1. Introduction
Retention mechanisms of various chromatographic methods such as gas chromatography, liquid chromatography, chiral chromatography, and affinity chromatography have been quantitatively studied using computational chemistry. The new approach has demonstrated the determination of the quantitative structure–retention relationship using chromatography [
1]. However, the system required is fairly expensive software, despite that the software can be run by a personal computer. Because the model phase requires more than 1 MB of memory and a longer calculation time, to date, simple molecules have been used to explain the basic retention mechanisms in chromatography. This simple approach was applied to explain the difference in retention mechanisms of hydrophobic (reversed-phase) and aqueous hydrophilic interaction, as well as the ion-exchange liquid chromatographic method. Ion-exchange liquid chromatography is a type of hydrophilic liquid chromatography, whereas ion-exchange liquid chromatography is an established liquid chromatography method [
2]. Therefore, ion-exchange liquid chromatography should remain classified as an independent chromatographic method. The retention mechanism of ion-exchange liquid chromatography occurs via the exchange of ions interacting (being adsorbed) with the ion-exchange groups of the ion-exchangers. However, the molecular forms of the analytes are retained on ion-exchangers by Lewis acid–base (charge-transfer) interactions, as well as by hydrogen-bonding. On the other hand, if the silanol effect of bonded-phase silica gel is eliminated, the retention mechanisms of hydrophilic-interaction liquid chromatography (HILIC) are hydrogen bonding (HB) and Lewis acid–base (charge-transfer) interactions that are dependent upon the chemical structures of the packing materials and analytes [
3]. The retention mechanism of HILIC-mode liquid chromatography was reviewed [
4,
5,
6,
7,
8,
9,
10]. The chromatographic behavior of selected compounds using silica-based stationary phases was studied, and it was concluded that the description of either partition or adsorption mechanisms were inconclusive in HILIC. Ion-exchange was a significant contributor to the retention of ionized bases for all the studied columns. Van Deemter plots of different columns were studied, but the results varied. The behavior could be influenced by the polymeric or monomeric nature of the different bonded phases, and the polymeric layers led to a somewhat reduced performance [
4].
The stationary and mobile phases used in HILIC were analyzed, and it was difficult to unambiguously define the HILIC retention mechanism. The term “HILIC” could be used as the reference to a plethora of techniques employing polar stationary phases in combination with aqueous–organic (or polar–organic) mobile phases, generally showing a normal-phase retention behavior, which could be to some extent affected by ion-exchange, ion-repulsion, size-exclusion, ion-pairing or reversed-phase mechanism contributions, regardless of whether the solute distribution between the stationary and the mobile phases was attributed to adsorption, partition, or a combination of the two effects. This “universal” understanding of HILIC separations might cover the gap between the non-aqueous normal-phase chromatography and aqueous reversed-phase chromatography [
7]. The measurement of adsorbed water in the stationary phase exhibited the properties of different stationary phases; however, such polar phases retain free silanol groups [
9]. The retention mechanism of HILIC in terms of HB, Coulombic interactions, and the phase ratio using linear solvation energy relationships between 23 packing materials were examined; however, no universal model that simultaneously comprises acidic, basic, and neutral analytes could be achieved. HILIC columns were classified using physico-chemical descriptors, and the retention mechanisms were intensely discussed; however, the results could not be adopted as a general rule, and may only be attributed to their specific test settings, varying under different elution conditions [
10]. These above-mentioned problems are the basic problems in quantitatively studying the stationary phase selectivity. The stability of silica gels and the polar bonded phases also hinder the reproducibility in normal-phase liquid chromatography. The results are varied when such columns are aged. Particularly, polar bonded silica gels are unstable in aqueous eluents containing buffer components. Moreover, the adsorption of water and a soluble silica matrix under such conditions render the stationary phase unstable. Therefore, it was difficult to fit the retention data to the equations. However, hydrophilic interactions and ion-exchange should be clearly explained from the nature of analytes and the used packing materials. The HILIC mode and HILIC mechanism should be carefully described and selected. Their conclusions may reflect that the specificity and lifetime of the polar bonded phases are not guaranteed by manufacturers, and the free silanol groups remain in the polar phases.
