An Alternative Use of Olive Pomace as a Wide-Ranging Bioremediation Strategy to Adsorb and Recover Disperse Orange and Disperse Red Industrial Dyes from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
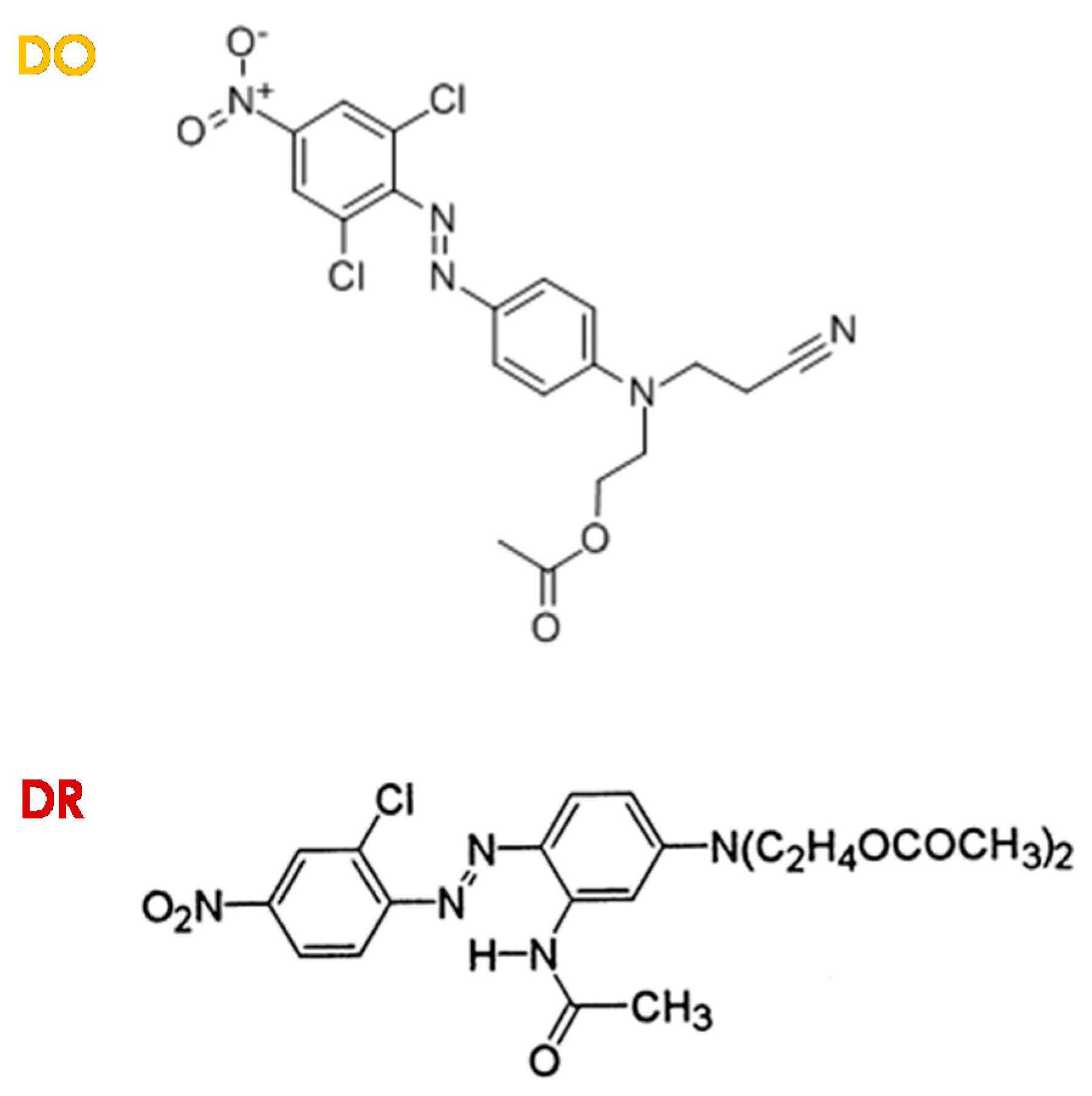
2.1. Chemicals
2.2. Preparation of the Biosorbent
2.3. Experimental Procedures
2.4. UV-Visible and Fourier Transform Infra-Red Spectroscopy in Total Attenuated Reflectance (FTIR-ATR) Measurements
2.5. X-Ray Photoelectron Spectroscopy (XPS) Analysis
2.6. Scanning Electron Microscopy (SEM)
2.7. Thermogravimetry Analysis (TGA)
2.8. Desorption Studies
3. Results and Discussion
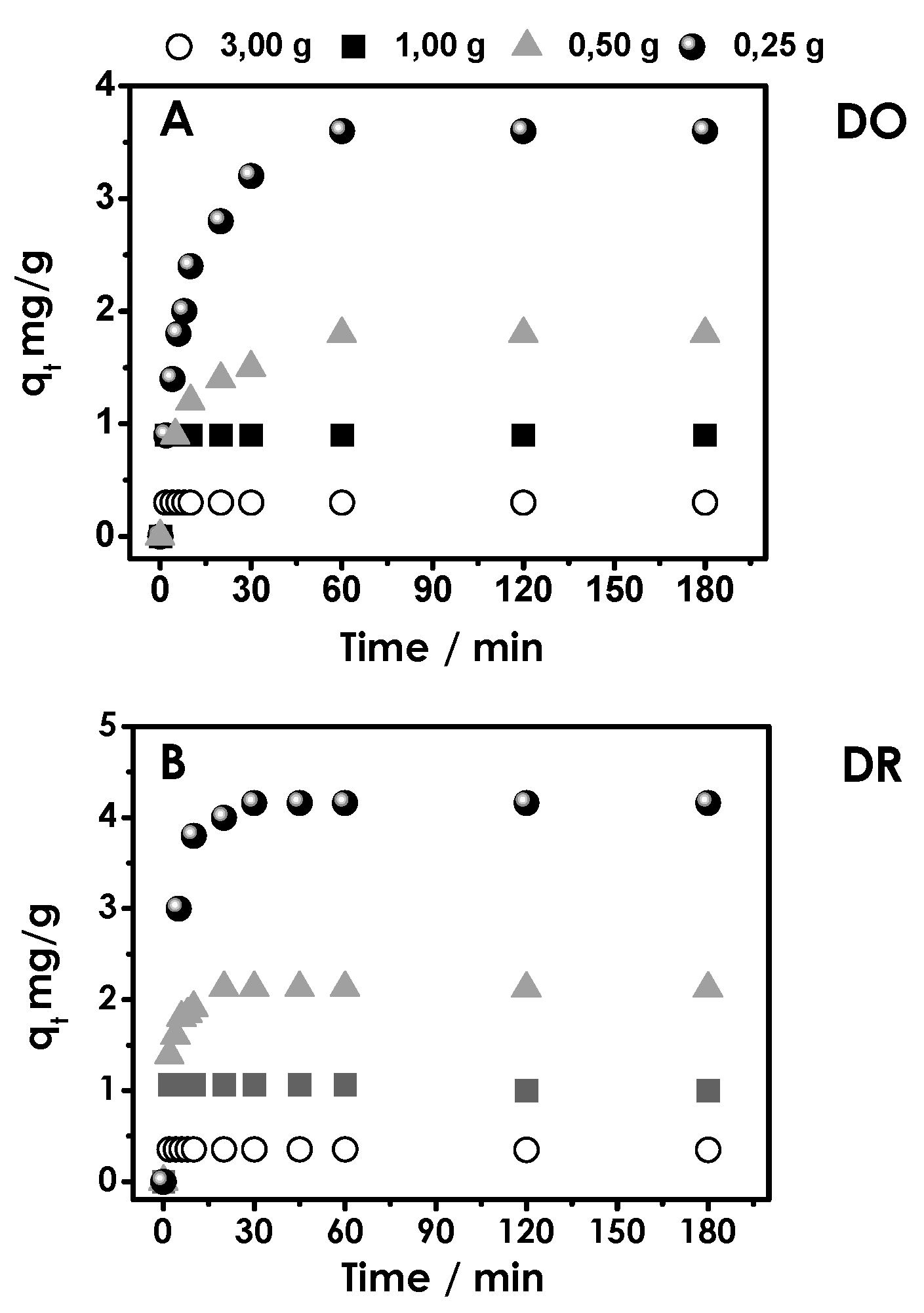
3.1. Cycles of Consecutive Adsorption of Disperse Orange and Red Dyes
3.2. Desorption Studies
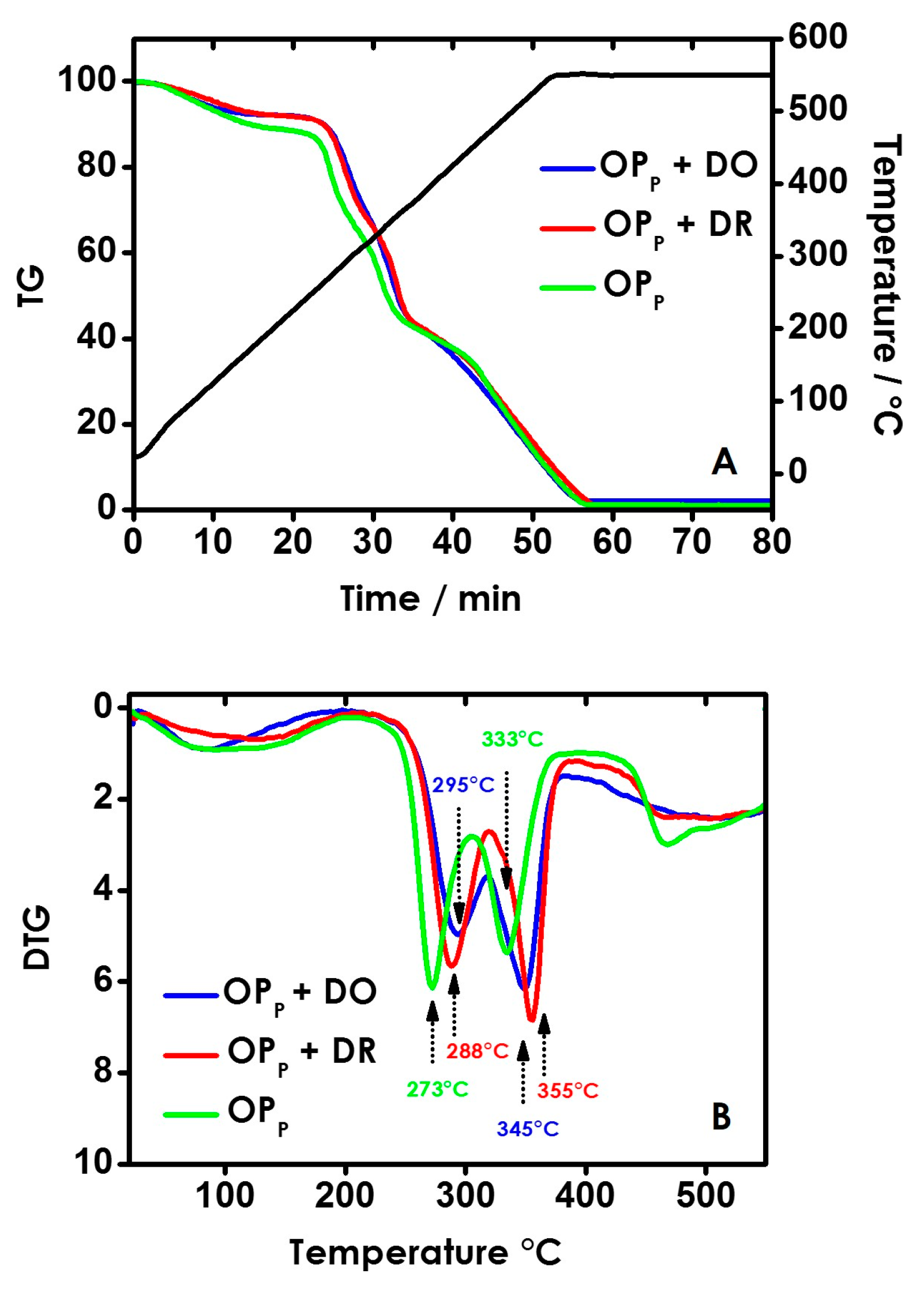
3.3. Thermogravimetric Analyses (TGA)
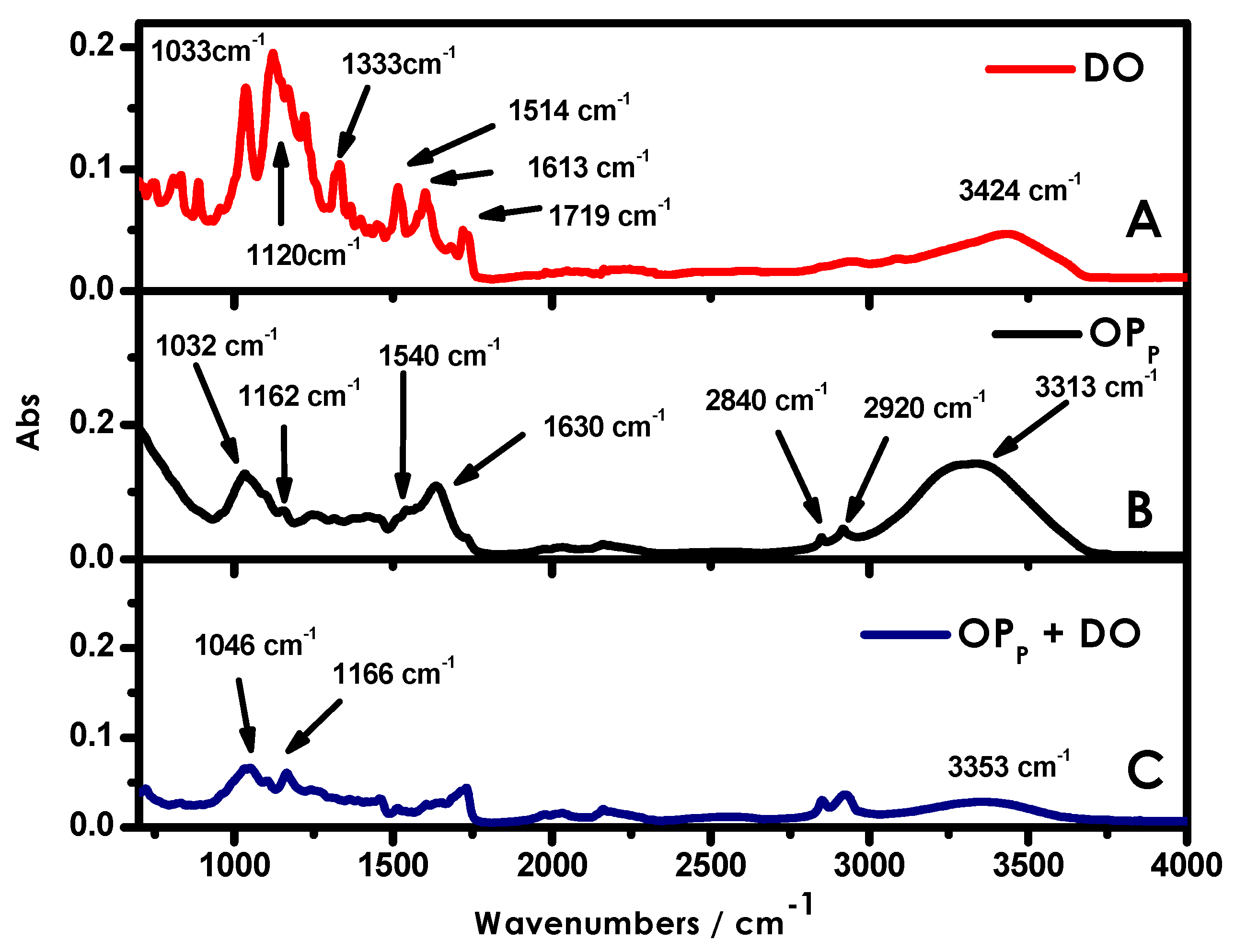
3.4. FTIR-ATR Spectroscopic Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holzman, D.C. Accounting for Nature’s Benefits: The Dollar Value of Ecosystem Services. Environ. Health Perspect. 2012, 120, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marin, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Merzouk, B.; Gourich, B.; Madani, K.; Vial, C.; Sekk, A. Removal of a disperse red dye from synthetic wastewater by chemical coagulation and continuous electrocoagulation, A comparative study. Desalination 2011, 272, 246–253. [Google Scholar] [CrossRef]
- Sirianuntapiboon, S.; Srisornsak, P. Removal of disperse dyes from textile wastewater using bio-sludge. Bioresour. Technol. 2007, 98, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Waghmode, T.R.; Kagalkar, A.N.; Govindwar, S.P. Decolorization of textile industry effluent containing disperse dye Scarlet RR by a newly developed bacterial-yeast consortium BL-GG. Chem. Eng. J. 2012, 184, 33–41. [Google Scholar] [CrossRef]
