Phytochemical Screening and Antimicrobial Activity of Various Extracts of Aerial Parts of Rhanterium epapposum
Abstract
:1. Introduction
2. Materials and Methods
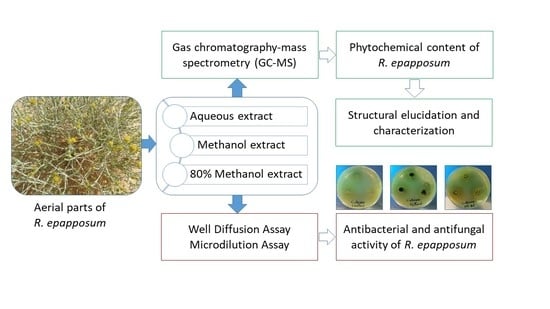
2.1. Plant Collection
2.2. Extract Preparation
2.3. Phytochemical Screening
2.4. Microorganisms
2.5. Well Diffusion Assay
2.6. Microdilution Assay
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Composition
3.2. Antimicrobial Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control. 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Narayana, D.V. A critical review on natural antimicrobial agents. World J. Pharm. Pharm. Sci. 2017, 329–341. [Google Scholar] [CrossRef]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: Possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist. Updat. 2020, 51, 100695. [Google Scholar] [CrossRef]
- Borris, R.P. Natural products research: Perspectives from a major pharmaceutical company. J. Ethnopharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Saganuwan, S.A. Some medicinal plants of Arabian Pennisula. J. Med. Plant Res. 2010, 4, 766–788. [Google Scholar] [CrossRef]
- El-shanawany, M.A.A. Plants Used in Saudi Folk Medicine; King Abdul-Aziz City for Science and Technology: Riyadh, Kingdom of Saudi Arabia, 1996. [Google Scholar]
- Phondani, P.C.; Bhatt, A.; Elsarrag, E.; Horr, Y.A. Ethnobotanical magnitude towards sustainable utilization of wild foliage in Arabian Desert. J. Tradit. Complement. Med. 2016, 6, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghmai, M.S.; Kolbadipour, S. Volatile components ofRhanterium epapposum oliv. Flavour Fragr. J. 1987, 2, 29–32. [Google Scholar] [CrossRef]
- Demirci, B.; Yusufoglu, H.S.; Tabanca, N.; Temel, H.E.; Bernier, U.; Agramonte, N.; Alqasoumi, S.I.; Al-Rehaily, A.J.; Başer, K.H.C.; Demirci, F. Rhanterium epapposum Oliv. essential oil: Chemical composition and antimicrobial, insect-repellent and anticholinesterase activities. Saudi Pharm. J. 2017, 25, 703–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeinab, E.B.E.; Abdelhafiz, A.D. Effectiveness of using oils extracts of Peganum harmala and Rhanterium epapposum against Khapra beetle (Coleoptera: Dermestidae) and their chemical compositions. Sci. Res. Essays 2019, 14, 68–73. [Google Scholar] [CrossRef]
- Adam, S.I.Y.; EL-Kamali, H.; Adama, S.E.I. Phytochemical screening and antibacterial activity of two Sudanese wild plants, Rhanterium epapposum and Trichodesma africanum. J. Fac. Sci. Tech. 2011, 2, 83–96. [Google Scholar]
- Al-yahya, M.A.; Al-meshal, I.A.; Mossa, J.S.; Al-badr, A.A.; Tariq, M. Saudi Plants, A Phytochemical and Biological Approach; King Saud University: Riyadh, Saudi Arabia, 1990; pp. 75–80. [Google Scholar]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Gatsing, D.; Tchakoute, V.; Ngamga, D.; Kuiate, J.R.; Tamokou, J.D.D.; Nji-nkah, B.F.; Tchouanguep, F.M.; Fodouop, C. In Vitro antibacterial activity of Crinum purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran. J. Med. Sci. 2009, 34, 126–136. [Google Scholar]
- Hazen, K.C. Fungicidal versus fungistatic activity of terbinafine and itraconazole: An In Vitro comparison. J. Am. Acad. Dermatol. 1998, 38, S37–S41. [Google Scholar] [CrossRef]
- Aldoweriej, A.M.; Alharbi, K.B.; Saeed, E.M.A.; El-ashmawy, I.M. Antimicrobial activity of various extracts from some plants native to Alqassim region, Saudi Arabia. J. Food Agric. Environ. 2016, 14, 14–19. [Google Scholar]
