] [CrossRef]
- Kovalenko, K.A.; Potapov, A.S.; Fedin, V.P. Micro- and mesoporous metal-organic coordination polymers for the separation of hydrocarbons. Russ. Chem. Rev. 2022, 91, 1–31. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Aljammal, N.; Jabbour, C.; Chaemchuen, S.; Juzsakova, T.; Verpoort, F. Flexibility in Metal–Organic Frameworks: A Basic Understanding. Catalysts 2019, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, L.; Sun, D. Stimuli-responsive structural changes in metal–organic frameworks. Chem. Commun. 2020, 56, 9416–9432. [Google Scholar] [CrossRef]
- Grebenyuk, D.; Zobel, M.; Polentarutti, M.; Ungur, L.; Kendin, M.; Zakharov, K.; Degtyarenko, P.; Vasiliev, A.; Tsymbarenko, D. A Family of Lanthanide Hydroxo Carboxylates with 1D Polymeric Topology and Ln4 Butterfly Core Exhibits Switchable Supramolecular Arrangement. Inorg. Chem. 2021, 60, 8049–8061. [Google Scholar] [CrossRef]
- Makal, T.A.; Yakovenko, A.A.; Zhou, H.-C. Isomerism in Metal–Organic Frameworks: “Framework Isomers”. J. Phys. Chem. Lett. 2011, 2, 1682–1689. [Google Scholar] [CrossRef]
- Widmer, R.N.; Lampronti, G.I.; Chibani, S.; Wilson, C.W.; Anzellini, S.; Farsang, S.; Kleppe, A.K.; Casati, N.P.M.; MacLeod, S.G.; Redfern, S.A.T.; et al. Rich Polymorphism of a Metal–Organic Framework in Pressure–Temperature Space. J. Am. Chem. Soc. 2019, 141, 9330–9337. [Google Scholar] [CrossRef] [Green Version]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Topological polymorphism and temperature-driven topotactic transitions of metal–organic coordination polymers. CrystEngComm 2020, 22, 6295–6301. [Google Scholar] [CrossRef]
- Bueken, B.; Van Velthoven, N.; Krajnc, A.; Smolders, S.; Taulelle, F.; Mellot-Draznieks, C.; Mali, G.; Bennett, T.D.; De Vos, D. Tackling the Defect Conundrum in UiO-66: A Mixed-Linker Approach to Engineering Missing Linker Defects. Chem. Mater. 2017, 29, 10478–10486. [Google Scholar] [CrossRef]
- Demakov, P.A.; Sapchenko, S.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Coordination polymers based on zinc(II) and manganese(II) with 1,4-cyclohexanedicarboxylic acid. Russ. Chem. Bull. 2018, 67, 490–496. [Google Scholar] [CrossRef]
- Reinsch, H.; Homburg, T.; Heidenreich, N.; Fröhlich, D.; Hennninger, S.; Wark, M.; Stock, N. Green Synthesis of a New Al-MOF Based on the Aliphatic Linker Mesaconic Acid: Structure, Properties and In Situ Crystallisation Studies of Al-MIL-68-Mes. Chem. Eur. J. 2018, 24, 2173–2181. [Google Scholar] [CrossRef]
- Macreadie, L.K.; Mensforth, E.J.; Babarao, R.; Konstas, K.; Telfer, S.G.; Doherty, C.M.; Tsanaktsidis, J.; Batten, S.R.; Hill, M.R. CUB-5: A Contoured Aliphatic Pore Environment in a Cubic Framework with Potential for Benzene Separation Applications. J. Am. Chem. Soc. 2019, 141, 3828–3832. [Google Scholar] [CrossRef]
- Llabres-Campaner, P.J.; Pitarch-Jarque, J.; Ballesteros-Garrido, R.; Abarca, B.; Ballesteros, R.; García-España, E. Bicyclo[2.2.2]octane-1,4-dicarboxylic acid: Towards transparent metal-organic frameworks. Dalton Trans. 2017, 46, 7397–7402. [Google Scholar] [CrossRef]
- Yin, J.; Yang, H.; Fei, H. Robust, Cationic Lead Halide Layered Materials with Efficient Broadband White-Light Emission. Chem. Mater. 2019, 31, 3909–3916. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent Lanthanide MOFs: A Unique Platform for Chemical Sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [Green Version]