However, it is challenging to explain the retention mechanisms of HILIC in various bonded-phase silica gels, and the experimental conditions must be known and the experimental conditions simplified, in order to explain the retention mechanisms quantitatively. Here, the experimental conditions were simplified, and the hydrophobic and hydrophilic retention mechanisms have been analyzed and explained quantitatively using a computational chemical method with simple model phases and analytes.
A basic quantitative explanation of molecular interactions (MIs) was applied to the quantitative analyses of HILIC retention mechanisms. The retention factors were based on the solubility factors that are commonly used to explain the retention mechanisms in liquid chromatography [
1]. The mechanisms are quantitatively explained using model compounds in silico, as used in organic chemistry. Different chromatography methods demonstrate the typical MI forces. If we can quantitatively reconstruct the obtained solubility factors, we can quantitatively analyze the chromatographic retention mechanisms. Computational chemical analysis methods provide the MI energy as the sum of mainly van der Waals (VW), HB, and electrostatic (ES) energy values. The VW energy is related to molecular size; hence, the contact surface area between an analyte and an adsorbent contribute to the MI energy. When HB exists between an analyte and an adsorbent, the HB energy contributes to the MI energy. When ion–ion interactions exist, ES energy contributes to the MI energy. The interaction degree can be obtained as ES energy values as calculated for the ion–ion interaction using molecular mechanics (MM) calculations. Here, a simple model experiment was carried out in silico to obtain a quantitative explanation of hydrophobic and hydrophylic mode chromatography, particularly for the effect of alkyl chain (ligand) length as described excluding ion-exchangers and zwitter ion phase used for HILIC; however the retention mechanism was inconclusive because unspecified experimental conditions were provided.
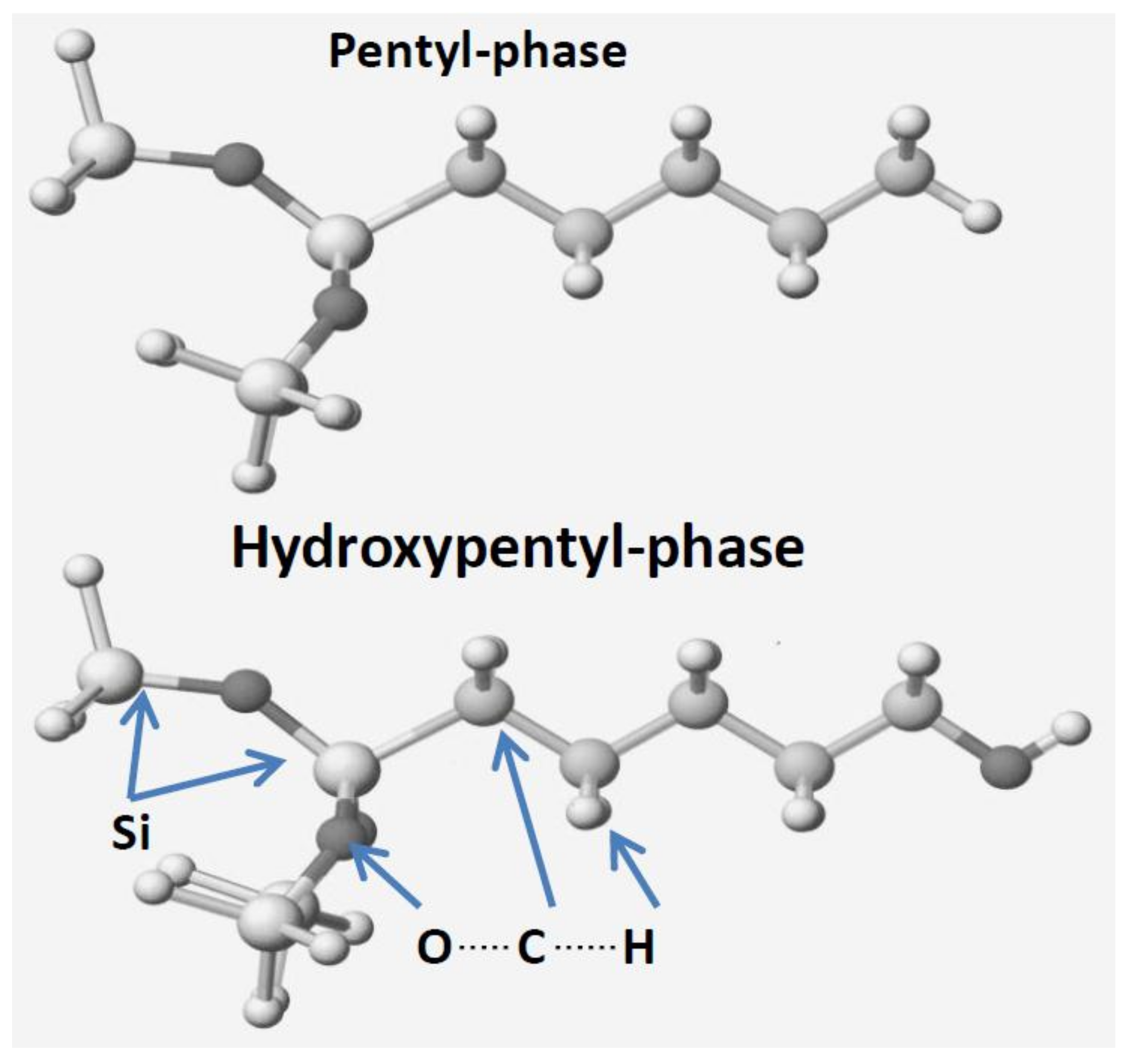
Pentyl-bonded silica gel is inert and stable compared to butyl-bonded silica gel [
11]. Therefore, the design of stable and polar bonded silica gels was studied to perform HILIC-mode liquid chromatography for the long-term operation. The limitation of the alkyl chain (ligand) length was studied using a computational chemical analysis.