- Gercel, O.; Gercel, H.F.; Savas Koparal, A.; Ogutveren, U.B. Removal of disperse dye from aqueous solution by novel adsorbent prepared from biomass plant material. J. Hazard. Mater. 2008, 160, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Suryavathi, V.; Sharma, S.; Saxena, P.; Pandey, S.; Grover, R.; Kumar, S.; Sharma, K.B. Acute toxicity of textile dye wastewaters (untreated and treated) of Sanganer on male reproductive systems of albino rats and mice. Reprod. Toxicol. 2005, 19, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, E.R.A.; Grando, M.D.; Oliveira, D.P. The azo dye Disperse Orange 1 induces DNA damage and cytotoxic effects but does not cause ecotoxic effects in Daphnia similis and Vibrio fischeri. J. Hazard. Mater. 2011, 192, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Chu, W. Dye removal from textile dye wastewater using recycled alum sludge. Water Res. 2001, 35, 3147–3152. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Arruda Guelli Ulson de Souza, S.M.; Forgiarini, E.; Ulson de Souza, A.A. Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). J. Hazard. Mater. 2007, 147, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.H.; Lang, L.S.; Asaari, F.A.H.; Aziz, H.A.; Ramli, N.A.; Dhas, J.P.A. Low cost removal of disperse dyes from aqueous solution using palm ash. Dyes Pigment. 2007, 74, 446–453. [Google Scholar]
- Rizzi, V.; Longo, A.; Fini, P.; Semeraro, P.; Cosma, P.; Franco, E.; García, R.; Ferrándiz, M.; Núñez, E.; Gabaldón, J.A.; et al. Applicative study (Part I): The excellent conditions to remove in batch direct textile dyes (Direct red Direct blue and Direct yellow) from aqueous solutions by adsorption processes on low-cost chitosan films under different conditions. Adv. Chem. Eng. Sci. 2014, 4, 454–469. [Google Scholar] [CrossRef]
