Determination of Posaconazole in Plasma/Serum by High-Performance Liquid Chromatography with Fluorescence Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation and Chromatographic Conditions
2.3. Preparation of Calibrator and Quality Control Samples
2.4. Sample Preparation Procedure
2.5. Method Validation
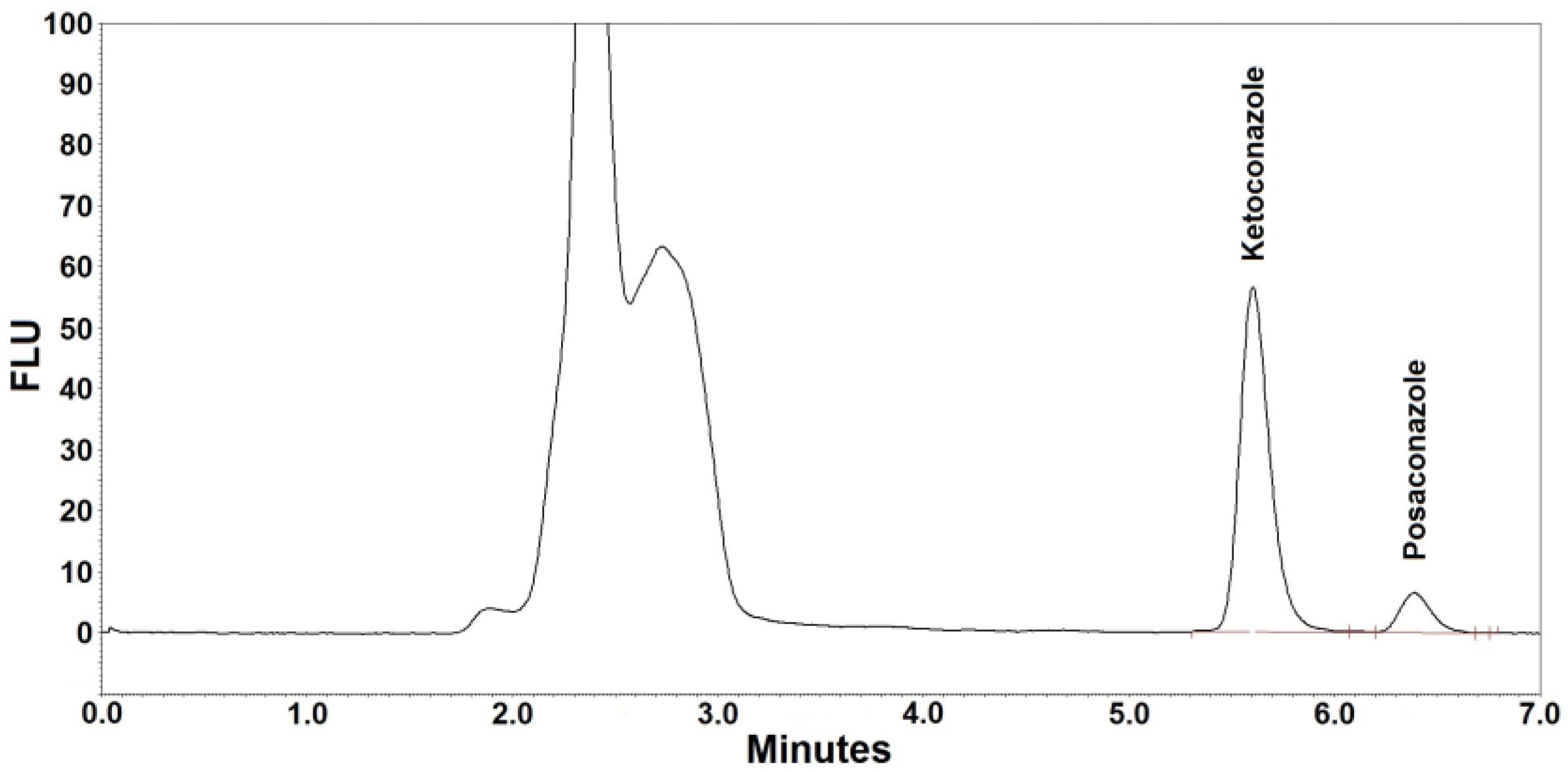
2.5.1. Selectivity
2.5.2. Calibration Curve
2.5.3. Recovery
2.5.4. Precision, Reproducibility, and Stability
2.5.5. Cross-Validation
3. Results and Discussion
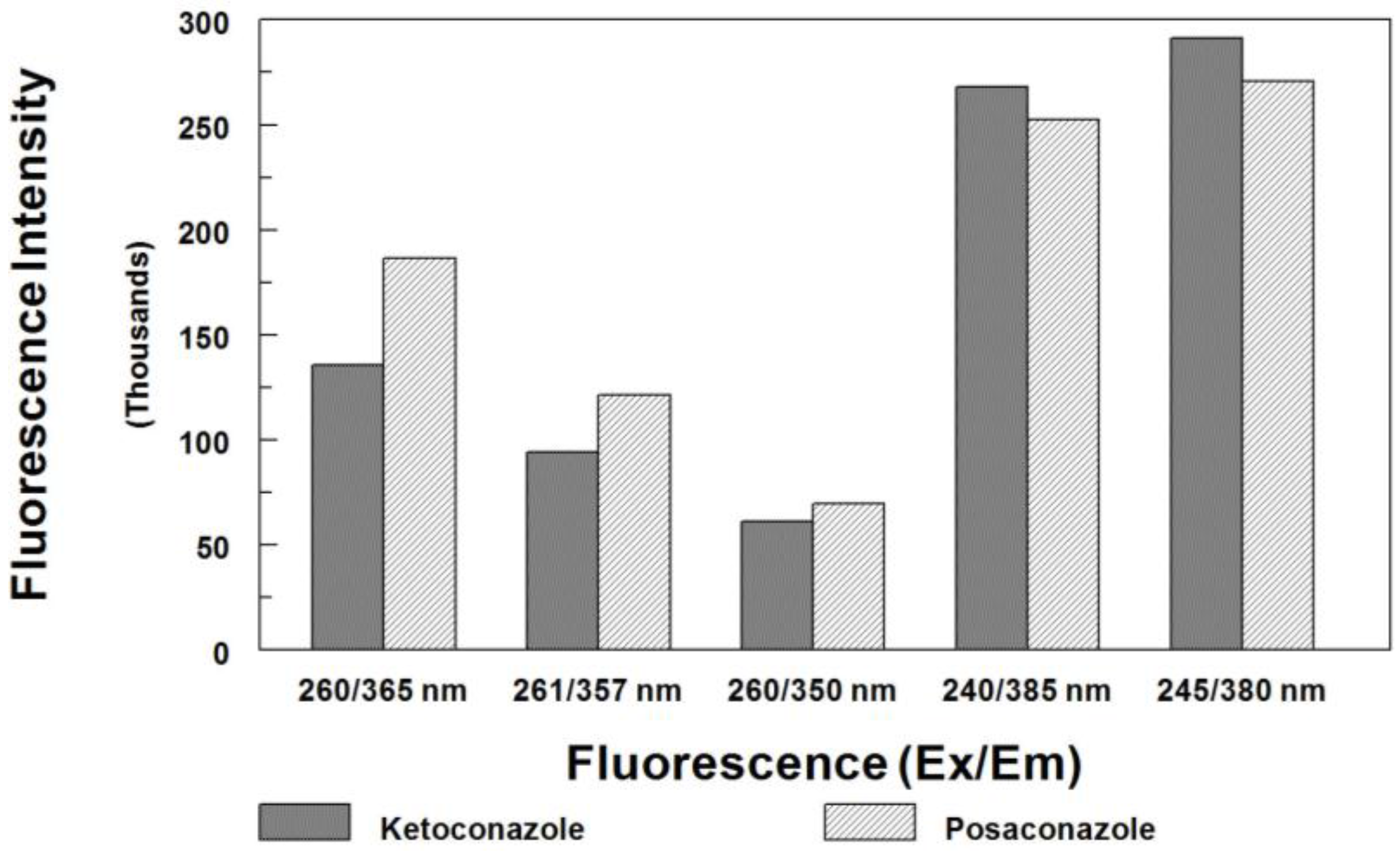
3.1. Internal Standard Alternative
3.2. Selectivity
3.3. Solvent Extraction in a Single Dilution Step
3.4. Calibration Curve
3.5. Reproducibility
3.6. Stability
3.7. Method Comparison
3.8. Cross-Validation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schiller, D.; Fung, H. Posaconazole: An extended-spectrum triazole antifungal agent. Clin. Ther. 2007, 29, 1862–1886. [Google Scholar] [CrossRef] [PubMed]
- Rachwalski, E.; Wieczorkiewicz, J.; Scheetz, M. Posaconazole: An oral triazole with an extended spectrum of activity. Ann. Pharmacother. 2008, 42, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brown, N.; Chau, A.; López-Ribot, J.; Ruesga, M.; Quindos, G.; Mendrick, C.; Hare, R.; Loebenberg, D.; DiDomenico, B.; et al. Changes in susceptibility to posaconazole in clinical isolates of Candida albicans. J. Antimicrob. Chemother. 2004, 53, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Raad, I.; Patterson, T.; Chandrasekar, P.; Donowitz, G.; Graybill, R.; Greene, R.; Hachem, R.; Hadley, S.; Herbrecht, R.; et al. Treatment of Invasive Aspergillosis with Posaconazole in Patients Who Are Refractory to or Intolerant of Conventional Therapy: An Externally Controlled Trial. Clin. Infect. Dis. 2007, 44, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.; Hachem, R.; Chemaly, R.; Kontoyiannis, D.; Raad, I. Posaconazole: A broad-spectrum triazole antifungal. Lancet Infect. Dis. 2005, 5, 775–785. [Google Scholar] [CrossRef]
- Lipp, H. Clinical pharmacodynamics and pharmacokinetics of the antifungal extended-spectrum triazole posaconazole: An overview. Br. J. Clin. Pharmacol. 2010, 70, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Theuretzbacher, U.; Clancy, C.; Nguyen, M.; Derendorf, H. Pharmacokinetic/pharmacodynamic profile of posaconazole. Clin. Pharmacokinet. 2010, 49, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Kraft, W.; Chang, P.; van Iersel, M.; Waskin, H.; Krishna, G.; Kersemaekers, W. Posaconazole tablet pharmacokinetics: Lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob. Agents Chemother. 