Numerical Study of Light Transport in Apple Models Based on Monte Carlo Simulations
Abstract
:1. Introduction
2. Methodology
2.1. Monte Carlo Algorithm

- The emission point of a photon in the Gaussian beam is randomly determined to generate the coordinates Given ε1 and ε2 independently uniformly distributed in [–1,1], set, and is close to the local curvature of the model. Then the coordinates and are computed as [41]
- To simulate propagation, Monte Carlo method requires defining a distance for the collision (step size). The step size of the photon is computed based on the sampling of the probability distribution for mean free path ., where is a random number uniformly distributed over [0,1].
- Once the photon has taken a step, some of the photon weight Wk (initial value W0 = 1) decrease due to absorption by tissue sample must occur. Therefore,until weight falls below a threshold value Wth (associated to a Russian roulette technique).
- Once the photon has been moved and its weight decremented, the photon is ready to be scattered. A random number is generated, and the selection of the deflection angle , is accomplished using the probability density function of scattering in tissue (Henyey-Greenstein) [42]where , and : uniformly distributed within the interval [0,2π]. Once the deflection and azimuthal angles are chosen, the new direction for the photon propagation is computed from the three direction cosines using the formulas [43,44]
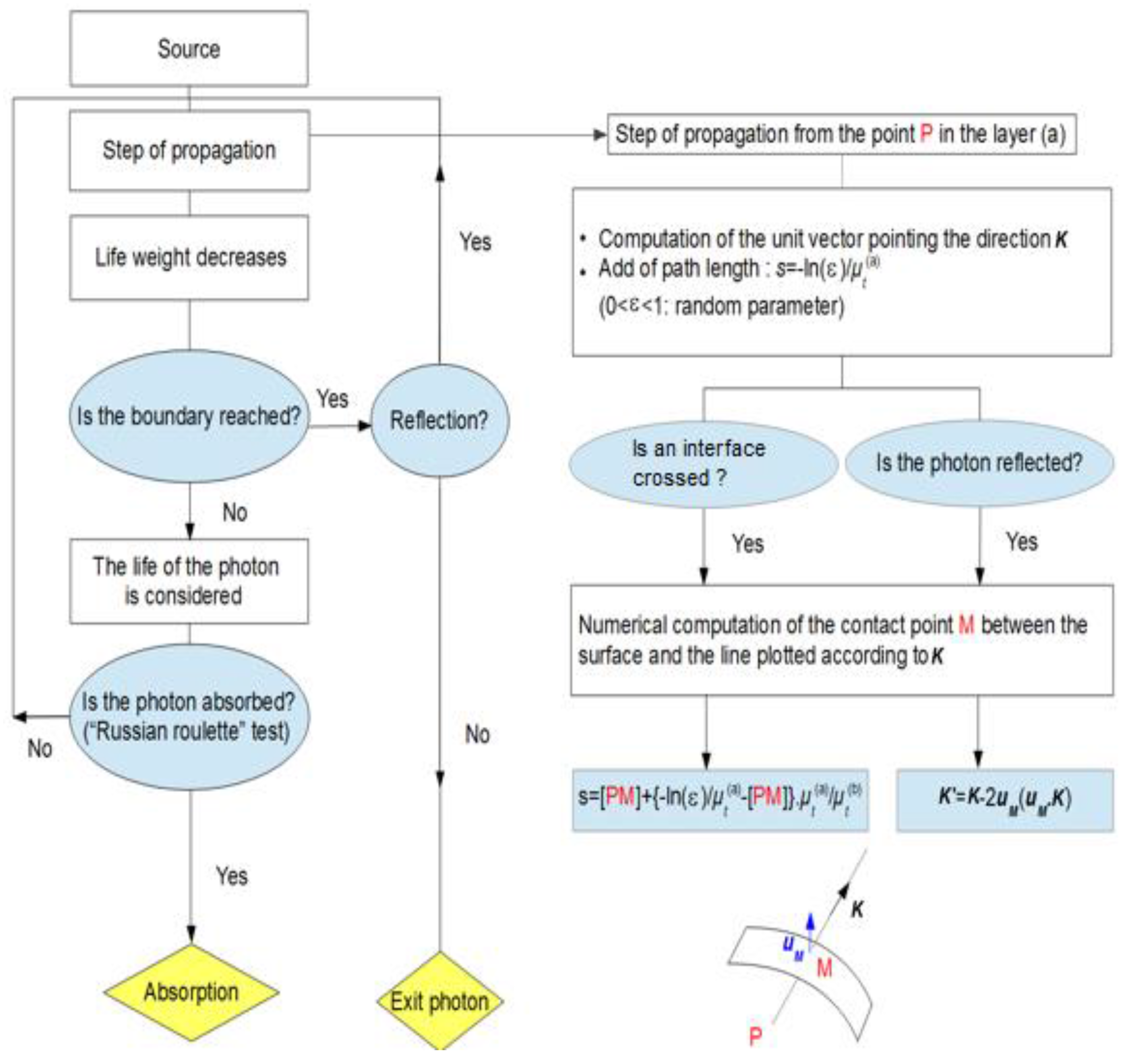
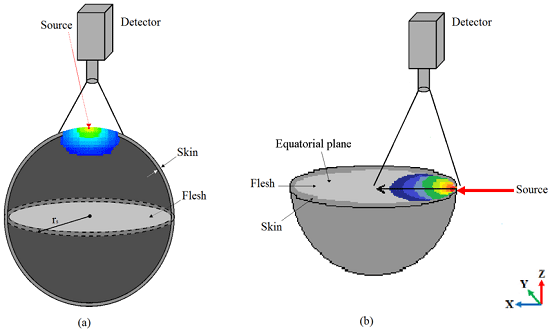
- During a step, a photon may attempt to escape the spherical apple model (with and without skin layer) at the air–tissue interface. Then, the photon may either escape and contributes to the observed reflectance or be internally reflected by the interface (flesh or skin). Snell’s law, which gives the relationship between the angle of incidence and the angle of transmittance (and refractive indexes), allows us to calculate the internal reflectance R(), according to the Fresnel’s law
2.2. Monte Carlo Input Data
| Apples | Skin Thickness (µm) |
|---|---|
| Royal Gala | 65.6 ± 13.3 |
| Granny Smith | 91.3 ± 13.8 |
| Golden Delicious | 78.1 ± 09.1 |
| Apples | Wavelengths | Flesh µa(f) | Flesh µ’s(f) | Skin µa(s) | Skin µ’s(s) |
|---|---|---|---|---|---|
| Gala | 750–850 nm | ~0.1 ± 0.1 (1) ~0.0125 (2) | ~1.2 (1) 1.15 ± 0.3 (2) | ~ 0.15 ± 0.1 (1) | 3.75 (1) |
| 633 nm | ~0.1 ± 0.1 (1) ~0.0125 (2) | ~1.2 (1) 1.2 ± 0.3 (2) | ~0.5 ± 0.1 (1) | ~4 (1) | |