- Saraci, F.; Quezada-Novoa, V.; Donnarumma, P.R.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Eliseeva, S.V.; Gładysiak, A.; Petoud, S.; Stylianou, K.C. Design of lanthanide-based metal–organic frameworks with enhanced near-infrared emission. J. Mater. Chem. A 2020, 8, 10188–10192. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide azolecarboxylate compounds: Structure, luminescent properties and applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Pan, M.; Liao, W.-M.; Yin, S.-Y.; Sun, S.-S.; Su, C.-Y. Single-Phase White-Light-Emitting and Photoluminescent Color-Tuning Coordination Assemblies. Chem. Rev. 2018, 118, 8889–8935. [Google Scholar] [CrossRef]
- Igoa, F.; Peinado, G.; Suescun, L.; Kremer, C.; Torres, J. Design of a white-light emitting material based on a mixed-lanthanide metal organic framework. J. Solid State Chem. 2019, 279, 120925. [Google Scholar] [CrossRef]
- Dong, Z.-P.; Zhao, F.; Zhang, L.; Liu, Z.-L.; Wang, Y.-Q. A white-light-emitting lanthanide metal–organic framework for luminescence turn-off sensing of MnO4− and turn-on sensing of folic acid and construction of a “turn-on plus” system. New J. Chem. 2020, 44, 10239–10249. [Google Scholar] [CrossRef]
- Sun, Z.; Khurshid, A.; Sohail, M.; Qiu, W.; Cao, D.; Su., S.-J. Encapsulation of Dyes in Luminescent Metal-Organic Frameworks for White Light Emitting Diodes. Nanomaterials 2021, 11, 2761. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ryadun, A.A.; Dorovatovskii, P.V.; Lazarenko, V.A.; Samsonenko, D.G.; Brylev, K.A.; Fedin, V.P.; Dybtsev, D.N. Intense multi-colored luminescence in a series of rare-earth metal–organic frameworks with aliphatic linkers. Dalton Trans. 2021, 50, 11899–11908. [Google Scholar] [CrossRef]
- Wang, S.; Sun, B.; Sun, J.; Hao, X.; Li, X.; Zhou, C.; Su, Z. Lanthanide metal−organic frameworks based on planar π-conjugated ligands for white light emission, temperature and chemical sensing. Dyes Pigm. 2022, 202, 110256. [Google Scholar] [CrossRef]
- Zehnder, R.A.; Jenkins, J.; Zeller, M.; Dempsey, C.; Kozimor, S.A.; Jackson, G.; Gilbert, K.; Smith, M. Conversion of lanthanide glutarate chlorides with interstitial THF into lanthanide glutarates with unprecedented topologies. Inorg. Chim. Acta 2018, 471, 502–512. [Google Scholar] [CrossRef]
- Kumar, M.; Qiu, C.-Q.; Zareba, J.K.; Frontera, A.; Kaur Jassal, A.; Sahoo, S.C.; Liu, S.-J.; Nawaz Sheikh, H. Magnetic, luminescence, topological and theoretical studies of structurally diverse supramolecular lanthanide coordination polymers with flexible glutaric acid as a linker. New J. Chem. 2019, 43, 14546–14564. [Google Scholar] [CrossRef]
- Long, D.-L.; Blake, A.J.; Champness, N.R.; Wilson, C.; Schröder, M. Unprecedented Seven- and Eight-Connected Lanthanide Coordination Networks. Angew. Chem. Int. Ed. 2001, 40, 2443–2447. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Lin, R.; Yao, Z.; Lin, Q.; Wang, L.; Zhang, Z.; Xiang, S. Two water-stable lanthanide metal–organic frameworks with oxygen-rich channels for fluorescence sensing of Fe(III) ions in aqueous solution. Dalton Trans. 2018, 47, 16190–16196. [Google Scholar] [CrossRef]
- Pérez, J.M.; Rojas, S.; García-García, A.; Montes-Andrés, H.; Martínez, C.R.; Romero-Cano, M.S.; Choquesillo-Lazarte, D.; Abdelkader-Fernández, V.K.; Pérez-Mendoza, M.; Cepeda, J.; et al. Catalytic Performance and Electrophoretic Behavior of an Yttrium–Organic Framework Based on a Tricarboxylic Asymmetric Alkyne. Inorg. Chem. 2022, 61, 1377–1384. [Google Scholar] [CrossRef]
- Lu, T.-Q.; Cheng, L.-T.; Wang, X.-T.; Chen, C.; Zheng, J.; Lu, D.-F.; Zheng, X.-Y. Lanthanide Metal–Organic Frameworks with High Chemical Stability as Multifunctional Materials: Cryogenic Magnetic Cooler and Luminescent Probe. Cryst. Growth Des. 2022, 22, 4917–4925. [Google Scholar] [CrossRef]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal-Organic Frameworks as Sensory Materials and Imaging Agents. Inorg. Chem. 2014, 53, 1916–1924. [Google Scholar] [CrossRef]