2014, 58, 4020–4025. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Cornely, O.; Ullmann, A.; Heinz, W.; Krishna, G.; Patino, H.; Caceres, M.; Kartsonis, N.; Waskin, H.; Robertson, M. Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob. Agents Chemother. 2014, 58, 3610–3617. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K. Posaconazole: A review of the gastro-resistant tablet and intravenous solution in invasive fungal infections. Drugs 2015, 75, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kersemaekers, W.; van Iersel, T.; Nassander, U.; O’Mara, E.; Waskin, H.; Caceres, M.; van Iersel, M. Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects. Antimicrob. Agents Chemother. 2015, 59, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Luke, D.; Tomaszewski, K.; Damle, B.; Schlamm, H. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD). J. Pharm. Sci. 2010, 99, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Dolton, M.; Brüggemann, R.; Burger, D.; McLachlan, A. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob. Agents Chemother. 2014, 58, 6879–6885. [Google Scholar] [CrossRef] [PubMed]
- Dolton, M.; Ray, J.; Marriott, D.; McLachlan, A. Posaconazole exposure-response relationship: Evaluating the utility of therapeutic drug monitoring. Antimicrob. Agents Chemother. 2012, 56, 2806–2813. [Google Scholar] [CrossRef] [PubMed]
- Dolton, M.; Ray, J.; Chen, S.; Ng, K.; Pont, L.; McLachlan, A. Multicenter study of posaconazole therapeutic drug monitoring: Exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 2012, 56, 5503–5510. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Mouton, J.; Verweij, P.; Brüggemann, R. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev. Anti-Infect. Ther. 2013, 11, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, H.; Barnes, R.; Johnson, E.; Richardson, M.; Gorton, R.; Hope, W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2013, 69, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, J.; Noren, C.; Hayes, R.; Clement, R.; Shen, J. A high-throughput LC-MS/MS method for the quantitation of posaconazole in human plasma: Implementing fused core silica liquid chromatography. J. Pharm. Biomed. Anal. 2009, 50, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Vogeser, M.; Rieger, C.; Ostermann, H.; Spöhrer, U. A routine method for the quantitation of the novel antimycotic drug posaconazole in plasma using liquid chromatography-tandem mass spectrometry. Clin. Chem. Lab. Med. 2009, 47, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Rochat, B.; Pascual, A.; Pesse, B.; Lamoth, F.; Sanglard, D.; Decosterd, L.; Bille, J.; Marchetti, O. Ultra-performance liquid chromatography mass spectrometry and sensitive bioassay methods for quantitation of posaconazole plasma concentrations after oral dosing. Antimicrob. Agents Chemother. 2010, 54, 5074–5081. [Google Scholar] [CrossRef] [PubMed]