2. Experimental
The model phases were constructed on the basis of bonded-phase silica gels. Model pentyl- and pentanol-bonded silica gels are shown as examples in
Figure 1. The siloxane-related atoms were locked (indicated by L on atoms) as solid silica gels. These model phases were considered as model phases without a silanol effect. Free silanol groups form strong interactions with amines. However, modern bonded-phase silica gels synthesized from pure silica gels are chemically treated and do not show the silanol effect. However, popular amino-, cyano-, and phenyl-silica gels are not treated using secondary silanization (end-capping), and thus, a long lifetime is not guaranteed in an aqueous solution containing buffer components. When the silanol effect is included, the analysis of chromatographic retention mechanisms becomes very complicated, and the basic mechanisms cannot be quantitatively analyzed. In this experiment, the model alkyl chain length was from ethyl to octadecyl. The analytes were benzoic acid, aniline, benzene, water, and acetonitrile. The molecular and ionized form of benzoic acid and aniline were used to make clear the effect of HB and ES energy changes. Their polar group effect for direct interactions with the model phase was also studied, and either the polar group of analytes directly faced the model phases or the aromatic ring directly faced the model phases. The MI energy values were calculated using the following equations. The HB, ES, and VW energy values were calculated using the CAChe MM program (Fujitsu, Japan). The computer was a PC model Prime INWIN BL672-4 with Intel Core i7 from Dospara, Yokohama, Japan. These MI energy values (kcal mol
−1), the sum of the solute and model phase energy values minus a complex energy value, were calculated according to the following equations [
1]. MIHB, MIES, and MIVW are the MI energy of HB, ES, and VW energy values, respectively.
MIHB = HB (molecule A) + HB (molecule B) − HB (molecule A and molecule B complex).
MIES = ES (molecule A) + ES (molecule B) − ES (molecule A and molecule B complex).
MIVW = VW (molecule A) + VW (molecule B) − VW (molecule A and molecule B complex). The relative MIHB, MIES, and MIVW values indicate the contribution level.