- Wang, H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Sava Gallis, D.F.; Rohwer, L.E.S.; Rodriguez, M.A.; Barnhart-Dailey, M.C.; Butler, K.S.; Luk, T.S.; Timlin, J.A.; Chapman, K.W. Multifunctional, Tunable Metal–Organic Framework Materials Platform for Bioimaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 22268–22277. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Zu, W.; Yang, H.; Wang, Y. One-pot synthesis of Eu-MOFs for bioimaging and drug delivery. Drug Dev. Ind. Pharm. 2021, 47, 1175–1182. [Google Scholar] [CrossRef]
- Cui, R.; Sun, W.; Liu, M.; Shi, J.; Liu, Z. Near-Infrared Emissive Lanthanide Metal–Organic Frameworks for Targeted Biological Imaging and pH-Controlled Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 59164–59173. [Google Scholar] [CrossRef]
- Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. [Google Scholar] [CrossRef]
- Anderegg, G. Pyridinderivate als Komplexbildner VI. Reaktionsenthalpie und entropie bei der Bildung der Metallkomplexe von 1,10-Phenanthrolin und α,α′-Dipyridyl. Helv. Chim. Acta 1963, 46, 2813–2822. [Google Scholar] [CrossRef]
- Bencini, A.; Lippolis, V. 1,10-Phenanthroline: A versatile building block for the construction of ligands for various purposes. Coord. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhou, J.; Zou, H.-H.; Fu, L.; Tang, Q.; Wang, P. A series of lanthanide glutarates: Lanthanide contraction effect on crystal frameworks of lanthanide glutarates. RSC Adv. 2017, 7, 17934–17940. [Google Scholar] [CrossRef] [Green Version]
- Demakov, P.A.; Sapchenko, S.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Gadolinium Break in a Series of Three-Dimensional trans-1,4-Cyclohexane Dicarboxylates of Rare Earth Elements. J. Struct. Chem. 2019, 60, 815–822. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-Z.; Xin, L.-Y.; Wang, L.-Y. Ancillary ligand-mediated syntheses and fluorescence properties of zinc(ii) complexes based on flexible benzene dicarboxylic acid. CrystEngComm 2011, 13, 3013–3020. [Google Scholar] [CrossRef]
- Manna, P.; Tripuramallu, B.K.; Bommakanti, S.; Das, S.K. Synthesis, characterization and magnetism of metal–organic compounds: Role of the positions of the coordinating groups of a meso-flexible ligand in placing anisotropy to exhibit spin-canting behaviour. Dalton Trans. 2015, 44, 2852–2864. [Google Scholar] [CrossRef]
- Singh, S.S. Infrared Spectra of 1:10 Phenanthroline and its Addition Compounds with Antimony Trichloride and Antimony Pentachloride. Z. Nat. A 1969, 24, 2015–2016. [Google Scholar] [CrossRef]
- Reiher, M.; Brehm, G.; Schneider, S. Assignment of Vibrational Spectra of 1,10-Phenanthroline by Comparison with Frequencies and Raman Intensities from Density Functional Calculations. J. Phys. Chem. A 2004, 108, 734–742. [Google Scholar] [CrossRef]
- Li, C.-T.; Zhao, Y.-F.; Hu, H.-M.; Zhao, H.; Wang, X.; Xue, G. Lanthanide coordination frameworks constructed from 3,3′,4,4′-diphenylsulfonetetracarboxylic and 1,10-phenanthroline: Synthesis, crystal structures and luminescence properties. Dalton Trans. 2016, 45, 15436–15444. [Google Scholar] [CrossRef]
- Zheng, K.; Liu, Z.-Q.; Huang, Y.; Chen, F.; Zeng, C.-H.; Zhong, S.; Ng, S.W. Highly luminescent Ln-MOFs based on 1,3-adamantanediacetic acid as bifunctional sensor. Sens. Actuators B Chem. 2018, 257, 705–713. [Google Scholar] [CrossRef]