- Alffenaar, J.; Wessels, A.; van Hateren, K.; Greijdanus, B.; Kosterink, J.; Uges, D. Method for therapeutic drug monitoring of azol antifungal drugs in human serum using LC/MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Baietto, L.; D’Avolio, A.; Ventimiglia, G.; De Rosa, F.; Siccardi, M.; Simiele, M.; Sciandra, M.; Di Perri, G. Development, validation, and routine application of a high-performance liquid chromatography method coupled with a single mass detector for quantification of itraconazole, voriconazole, and posaconazole in human plasma. Antimicrob. Agents Chemother. 2010, 54, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- Beste, K.; Burkhardt, O.; Kaever, V. Rapid HPLC-MS/MS method for simultaneous quantitation of four routinely administered triazole antifungals in human plasma. Clin. Chim. Acta 2012, 413, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Baietto, L.; D’Avolio, A.; Marra, C.; Simiele, M.; Cusato, J.; Pace, S.; Ariaudo, A.; De Rosa, F.; Di Perri, G. Development and validation of a new method to simultaneously quantify triazoles in plasma spotted on dry sample spot devices and analysed by HPLC-MS. J. Antimicrob. Chemother. 2012, 67, 2645–2649. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Cho, S.; Yu, H.; Cha, K.; Lee, S.; Kim, M.; Kim, Y.; Kim, Y.J.; Kim, H.J.; Lee, D.G. Determination of posaconazole concentration with LC-MS/MS in adult patients with hematologic malignancy. Clin. Chim. Acta 2015, 450, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Chhun, S.; Rey, E.; Tran, A.; Lortholary, O.; Pons, G.; Jullien, V. Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Storzinger, D.; Swoboda, S.; Lichtenstern, C.; Müller, C.; Weigand, M.; Hoppe-Tichy, T. Development and validation of a high-performance liquid chromatography assay for posaconazole utilizing solid-phase extraction. Clin. Chem. Lab. Med. 2008, 46, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.; Langmann, P.; Schirmer, D.; Lenker, U.; Keller, D.; Helle, A.; Klinker, H.; Heinz, W. Simultaneous determination of voriconazole and posaconazole concentrations in human plasma by high-performance liquid chromatography. Antimicrob. Agents Chemother. 