3. Results and Discussion
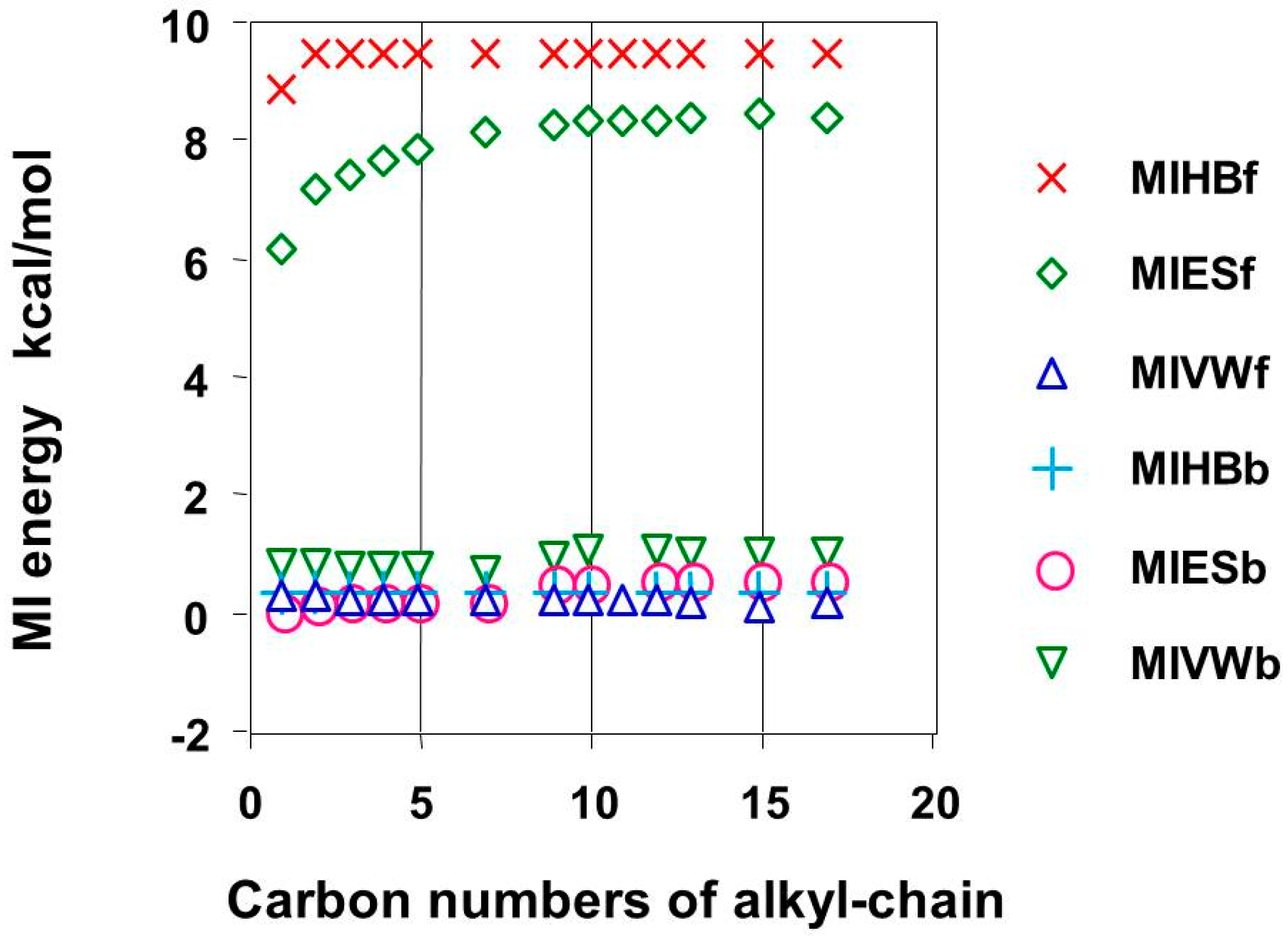
First, the alkyl chain-length effect, which may contribute to the MIs based on the electron localization of the alkyl group, was studied, because the alkyl chain length contributes to the HB of alkyl alcohols. The HB of alkyl alcohols on the alkyl chain length and up to four methylene units can affect the HB based on calorimetric experiments. The result could be explained by the energy change calculated using the MM [
12]. The calculated MI energy values are shown in
Table 1 and
Table 2 with the corresponding alkyl chain numbers. The complex conformation was designed to form head-to-head complexes but not side-to-side complexes. This is because the side-to-side complexes are related to the contact surface area but are not direct indications of the electron localization at the head group of the model phase. The practical alkyl chain length in reversed-phase liquid chromatography was demonstrated, and the proposed chain length was 11 [
13]. The quantitative analysis was achieved using dodecane as the brush for the model bonded phase, and the MI between the dodecane and a variety of analytes, such as alkanes, alkenes, and alkyl alcohols, but not aromatic compounds, as a result of the requirement of steric effects, was quantitatively analyzed using MM calculations. As the results, VW energy was the predominant MI. The alkyl chain length affected the retention of a variety of compounds. The MIVW of larger compounds was constant when a shorter alkyl chain bonded phase was used. MIVW energy values of alkenes were less than those of their related alkanes. The result supports the idea that the hydrophobic interaction due to MIVW is the predominant MI in reversed-phase liquid chromatography [
1].
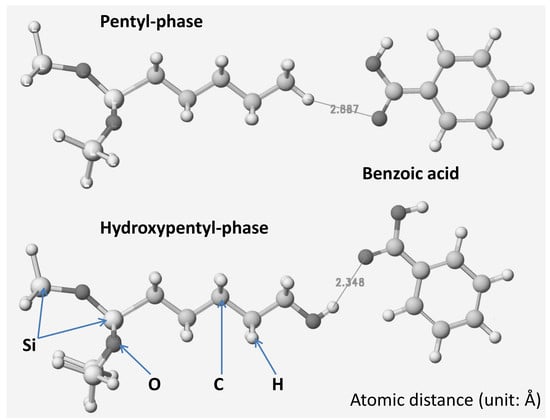