| Granny | 750–850 nm | ~0.04 ± 0.03 (1) ~0.004 (4) | ~1.2 (1) ~2 (4) | ~0.075 ± 0.03 (1) | 4.25 (1) |
| 633 nm | ~0.1 ± 0,1 (1) 0.005 ± 0.0025 (5) | ~1.2 (1) 1.1 ± 0.1 (5) | ~0.5 ± 0.1 (1) | ~4 (1) | |
| Golden | 750–850 nm | ~0.01 (3) ~0.004 (4) | 0.8 ± 0.1 (3) ~2 (4) | ||
| 633 nm | ~0.04 (3) 0.004 ± 0.0025 (5) | 0.85 ± 0.15 (3) 1.4 ± 0.15 (5) | |||
3. Results and Discussion
3.1. Imaging Whole Apple
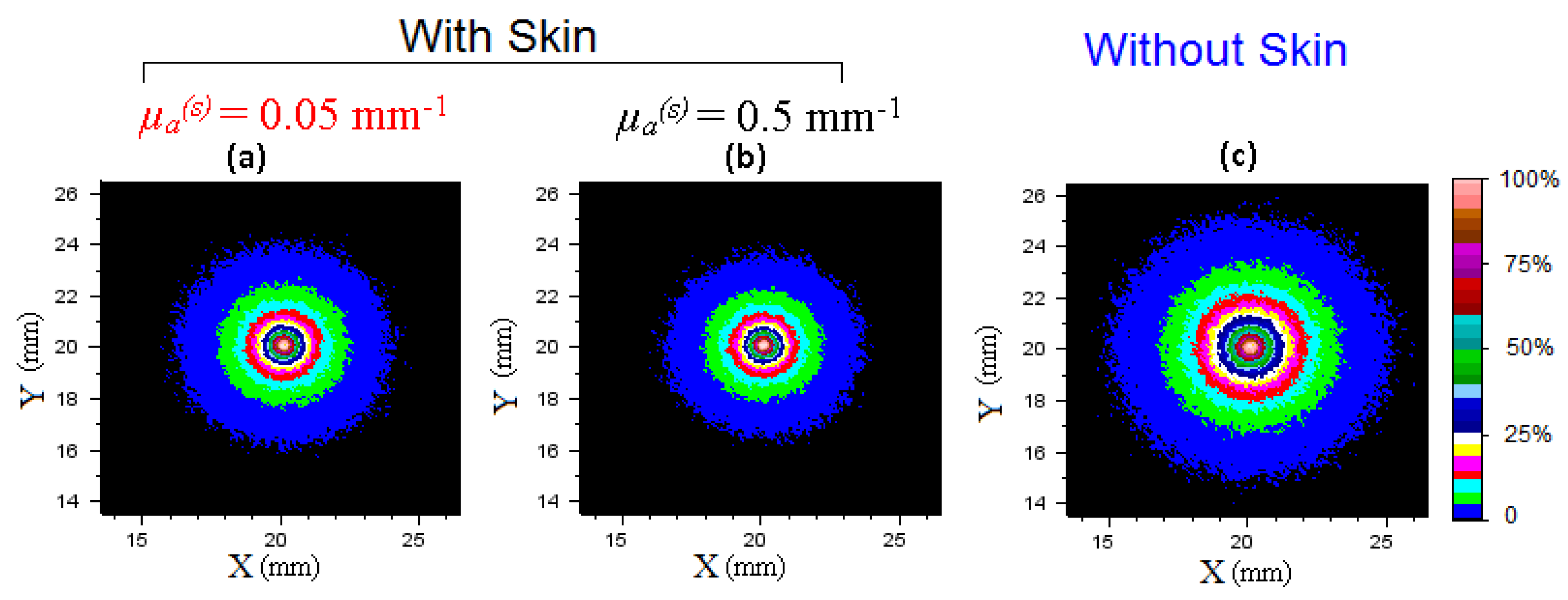

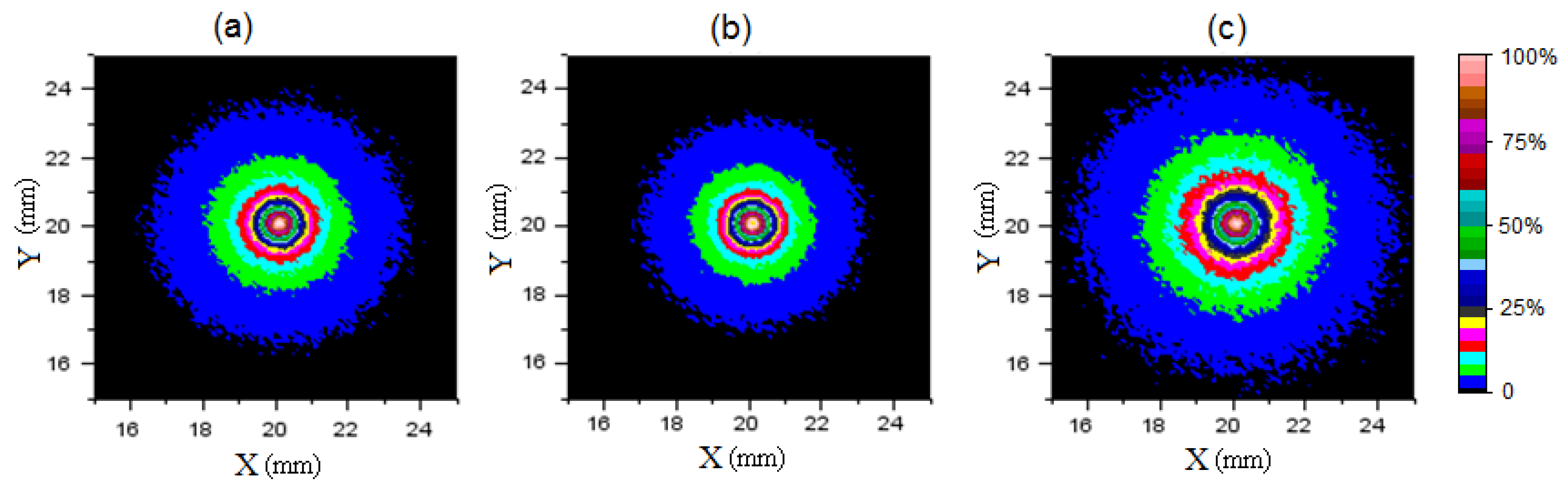

3.2. Imaging of Half-Cut Apple

3.3. Assessment of Internal Optical Properties

| Input data | Retrieved Data | Relative Error (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fit Range (2.8–10 mm) | ||||||||
| d | µa(s) | µ’s(s) | µa(f) | µ’s(f) | µa | µ’s | Δµa | Δµ’s |
| 0 | - | - | 0.0075 | 1.25 | 0.0076 | 1.01 | 1.33% | 19.58% |
| 80 | 0.05 | 4 | 0.0075 | 1.25 | 0.0111 | 1.09 | 48.00% | 12.92% |
| 80 | 0.5 | 4 | 0.0075 | 1.25 | 0.0125 | 1.09 | 66.67% | 13.00% |
| 150 | 0.05 | 4 | 0.0075 | 1.25 | 0.0102 | 1.09 | 36.00% | 12.70% |
| 150 | 0.5 | 4 | 0.0075 | 1.25 | 0.0124 | 1.08 | 65.33% | 13.45% |
| 150 | 0.05 | 2.5 | 0.0075 | 1.25 | 0.0134 | 0.71 | 78.67% | 42.92% |
| 0 | - | - | 0.0225 | 1.25 | 0.0203 | 1.19 | 9.78% | 4.74% |
| 80 | 0.05 | 4 | 0.0225 | 1.25 | 0.0233 | 1.18 | 3.56% | 5.29% |
| 150 | 0.5 | 4 | 0.0225 | 1.25 | 0.0264 | 1.20 | 17.33% | 3.63% |
| 0 | - | - | 0.0150 | 1.15 | 0.0144 | 1.14 | 4.00% | 0.64% |
| 80 | 0.05 | 4 | 0.0150 | 1.15 | 0.0159 | 1.14 | 6.00% | 0.81% |
| 80 | 0.5 | 4 | 0.0150 | 1.15 | 0.0163 | 1.14 | 8.67% | 0.84% |
| 150 | 0.05 | 4 | 0.0150 | 1.15 | 0.0163 | 1.14 | 8.87% | 0.81% |
| 150 | 0.5 | 4 | 0.0150 | 1.15 | 0.0164 | 1.16 | 9.93% | 0.88% |
| 150 | 0.05 | 2.5 | 0.0150 | 1.15 | 0.0173 | 0.95 | 15.47% | 17.14% |
| 0 | - | - | 0.0075 | 0.75 | 0.0061 | 0.60 | 18.67% | 19.67% |
| 80 | 0.05 | 4 | 0.0075 | 0.75 | 0.0192 | 0.60 | 156.00% | 19.89% |
| 150 | 0.5 | 4 | 0.0075 | 0.75 | 0.0213 | 0.60 | 184.00% | 19.96% |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Dover, C.; Johnson, D.; Ruiz-Altisent, M.; Valero, C. Nondestructive quantification of chemical and physical properties of fruits by time-resolved reflectance spectroscopy in the wavelength range 650–1000 nm. Appl. Opt. 2001, 40, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Ruiz-Altisent, M.; Valero, C.; Ortiz, C.; Dover, C. Time-resolved reflectance spectroscopy applied to the nondestructive monitoring of the internal optical properties in apples. Appl. Spectrosc. 2001, 55, 1368–1374. [Google Scholar] [CrossRef] [Green Version]