2009, 53, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Cendejas-Bueno, E.; Forastiero, A.; Rodriguez-Tudela, J.; Cuenca-Estrella, M.; Gomez-Lopez, A. HPLC/UV or bioassay: Two valid methods for posaconazole quantification in human serum samples. Clin. Microbiol. Infect. 2012, 18, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Wissen, C.; Burger, D.; Verweij, P.; Aarnoutse, R.; Brüggemann, R. Simultaneous determination of the azoles voriconazole, posaconazole, isavuconazole, itraconazole and its metabolite hydroxyl-itraconazole in human plasma by reversed phase ultra-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 887–888, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Moore, G.; Barclay, M.; Begga, E. A simple high-performance liquid chromatography method for simultaneous determination of three triazole antifungals in human plasma. Antimicrob. Agents Chemother. 2013, 57, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Arndt, M.; Queckenberg, C.; Cornely, O.; Theisohn, M. HPLC analysis of the antifungal agent posaconazole in patients with haematological diseases. Mycoses 2006, 49 (Suppl. 1), 17–22. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, W.; Konig, A.; Bolek, R.; Trittler, R.; Engelhardt, M.; Jung, M.; Kümmerer, K. Determination of the antifungal agent posaconazole in human serum by HPLC with parallel column-switching technique. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2493–2498. [Google Scholar] [CrossRef] [PubMed]
- Buckner, S.; Ceesay, M.; Pagliuca, A.; Morgan, P.; Flanagan, R. Measurement of posaconazole, itraconazole, and hydroxyitraconazole in plasma/serum by high-performance liquid chromatography with fluorescence detection. Ther. Drug Monit. 2011, 33, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Hens, B.; Corsetti, M.; Brouwers, J.; Augustijns, P. Gastrointestinal and systemic monitoring of posaconazole in humans after fasted and fed state administration of a solid dispersion. J. Pharm. Sci. 2016, 105, 2904–2912. [Google Scholar] [CrossRef] [PubMed]
- Remmel, R.; Amoh, K.; Abdel-Monem, M. The disposition and pharmacokinetics of ketoconazole in the rat. Drug Metab. Dispos. 1987, 15, 735–739. [Google Scholar] [PubMed]
- Khashaba, P.; El-Shabouri, S.; Emara, K.; Mohamed, A. Analysis of some antifungal drugs by spectrophotometric and spectrofluorimetric methods in different pharmaceutical dosage forms. J. Pharm. Biomed. Anal. 2000, 22, 363–376. [Google Scholar] [CrossRef]
- Tang, P.; Miles, M.; DeGrauw, A.; Hershey, A.; Pesce, A. HPLC analysis of reduced and oxidized coenzyme Q10 in human plasma. Clin. Chem. 2001, 47, 256–265. [Google Scholar] [PubMed]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry: Bioanalytical Method Validation. 2001. [Google Scholar]