- Semeraro, P.; Rizzi, V.; Fini, P.; Matera, S.; Cosma, P.; Franco, E.; García, R.; Ferrándiz, M.; Núñez, E.; Gabaldón, J.A.; et al. Interaction of industrial textile dyes and cyclodextrins. Dyes Pigment. 2015, 119, 84–94. [Google Scholar] [CrossRef]
- Rizzi, V.; D’Agostino, F.; Fini, P.; Semeraro, P.; Cosma, P. An interesting environmental friendly cleanup: The excellent potential of olive pomace for disperse blue adsorption/desorption from wastewater. Dyes Pigment. 2017, 140, 480–490. [Google Scholar] [CrossRef]
- Rizzi, V.; Mongiovì, C.; Fini, P.; Petrella, A.; Semeraro, P.; Cosma, P. Operational parameters affecting the removal and recycling of direct blue industrial dye from wastewater using bleached oil mill waste as alternative adsorbent material. IJEAB 2017, 2, 1560–1572. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kaczala, F.; Hogland, W.; Marques, M.; Paraskeva, C.A.; Papadakis, V.G.; Sillanpää, M. Valorization of solid waste products from olive oil industry as potential adsorbents for water pollution control—A review. Environ. Sci. Pollut. Res. 2014, 21, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Vegliò, F.; Beolchini, F.; Prisciandaro, M. Sorption of copper by olive mill residues. Water Res. 2003, 37, 4895–4903. [Google Scholar] [CrossRef]