- Nicolaï, B.M.; Verlinden, B.E.; Desmet, M.; Saevels, S.; Saeys, W.; Theron, K.; Cubeddu, R.; Pifferi, A.; Torricelli, A. Time-resolved and continuous wave NIR reflectance spectroscopy to predict soluble solids content and firmness of pear. Postharvest Biol. Technol. 2008, 47, 68–74. [Google Scholar] [CrossRef]
- McGlone, V.; Abe, H.; Kawano, S. Kiwifruit firmness by near infrared light scattering. J. Near Infrared Spectrosc. 1997, 5, 83–90. [Google Scholar] [CrossRef]
- Il’iasov, S.; Krasnikov, V.V. Physical Principles of Infrared Irradiation of Foodstuffs; Hemisphere Pub. Corp.: Summit, NJ, USA, 1991. [Google Scholar]
- Watté, R.; Aernouts, B.; Van Beers, R.; Herremans, E.; Ho, Q.T.; Verboven, P.; Nicolaï, B.; Saeys, W. Modeling the propagation of light in realistic tissue structures with mmc-fpf: A meshed monte carlo method with free phase function. Opt. Express 2015, 23, 17467–17486. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V.; Maksimova, I.L.; Zimnyakov, D.A.; Kon, I.L.; Mavlyutov, A.H.; Mishin, A.A. Light propagation in tissues with controlled optical properties. J. Biomed. Opt. 1997, 2, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.-F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef]
- Welch, A.J.; Van Gemert, M.J. Optical-Thermal Response of Laser-Irradiated Tissue; Springer: New York, NY, USA, 1995; Vol. 1. [Google Scholar]
- Gibson, A.; Hebden, J.; Arridge, S.R. Recent advances in diffuse optical imaging. Phys. Med. Biol. 2005, 50. [Google Scholar] [CrossRef]
- Kienle, A.; Patterson, M.S. Improved solutions of the steady-state and the time-resolved diffusion equations for reflectance from a semi-infinite turbid medium. J. Opt. Soc. Am. A 1997, 14, 246–254. [Google Scholar] [CrossRef]
- Farrell, T.J.; Patterson, M.S.; Wilson, B. A diffusion theory model of spatially resolved, steady-state diffuse reflectance for the noninvasive determination of tissue optical properties in vivo. Med. Phys. 1992, 19, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Contini, D.; Martelli, F.; Zaccanti, G. Photon migration through a turbid slab described by a model based on diffusion approximation. I. Theory. Appl. Opt. 1997, 36, 4587–4599. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Lu, R. Hyperspectral diffuse reflectance imaging for rapid, noncontact measurement of the optical properties of turbid materials. Appl. Opt. 2006, 45, 8366–8373. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lu, R. Analysis of spatially resolved hyperspectral scattering images for assessing apple fruit firmness and soluble solids content. Postharvest Biol. Technol. 2008, 48, 52–62. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Mendoza, F.; Beaudry, R.M. Relationship of the optical absorption and scattering properties with mechanical and structural properties of apple tissue. Postharvest Biol. Technol. 2013, 85, 30–38. [Google Scholar] [CrossRef]