| Parameters | Conditions |
|---|---|
| Column | ODS HYPERSIL, 5-µm, 250 × 4.6 mm |
| Mobile phase | Ammonium acetate (0.1 M): water:acetonitrile:TFA (409/590/1, v/v/v) |
| Mode | Isocratic elution |
| Oven | 45 °C |
| Autosampler | 5 °C |
| Flow rate | 1.1 mL/min |
| Injection | 10 µL |
| Detector settings | 245 nm (Ex) and 380 nm (Em) |
| Run time | 7 min |
| Sample | Sample Concentration (µg/mL) | Observed Concentration (µg/mL) | Standard Deviation (µg/mL) | CV (%) |
|---|---|---|---|---|
| LLOQ (n = 10) | 0.10 | 0.08–0.12 | 0.02 | 19.0 |
| ULOQ (n = 10) | 10.00 | 9.65–10.44 | 0.39 | 3.9 |
| Patient #1 (n = 5) | 1.23 | 1.15–1.30 | 0.06 | 4.9 |
| Patient #2 (n = 5) | 0.67 | 0.63–0.72 | 0.04 | 6.0 |
| Patient #3 (n = 5) | 0.96 | 0.92–1.01 | 0.05 | 5.2 |
| Run | Nominal Concentration (µg/mL) | Observed Concentration (µg/mL) | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Within-run (n = 5) | 0.300 | 0.303 | 101.1 | 4.5 |
| 2.00 | 1.95 | 97.4 | 5.2 | |
| 4.00 | 4.16 | 104.1 | 3.6 | |
| 8.00 | 8.04 | 100.6 | 2.9 | |
| Between-run (n = 5) | 0.300 | 0.293 | 97.7 | 5.3 |
| 2.00 | 2.11 | 105.5 | 5.8 | |
| 4.00 | 4.09 | 102.3 | 4.1 | |
| 8.00 | 8.17 | 102.2 | 4.0 |
| Nominal (µg/mL) | Measured Concentration (µg/mL) | ||
|---|---|---|---|
| Freeze-thaw stability (n = 3 at each concentration) | |||
| Cycle 1 | Cycle 2 | Cycle 3 | |
| 0.300 | 0.296 | 0.302 | 0.304 |
| 2.00 | 2.16 | 1.98 | 2.02 |
| 4.00 | 4.05 | 4.12 | 3.88 |
| 8.00 | 7.85 | 8.07 | 7.96 |
| Short-term stability at room temperature (n = 3 at each concentration) | |||
| 24 h | |||
| 0.300 | 0.284 | ||
| 2.00 | 2.15 | ||
| 4.00 | 4.18 | ||
| 8.00 | 8.29 | ||
| Short-term stability at refrigeration (2–8 °C, n = 3 at each concentration) | |||
| Day 2 | Day 5 | ||
| 0.300 | 0.288 | 0.292 | |
| 2.00 | 2.08 | 2.14 | |
| 4.00 | 4.26 | 4.09 | |
| 8.00 | 7.95 | 8.16 | |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.H. Determination of Posaconazole in Plasma/Serum by High-Performance Liquid Chromatography with Fluorescence Detection. Separations 2017, 4, 16. https://doi.org/10.3390/separations4020016
Tang PH. Determination of Posaconazole in Plasma/Serum by High-Performance Liquid Chromatography with Fluorescence Detection. Separations. 2017; 4(2):16. https://doi.org/10.3390/separations4020016
Chicago/Turabian StyleTang, Peter H. 2017. "Determination of Posaconazole in Plasma/Serum by High-Performance Liquid Chromatography with Fluorescence Detection" Separations 4, no. 2: 16. https://doi.org/10.3390/separations4020016