- Gharaibeh, S.H.; Abu-el-sha’r, W.Y.; Al-Kofahi, M.M. Removal of selected heavy metals from aqueous solutions using processed solid residue of olive mill products. Water Res. 1998, 32, 498–502. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Toro, L.; Veglio, F. Olive mill solid residues as heavy metal sorbent material: A preliminary study. Waste Manag. 2002, 22, 901–907. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Mainelli, S.; Veglio, F.; Toro, L. Heavy metal removal by olive pomace: Biosorbent characterization and equilibrium modelling. Chem. Eng. Sci. 2003, 58, 4709–4717. [Google Scholar] [CrossRef]
- Hawari, A.; Rawajfih, Z.; Nsour, N. Equilibrium and thermodynamic analysis of zinc ions adsorption by olive oil mill solid residues. J. Hazard. Mater. 2009, 168, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Nuhoglu, Y.; Malkoc, E. Thermodynamic and kinetic studies for environmentaly friendly Ni (II) biosorption using waste pomace of olive oil factory. Bioresour. Technol. 2009, 100, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Anbia, M.; Asl Hariri, S. Removal of methylene blue from aqueous solution using nanoporous SBA-3. Desalination 2010, 261, 61–65. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Kalaamani, P.; Subburaam, C.V. Liquid phase adsorption of crystal violet onto activated carbons derived from male flowers of coconut tree. J. Hazard. Mater. 2006, 136, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Cinar, M.; Coruh, A.; Karabacak, M. A comparative study of selected disperse azo dye derivatives based on spectroscopic (FT-IR NMR and UV–Vis) and nonlinear optical behaviors. Spectrochim. Acta Part A 2014, 122, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Raditoiu, V.; Raditoiu, A.; Wagner, L.; Amariutei, V.; Raduly, M.; Nicolaerev, C.A. Disperse Dyes Functionalized with Trialkoxysilane Groups. Rev. Chim. (Buchar.) 2011, 62, 409–414. [Google Scholar]