- Lorente, D.; Zude, M.; Idler, C.; Gómez-Sanchis, J.; Blasco, J. Laser-light backscattering imaging for early decay detection in citrus fruit using both a statistical and a physical model. J. Food Eng. 2015, 154, 76–85. [Google Scholar] [CrossRef]
- Valero, C.; Ruiz-Altisent, M.; Cubeddu, R.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Johnson, D.; Dover, C. Selection models for the internal quality of fruit, based on time domain laser reflectance spectroscopy. Biosyst. Eng. 2004, 88, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Chauchard, F.; Roger, J.; Bellon-Maurel, V.; Abrahamsson, C.; Andersson-Engels, S.; Svanberg, S. Madstress: A linear approach for evaluating scattering and absorption coefficients of samples measured using time-resolved spectroscopy in reflection. Appl. Spectrosc. 2005, 59, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Vaudelle, F.; L’Huillier, J.-P. Influence of the size and skin thickness of apple varieties on the retrieval of internal optical properties using VIS/NIR spectroscopy: A monte carlo-based study. Comput. Electron. Agric. 2015, 116, 137–149. [Google Scholar] [CrossRef]
- Schmitt, J.; Wall, R.; Zhou, G.; Walker, E. Multilayer model of photon diffusion in skin. JOSA A 1990, 7, 2141–2153. [Google Scholar] [CrossRef]
- Kienle, A.; Patterson, M.S.; Dögnitz, N.; Bays, R.; Wagnieres, G.; van Den Bergh, H. Noninvasive determination of the optical properties of two-layered turbid media. Appl. Opt. 1998, 37, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Lu, R. Quantification of the optical properties of two-layer turbid materials using a hyperspectral imaging-based spatially-resolved technique. Appl. Opt. 2009, 48, 5612–5623. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.; Schweiger, M.; Hiraoka, M.; Delpy, D. A finite element approach for modeling photon transport in tissue. Med. Phys. 1993, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Katsimichas, S.; de Oliveira, C. Time-dependent diffusion and transport calculations using a finite-element-spherical harmonics method. J. Quant. Spectrosc. Radiat. Transf. 2005, 95, 349–363. [Google Scholar] [CrossRef]