- Feng, X.; Shang, Y.; Zhang, H.; Li, R.; Wang, W.; Zhang, D.; Wang, L.; Li, Z. Enhanced luminescence and tunable magnetic properties of lanthanide coordination polymers based on fluorine substitution and phenanthroline ligand. RSC Adv. 2019, 9, 16328–16338. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.-Y.; Xue, L.-L.; Hu, H.-M.; Zheng, L.-N.; Wang, X.; Xue, G. Syntheses, structures, fluorescence sensing properties and white-light emission of lanthanide coordination polymers assembled from imidazophenanthroline derivative and isophthalate ligands. J. Solid State Chem. 2019, 276, 6–18. [Google Scholar] [CrossRef]
- An, R.; Wang, X.; Hu, H.-M.; Zhao, Z.; Bai, C.; Xue, G. Controllable synthesis of four series of lanthanide coordination polymers: Synthesis, structures, luminescent and magnetic properties. CrystEngComm 2015, 17, 8289–8299. [Google Scholar] [CrossRef]
- Litvinova, Y.M.; Gayfulin, Y.M.; Brylev, K.A.; Piryazev, D.A.; Van Leusen, J.; Kögerler, P.; Mironov, Y.V. Metal–organic frameworks with solvent-free lanthanide coordination environments: Synthesis from aqueous ethanol solutions. CrystEngComm 2020, 22, 7935–7943. [Google Scholar] [CrossRef]
- Roitershtein, D.M.; Puntus, L.N.; Varaksina, E.A.; Taidakov, I.V.; Lysenko, K.A. Lanthanide Coordination Polymers Based on Dicyanamide Ligand. Russ. J. Coord. Chem. 2020, 46, 15–22. [Google Scholar] [CrossRef]
- But, S.; Lis, S.; Van Deun, R.; Parac-Vogt, T.N.; Görller-Walrand, C.; Binnemans, K. Spectroscopic properties of neodymium(III)-containing polyoxometalates in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 62, 478–482. [Google Scholar] [CrossRef]
- Zapata-Lizama, M.; Hermosilla-Ibáñez, P.; Venegas-Yazigi, D.; Espallargas, G.M.; Maia, L.J.Q.; Gasparotto, G.; De Santana, R.C.; Cañón-Mancisidor, W. A systematic study of the optical properties of mononuclear hybrid organo–inorganic lanthanoid complexes. Inorg. Chem. Front. 2020, 7, 3049–3062. [Google Scholar] [CrossRef]
- Metlina, D.A.; Metlin, M.T.; Ambrozevich, S.A.; Selyukov, A.S.; Datskevich, N.P.; Aminev, D.F.; Goryachii, D.O.; Lyssenko, K.A.; Pavlov, A.A.; Dmitrienko, A.O.; et al. Bright NIR-luminescent Nd3+ complexes with pyrazole-substituted 1,3-diketones demonstrated an unusual spectral lines branching ratios. Dyes Pigm. 2020, 181, 108558. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.-B.; Zou, Y.-Q. Hydrothermal synthesis, crystal structure, and luminescence of lanthanide(III) coordination polymers with tetrafluorosuccinate and 1,10-phenanthroline. J. Mol. Struct. 2009, 919, 277–283. [Google Scholar] [CrossRef]
- Wang, X.-J.; Jiang, Z.-G.; Chen, J.; Feng, Y.-L. Lanthanide complexes with disulfide ligand generated in situ: Syntheses, crystal structures and fluorescent properties. Inorg. Chim. Acta 2011, 373, 270–275. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, G.-H.; Xie, L.-Q.; Wu, Y.-S.; Wu, H.-L.; Zhou, A.-J.; Wu, Z.-Y.; Wang, J.; Chen, Y.-C.; Tong, M.-L. Magnetic and luminescent properties of lanthanide coordination polymers with asymmetric biphenyl-3,2′,5′-tricarboxylate. Dalton Trans. 2015, 44, 14424–14435. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Zhang, Y.-H.; Huo, R.; Ma, D. White light emission by a lanthanide doped Sm(III) framework constructed from 4-sulfobenzoate and 1H-imidazo[4,5-f][1,10]-phenanthroline. Dalton Trans. 2014, 43, 5974–5977. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Q.; Hu, H.-M.; An, R.; Wang, X.; Xue, G. Hydrothermal Syntheses, Crystal Structures, and Luminescence Properties of Lanthanide-Based Coordination Polymers Constructed by Sulfonate Functionalized Imidazophenanthroline Derivative Ligand. Cryst. Growth Des. 2015, 15, 2318–2329. [Google Scholar] [CrossRef]