- Ip, A.W.M.; Barford, J.P.; McKay, G. Reactive Black dye adsorption/desorption onto different adsorbents, Effect of salt surface chemistry pore size and surface area. J. Colloid Interface Sci. 2009, 337, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Tawarah, M.K.; Rababah, R.A. Characterization of some Jordanian crude and exhausted olive pomace samples. Green Sustain. Chem. 2013, 3, 146–162. [Google Scholar] [CrossRef]
- Kabakci, S.B.; Aydemir, H. Pyrolysis of Olive Pomace and Copyrolysis of Olive Pomace with Refuse Derived Fuel. Environ. Prog. Sustain. Energy 2013, 33, 649–656. [Google Scholar] [CrossRef]
- Sundberg, B.; Mellerowicz, E.; Persson, P. XPS study of living tree. Surf. Interface Anal. 2002, 34, 284–288. [Google Scholar]
- La Rubia-García, M.D.; Yebra-Rodríguez, A.; Eliche-Quesada, D.; Corpas-Iglesias, F.A.; Lopez-Galindo, A. Assessment of olive mill solid residue (pomace) as an additive in lightweight brick production. Constr. Build. Mater. 2012, 36, 495–500. [Google Scholar] [CrossRef]
- Mao, H.; Wang, C.; Wang, Y. Synthesis of polymeric dyes based on waterborne polyurethane for improved color stability. New J. Chem. 2015, 39, 3543–3550. [Google Scholar] [CrossRef]
- Mao, H.; Yang, F.; Wang, C.; Wang, Y.; Yaob, D.; Yina, Y. Anthraquinone chromophore covalently bonded blocked waterborne polyurethanes, synthesis and application. RSC Adv. 2015, 5, 30631–30639. [Google Scholar] [CrossRef]
- Akar, T.; Tosun, I.; Kaynak, Z.; Ozkara, E.; Yeni, O.; Sahin, E.N.; Akar, S.T. An attractive agro-industrial by-product in environmental cleanup, dye biosorption potential of untreated olive pomace. J. Hazard. Mater. 2009, 166, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Pérez, A.; Blázquez, G.; Ronda, A.; Martín-Lara, M.A. Characterization of chemically modified biosorbents from olive tree pruning for the biosorption of lead. Ecol. Eng. 2013, 58, 344–354. [Google Scholar] [CrossRef]