- Deulin, X.; L’Huillier, J.P. Finite element approach to photon propagation modeling in semi-infinite homogeneous and multilayered tissue structures. Eur. Phys. J. Appl. Phys. 2006, 33, 133–146. [Google Scholar] [CrossRef]
- Wang, L.; Jacques, S.L.; Zheng, L. MCML-Monte Carlo modeling of light transport in multi-layered tissues. Comput. Methods Prog. Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Okada, K. Extended time dependent dielectric breakdown model based on anomalous gate area dependence of lifetime in ultra thin silicon dioxides. Jpn. J. Appl. Phys. 1997, 36, 1443–1447. [Google Scholar] [CrossRef]
- Mansouri, C.; L’Huillier, J.-P.; Kashou, N.H.; Humeau, A. Depth sensitivity analysis of functional near-infrared spectroscopy measurement using three-dimensional monte carlo modelling-based magnetic resonance imaging. Lasers Med. Sci. 2010, 25, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaudelle, F.; L’huillier, J.-P. Time-resolved optical fluorescence spectroscopy of heterogeneous turbid media with special emphasis on brain tissue structures including diseased regions: A sensitivity analysis. Opt. Commun. 2013, 304, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Eze, R.; Kumar, S. Laser transport through thin scattering layers. Appl. Opt. 2010, 49, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Rojas, E.; Garrido-Varo, A.; Aernouts, B.; Pérez-Marín, D.; Saeys, W.; Yamada, Y.; Guerrero-Ginel, J.E. Understanding near infrared radiation propagation in pig skin reflectance measurements. Innov. Food Sci. Emerg. Technol. 2014, 22, 137–146. [Google Scholar] [CrossRef]
- Meglinski, I.V.; Matcher, S.J. Quantitative assessment of skin layers absorption and skin reflectance spectra simulation in the visible and near-infrared spectral regions. Physiol. Meas. 2002, 23, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.; Culver, J.; Stott, J.; Dunn, A. Three dimensional monte carlo code for photon migration through complex heterogeneous media including the adult human head. Opt. Express 2002, 10, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Lu, R. Monte carlo simulation for quantification of light transport features in apples. Comput. Electron. Agric. 2009, 68, 44–51. [Google Scholar] [CrossRef]
- Baranyai, L.; Zude, M. Analysis of laser light propagation in kiwifruit using backscattering imaging and monte carlo simulation. Comput. Electron. Agric. 2009, 69, 33–39. [Google Scholar] [CrossRef]