- Li, J.-J.; Fan, T.-T.; Qu, X.-L.; Han, H.-L.; Li, X. Temperature-induced 1D lanthanide polymeric frameworks based on Lnn (n = 2, 2, 4, 6) cores: Synthesis, crystal structures and luminescence properties. Dalton Trans. 2016, 45, 2924–2935. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Li, X.; Song, S.; Yang, H.-Y.; Ma, D.; Liu, Y.-H. Lanthanide coordination frameworks: Crystal structure, down- and up-conversion luminescence and white light emission. CrystEngComm 2014, 16, 8390–8397. [Google Scholar] [CrossRef]
- Smirnova, K.S.; Ivanova, E.A.; Sukhikh, T.S.; Pozdnyakov, I.P.; Dotsenko, V.V.; Lider, E.V. Luminescent properties of Ln(III) complexes with 2-[(phenylamino)methylene]-5,5-dimethyl-cyclohexane-1,3-dione as an antenna. Inorg. Chim. Acta 2021, 525, 120490. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.-B.; Tao, X.-M.; Su, J.; Ning, P.-F.; Xu, X.-F.; Zhou, Y.; Gu, W.; Liu, X. Novel single component tri-rare-earth emitting MOF for warm white light LEDs. Dalton Trans. 2018, 47, 8427–8433. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Gontcharenko, V.E.; Lunev, A.M.; Sidoruk, A.V.; Arkhipov, I.A.; Taydakov, I.V.; Belousov, Y.A. New Carboxylate Anionic Sm-MOF: Synthesis, Structure and Effect of the Isomorphic Substitution of Sm3+ with Gd3+ and Tb3+ Ions on the Luminescent Properties. Inorganics 2022, 10, 104. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Li, X.; Song, S. White light emission based on a single component Sm(III) framework and a two component Eu(III)-doped Gd(III) framework constructed from 2,2′-diphenyl dicarboxylate and 1H-imidazo[4,5-f][1,10]-phenanthroline. Chem. Commun. 2013, 49, 10397–10399. [Google Scholar] [CrossRef]
- Luo, L.-L.; Qu, X.-L.; Li, Z.; Li, X.; Sun, H.-L. Isostructural lanthanide-based metal–organic frameworks: Structure, photoluminescence and magnetic properties. Dalton Trans. 2018, 47, 925–934. [Google Scholar] [CrossRef]
- Xu, Q.-W.; Dong, G.; Cui, R.; Li, X. 3D lanthanide-coordination frameworks constructed by a ternary mixed-ligand: Crystal structure, luminescence and luminescence sensing. CrystEngComm 2020, 22, 740–750. [Google Scholar] [CrossRef]
- Han, Y.; Yan, P.; Sun, J.; An, G.; Yao, X.; Li, Y.; Li, G. Luminescence and white-light emitting luminescent sensor of tetrafluoroterephthalate-lanthanide metal–organic frameworks. Dalton Trans. 2017, 46, 4642–4653. [Google Scholar] [CrossRef]
- CrysAlisPro 1.171.38.46; Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Lazarenko, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Burlov, A.S.; Koshchienko, Y.V.; Vlasenko, V.G.; Khrustalev, V.N. High-Throughput Small-Molecule Crystallography at the ‘Belok’ Beamline of the Kurchatov Synchrotron Radiation Source: Transition Metal Complexes with Azomethine Ligands as a Case Study. Crystals 2017, 7, 325. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, W. XDS. Acta Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Cambridge Crystallographic Data Center (CCDC) Structure Search. Available online: https://www.ccdc.cam.ac.uk/structures/ (accessed on 30 September 2022).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demakov, P.A.; Ryadun, A.A.; Fedin, V.P. Aliphatic-Bridged Early Lanthanide Metal–Organic Frameworks: Topological Polymorphism and Excitation-Dependent Luminescence. Inorganics 2022, 10, 163. https://doi.org/10.3390/inorganics10100163
Demakov PA, Ryadun AA, Fedin VP. Aliphatic-Bridged Early Lanthanide Metal–Organic Frameworks: Topological Polymorphism and Excitation-Dependent Luminescence. Inorganics. 2022; 10(10):163. https://doi.org/10.3390/inorganics10100163
Chicago/Turabian StyleDemakov, Pavel A., Alexey A. Ryadun, and Vladimir P. Fedin. 2022. "Aliphatic-Bridged Early Lanthanide Metal–Organic Frameworks: Topological Polymorphism and Excitation-Dependent Luminescence" Inorganics 10, no. 10: 163. https://doi.org/10.3390/inorganics10100163