- Timofei, S.; Schmidt, W.; Kurunczi, L.; Simon, Z. A review of QSAR for dye affinity for cellulose fibres. Dyes Pigment. 2000, 47, 5–16. [Google Scholar] [CrossRef]
- Dollendorf, C.; Kreth, S.K.; Choi, S.W.; Ritter, H. Polymerization of novel methacrylated anthraquinone dyes. Beilstein J. Org. Chem. 2013, 9, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Fini, P.; Fanelli, F.; Placido, T.; Semeraro, P.; Sibillano, T.; Fraix, A.; Sortino, S.; Agostiano, A.; Giannini, C.; et al. Molecular interactions characterization and photoactivity of Chlorophyll a/chitosan/2-HP-β-cyclodextrin composite films as functional and active surfaces for ROS production. Food Hydrocoll. 2016, 58, 98–112. [Google Scholar] [CrossRef]
| OP or OPP | OP + DO | OP + DR | ||||||
|---|---|---|---|---|---|---|---|---|
| g | % | OP Time (min) | % | OPP Time (min) | % | OP Time (min) | % | OPP Time (min) |
| 0.25 | 85 | 180 | 98 | 40 | 99 | 180 | 98 | 30 |
| 0.50 | 99 | 180 | 97 | 30 | 97 | 120 | 97 | 20 |
| 1.00 | 97 | 60 | 99 | 10 | 98 | 30 | 99 | 10 |
| 3.00 | 98 | 30 | 97 | 10 | 97 | 10 | 97 | 10 |
| Cycles | OPP + DO | OPP + DR | ||
|---|---|---|---|---|
| Efficiency (%) | Time (min) | Efficiency (%) | Time (min) | |
| 1 | 97 | 10 | 97 | 10 |
| 2 | 98 | 10 | 98 | 10 |
| 3 | 96 | 20 | 95 | 20 |
| 4 | 95 | 20 | 95 | 20 |
| 5 | 95 | 20 | 96 | 20 |
| Cycles | Adsorption/Desorption | OPP + DO | OPP + DR | ||
|---|---|---|---|---|---|
| Efficiency (%) | Time (min) | Efficiency (%) | Time (min) | ||
| 1 | Adsorption | 99 | 10 | 97 | 10 |
| Desorption | 98 | 120 | 86 | 120 | |
| 2 | Adsorption | 98 | 20 | 98 | 30 |