- Wang, W.; Li, C. Measurement of the light absorption and scattering properties of onion skin and flesh at 633 nm. Postharvest Biol. Technol. 2013, 86, 494–501. [Google Scholar] [CrossRef]
- Ishimaru, A. Wave Propagation and Scattering In Random Media. Volume I-Single Scattering And Transport Theory; New York Academic Press Inc.: New York, NY, USA, 1978; p. 267. [Google Scholar]
- Askoura, M.L.; Piron, V.; Vaudelle, F.; L’Huillier, J.-P.; Madieta, E.; Mehinagic, E. Experimental investigation on light propagation through apple tissue structures. Proc. SPIE 2015, 9542. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, R. Improving apple fruit firmness predictions by effective correction of multispectral scattering images. Postharvest Biol. Technol. 2006, 41, 266–274. [Google Scholar] [CrossRef]
- Zhang, F.; Kinnunen, M.; Popov, A.; Myllylä, R. Monte carlo method for simulating optical coherence tomography signal in homogeneous turbid media. Proc. SPIE 2007, 7022. [Google Scholar] [CrossRef]
- Prahl, S.A.; Keijzer, M.; Jacques, S.L.; Welch, A.J. A monte carlo model of light propagation in tissue. Dosim. Laser Radiat. Med. Biol. 1989, 5, 102–111. [Google Scholar]
- Prahl, S.A.; van Gemert, M.J.; Welch, A.J. Determining the optical properties of turbid media by using the adding-doubling method. Appl. Opt. 1993, 32, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Witt, A.N. Multiple scattering in reflection nebulae. Ii. Uniform plane-parallel nebulae with foreground stars. Astrophys. J. Suppl. Ser. 1977, 35, 7–19. [Google Scholar] [CrossRef]
- Castro, D.C.; Álvarez, N.; Gabriel, P.; Micheloud, N.; Buyatti, M.; Gariglio, N. Crop loading studies on “caricia” and “eva” apples grown in a mild winter area. Sci. Agric. 2015, 72, 237–244. [Google Scholar] [CrossRef]
- Konarska, A. The structure of the fruit peel in two varieties of malus domestica borkh.(rosaceae) before and after storage. Protoplasma 2013, 250, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Homutova, I.; Blažek, J. Differences in fruit skin thickness between selected apple (malus domestica borkh.) cultivars assessed by histological and sensory methods. Hortic. Sci. 2006, 33, 108–113. [Google Scholar]
- Rowe, P.I.; Künnemeyer, R.; McGlone, A.; Talele, S.; Martinsen, P.; Seelye, R. Relationship between tissue firmness and optical properties of “royal gala” apples from 400 to 1050 nm. Postharvest Biol. Technol. 2014, 94, 89–96. [Google Scholar] [CrossRef]
- Lu, R.; Cen, H.; Huang, M.; Ariana, D.P. Spectral absorption and scattering properties of normal and bruised apple tissue. Trans. ASABE 2010, 53, 263–269. [Google Scholar] [CrossRef]