When the phenyl group of benzoic acid was in contact with the alkyl group at the head of the alkyl chain, the MIVW was about 0.9 kcal mol−1, and even benzoic acid was ionized. The results indicated that the hydrophobic interaction was about the same for both molecular and ionized benzoic acids in the head-to-head complex. The MIVW values for molecular and ionized forms of benzoic acid complexes between the carboxyl group and the head of the alkyl chain were 0.7 and 0.65 kcal mol−1, respectively. The MIHB and MIES values were about zero for these complexes. These values indicate that the VW interactions of the head-to-head complex are weak, and the simple structure does not have a larger surface area than that of the carboxyl group. When the carboxyl group was ionized, the behavior was reversed. The results can be used to understand the chromatographic behavior of acidic compounds in reversed-phase liquid chromatography. The non-polar group is in contact with the non-polar phase, and the polar group does not face the non-polar phase. Further, the ionization reduces the retention time. Thus, this simple model analysis predicts the chromatographic behavior of acidic compounds in the reversed-phase mode.
When a hydroxyl group was introduced to the head of model alkyl group instead of the methyl group, the results were reversed. The MI energy between the polar group and the hydroxyl group was higher than that between the phenyl group and hydroxyl group. The MIHB, MIES, and MIVW values of the carboxyl and hydroxyl group complexes were about 2.6, 1.3, and 0.8 kcal mol
−1, respectively, and those of the phenyl and hydroxyl group complexes had values of 0.3, 0, and 0.7 kcal mol
−1, respectively. The hydroxyl group contribution to the retention is clear from these results. The polar group is favorable to the polar group. The calculated MI energy values also demonstrate the alkyl group contribution to the polar group interactions as well as to HB of alkyl alcohols [
3]. The MI energy-related alkyl chain length is shown in
Figure 2. A similar experiment was carried out using alkyl alcohols without silicon dioxide, but the flexibility of short-chain alkyl groups made it difficult to obtain similar complex conformations to those of longer alkyl chains.