| Desorption | 97 | 120 | 85 | 120 | |
| 3 | Adsorption | 97 | 30 | 96 | 30 |
| Desorption | 98 | 120 | 84 | 120 | |
| 4 | Adsorption | 96 | 40 | 99 | 60 |
| Desorption | 97 | 120 | 98 | 120 | |
| 5 | Adsorption | 97 | 30 | 96 | 60 |
| Desorption | 98 | 120 | 75 | 120 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, V.; D’Agostino, F.; Gubitosa, J.; Fini, P.; Petrella, A.; Agostiano, A.; Semeraro, P.; Cosma, P. An Alternative Use of Olive Pomace as a Wide-Ranging Bioremediation Strategy to Adsorb and Recover Disperse Orange and Disperse Red Industrial Dyes from Wastewater. Separations 2017, 4, 29. https://doi.org/10.3390/separations4040029
Rizzi V, D’Agostino F, Gubitosa J, Fini P, Petrella A, Agostiano A, Semeraro P, Cosma P. An Alternative Use of Olive Pomace as a Wide-Ranging Bioremediation Strategy to Adsorb and Recover Disperse Orange and Disperse Red Industrial Dyes from Wastewater. Separations. 2017; 4(4):29. https://doi.org/10.3390/separations4040029
Chicago/Turabian StyleRizzi, Vito, Francesco D’Agostino, Jennifer Gubitosa, Paola Fini, Andrea Petrella, Angela Agostiano, Paola Semeraro, and Pinalysa Cosma. 2017. "An Alternative Use of Olive Pomace as a Wide-Ranging Bioremediation Strategy to Adsorb and Recover Disperse Orange and Disperse Red Industrial Dyes from Wastewater" Separations 4, no. 4: 29. https://doi.org/10.3390/separations4040029
APA StyleRizzi, V., D’Agostino, F., Gubitosa, J., Fini, P., Petrella, A., Agostiano, A., Semeraro, P., & Cosma, P. (2017). An Alternative Use of Olive Pomace as a Wide-Ranging Bioremediation Strategy to Adsorb and Recover Disperse Orange and Disperse Red Industrial Dyes from Wastewater. Separations, 4(4), 29. https://doi.org/10.3390/separations4040029