- Saeys, W.; Velazco-Roa, M.A.; Thennadil, S.N.; Ramon, H.; Nicolaï, B.M. Optical properties of apple skin and flesh in the wavelength range from 350 to 2200 nm. Appl. Opt. 2008, 47, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Keijzer, M.; Jacques, S.L.; Prahl, S.A.; Welch, A.J. Light distributions in artery tissue: Monte carlo simulations for finite-diameter laser beams. Lasers Surg. Med. 1989, 9, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kienle, A.; Lilge, L.; Patterson, M.S.; Hibst, R.; Steiner, R.; Wilson, B.C. Spatially resolved absolute diffuse reflectance measurements for noninvasive determination of the optical scattering and absorption coefficients of biological tissue. Appl. Opt. 1996, 35, 2304–2314. [Google Scholar] [CrossRef] [PubMed]
- Haskell, R.C.; Svaasand, L.O.; Tsay, T.-T.; Feng, T.-C.; McAdams, M.S.; Tromberg, B.J. Boundary conditions for the diffusion equation in radiative transfer. J. Opt. Soc. Am. A 1994, 11, 2727–2741. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Postharvest Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Lee, K. Optical mammography: Diffuse optical imaging of breast cancer. World J. Clin. Oncol. 2011, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zeng, F.-A.; Chance, B. Photon migration in the presence of a single defect: A perturbation analysis. Appl. Opt. 1995, 34, 3826–3837. [Google Scholar] [CrossRef] [PubMed]
- Do Trong, N.N.; Rizzolo, A.; Herremans, E.; Vanoli, M.; Cortellino, G.; Erkinbaev, C.; Tsuta, M.; Spinelli, L.; Contini, D.; Torricelli, A. Optical properties-microstructure-texture relationships of dried apple slices: Spatially resolved diffuse reflectance spectroscopy as a novel technique for analysis and process control. Innov. Food Sci. Emerg. Technol. 2014, 21, 160–168. [Google Scholar] [CrossRef] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askoura, M.L.; Vaudelle, F.; L’Huillier, J.-P. Numerical Study of Light Transport in Apple Models Based on Monte Carlo Simulations. Photonics 2016, 3, 2. https://doi.org/10.3390/photonics3010002
Askoura ML, Vaudelle F, L’Huillier J-P. Numerical Study of Light Transport in Apple Models Based on Monte Carlo Simulations. Photonics. 2016; 3(1):2. https://doi.org/10.3390/photonics3010002
Chicago/Turabian StyleAskoura, Mohamed Lamine, Fabrice Vaudelle, and Jean-Pierre L’Huillier. 2016. "Numerical Study of Light Transport in Apple Models Based on Monte Carlo Simulations" Photonics 3, no. 1: 2. https://doi.org/10.3390/photonics3010002