When benzoic acid was ionized, the MI energy values of the phenyl and the hydroxyl group complexes were approximately the same as those of molecular-form benzoic acid. However, the MI energy values of the complexes between the hydroxyl group and ionized carboxyl group were dramatically changed. The MIHB, MIES, and MIVW values of the hydroxyl and ionized carboxyl groups were 3.6, 6, and 0.2 times those of the phenyl and the hydroxyl group complexes, respectively. Such phenomena have been observed in HILIC-mode liquid chromatography. The contribution of the HB energy value is very high, whereas that of the VW value is small.
Similar results were obtained for complexes of aniline and these alkyl- and hydroxyl- phases. When the phenyl group of aniline faced toward the methyl group of the alkyl chain, and the MIVW value was about 0.75 kcal mol−1 for both molecular and ionized anilines. The MIHB value was about zero. The MIES value of molecular-form aniline was about zero; however, the MIES value for ionized aniline decreased from 0.35 to 0 kcal mol−1, corresponding to the alkyl chain length. This result indicates that inductive effects of the alkyl chain are manifested as a change in the ES energy. When the phenyl group of aniline was aligned towards the hydroxyl group of the alkyl chain, the MIHB, MIES, and MIVW values were about 0.3, 0, and 0.7 kcal mol−1, respectively; however, when aniline was ionized, these energy values were 0.3, −0.5, and 0.7 kcal mol−1, respectively. The negative MIES value was due to the imperfect conformation of the complex. Because this analysis focused on the inductive effect of the alkyl chain length, aniline formed a head-to-head complex rather than a side-to side complex, resulting in higher MI energy values. When the amino group faced toward the hydroxyl group of the alkyl chain, the MIHB and MIES values for ionized aniline were 10.1 and 7.0 kcal mol−1, which were higher than those of molecular-form aniline, whose MIHB and MIES values were 7.0 and 1 kcal mol−1. The MIVW values of both molecular and ionized aniline were about 0 kcal mol−1. The inductive effect of the alkyl chain was also observed as the MIES value changed. However, the trend for ionized aniline was the opposite of that of molecular-form aniline. The MIES value decreased with increasing chain length for ionized aniline. Such a phenomenon was not observed for the MIHB value.
Benzene was used as a neutral compound to eliminate the HB and ionization. The MIVW value with these model alkyl phases was about 0.8 kcal mol−1. An interesting result was obtained for acetonitrile. When the methyl group formed a complex with the model alkyl phases, the MIVW value was about 3 times that of the cyano group and the model alkyl phases, about 0.75 kcal mol−1. The MIES value of the methyl and the alkyl group complexes decreased with increasing alkyl chain length, but the result was the opposite for the cyano-alkyl group complexes. However, for the model alkanol phase, the complex with benzene indicated the contribution of the MIVW; however, this was only 1 kcal mol−1, and both the MIHB and MIES were negligible.
In the pure HILIC mode, these active sites become inactive as they are filled with acetonitrile, which is in excess and prevents hydrophobic interactions between silica and pyridine [
14]. Therefore, the contribution of acetonitrile was analyzed. Acetonitrile also contributed to HB (MIHB of 3.2 kcal mol
−1, MIES of 1.9 kcal mol
−1, and weak MIVW of 0.35 kcal mol
−1). The above results indicate that the acetonitrile methyl group was in contact with the aliphatic alkyl chain and had a MIVW of 0.75 kcal mol
−1, and the acetonitrile cyano group was in contact with the alkyl chain hydroxyl group, having a MIHB of 3.2 kcal mol
−1. Water was also used as a solvent molecule, and the MIVW with these model alkyl phases was about 0.35 kcal mol
−1. The complex with water demonstrated a strong MIHB contribution (5.3 kcal mol
−1), but weak MIES (1.4 kcal mol
−1), and negligible MIVW contributions. However, the MIHB of molecular benzoic acid was only 2.6 kcal mol
−1; that of ionized benzoic acid was 9 kcal mol
−1. Therefore, ionized acids should form a strong complex in non-aqueous condition. This is a fundamental retention mechanism of hydrophilic-mode liquid chromatography. The results were the same for aniline.