- Mohammed, H.; Al-Omer, M.S.; Ahmed, A.M.; Hashish, N.E.; Alsaedi, H.M.; Alghazy, S.A.; Abdellatif, A.A.H. Comparative Study for the Volatile Oil Constituents and Antimicrobial Activity of Rhanterium epapposum Oliv. Growing in Qassim, Saudi Arabia. Pharmacogn. J. 2019, 11, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef]



| Compound Name | % Methanol | RT (mn) | MW (g/mol) | Chemical Formula | |
|---|---|---|---|---|---|
| 1 | Ethanethiol, 2-(dimethylamino)- | 1.98 | 3.246 | 105.061 | C4H11NS |
| 2 | Ethanol, 2-methoxy-, acetate | 2.16 | 3.526 | 118.063 | C5H10O3 |
| 3 | Ethene, (2-ethoxy-1-methoxyethoxy)- | 2.11 | 4.003 | 146.094 | C7H14O3 |
| 4 | 12-Crown-4 | 1.44 | 4.347 | 176.105 | C8H16O4 |
| 5 | 2-Propanone, 1-(dimethylamino)- | 1.08 | 5.492 | 101.084 | C5H11NO |
| 6 | 3-Piperidinol, 1,4-dimethyl-, trans- | 0.20 | 10.829 | 129.115 | C7H15NO |
| 7 | endo-2-Methyl-2-norbornanol | 1.53 | 11.224 | 126.104 | C8H14O |
| 8 | 2(5H)-Furanone,3-bromo-5-((dimethylamino)methyl)-4,5-dimethyl- | 0.16 | 11.599 | 247.021 | C9H14BrNO2 |
| 9 | Naphthalene, 2,7-bis(1,1-dimethylethyl)- | 1.07 | 12.204 | 240.188 | C18H24 |
| 10 | Ethanone, 1-(6,6-dimethylbicyclo[3.1.0]hex-2-en-2-yl)- | 0.32 | 13.629 | 150.104 | C10H14O |
| 11 | 6-Hydroxymethaqualone | 1.80 | 15.556 | 266.106 | C16H14N2O2 |
| 12 | Thiazole, 4,5-dihydro-2-methylamino- | 0.26 | 17.306 | 116.041 | C4H8N2S |
| 13 | 4-Cyclopropylmethylbenzonitrile | 0.25 | 17.586 | 157.089 | C11H11N |
| 14 | Acetamide, N-[(4.alpha.,5.alpha.)-cholestan-4-yl]- | 0.72 | 20.621 | 429.397 | C29H51NO |
| 15 | 4-Methyl-1,4-heptadiene | 1.78 | 20.792 | 110.11 | C8H14 |
| 16 | Tetrahydrofuran-2-carboxylic acid, dibenzofuran-3-ylamide | 0.75 | 23.795 | 281.105 | C17H15NO3 |
| Compound Name | % Methanol 80% | RT (mn) | MW (g/mol) | Chemical Formula | |
|---|---|---|---|---|---|
| 1 | Propanoic acid, 2-oxo-, methyl ester | 2.051 | 4.028 | 102.032 | C4H6O3 |
| 2 | 2-Propanol, 1-(2-propenyloxy)- | 0.710 | 4.149 | 116.084 | C6H12O2 |
| 3 | Phthalic acid, butyl 2-methoxyethyl ester | 0.342 | 5.797 | 280.131 | C15H20O5 |
| 4 | .beta.-Myrcene | 0.271 | 7.928 | 136.125 | C10H16 |
| 5 | Triethylenediamine | 0.588 | 8.189 | 112.1 | C6H12N2 |
| 6 | 2-Methyl-1-nonene-3-yne | 0.261 | 8.399 | 136.125 | C10H16 |
| 7 | 2-Propanol, 1-(dimethylamino)- | 0.256 | 9.347 | 103.1 | C5H13NO |
| 8 | N,N-Dimethylglycine | 0.842 | 9.563 | 103.063 | C4H9NO2 |
| 9 | 3-Cyclohexene-1-methanol, .alpha.,.alpha.4-trimethyl- | 1.001 | 11.211 | 154.136 | C10H18O |
| 10 | N,N,N’-Trimethyl-1,3-propanediamine | 0.215 | 11.949 | 116.131 | C6H16N2 |
| 11 | 1,3,6-Heptatriene, 2,5,5-trimethyl- | 0.471 | 15.785 | 136.125 | C10H16 |
| 12 | Bicyclo[3.1.1]heptane, 2,6,6-trimethyl-, (1.alpha.,2.beta.,5.alpha.)- | 5.009 | 20.792 | 138.141 | C10H18 |
| 13 | n-Hexadecanoic acid | 6.665 | 22.255 | 256.24 | C16H32O2 |
| 14 | Heptadecanoic acid, 10-methyl-, methyl ester | 0.409 | 23.795 | 298.287 | C19H38O2 |
| 15 | 2,15-Hexadecanedione | 0.652 | 25.246 | 254.225 | C16H30O2 |
| 16 | Tricosane | 0.475 | 25.335 | 324.376 | C23H48 |
| 17 | Carbamic acid, N-phenyl-, 1-methyl-1-(4-methylcyclohex-3-enyl)ethyl ester | 5.590 | 26.632 | 273.173 | C17H23NO2 |
| 18 | Pentacosane | 2.486 | 26.976 | 352.407 | C25H52 |
| 19 | Heptacosane | 5.666 | 28.496 | 380.438 | C27H56 |
| 20 | Octadecane | 0.826 | 29.215 | 254.297 | C18H38 |
| 21 | 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl- | 0.963 | 29.476 | 222.198 | C15H26O |
| 22 | Nonacosane | 2.908 | 29.902 | 408.47 | C29H60 |
| 23 | dl-.alpha.-Tocopherol | 0.745 | 31.938 | 430.381 | C29H50 |
| Compound Name | % Water | RT (mn) | MW (g/mol) | Chemical Formula | |
|---|---|---|---|---|---|
| 1 | 2,3-Butanediol | 15.402 | 5.339 | 90.068 | C4H10O2 |
| 2 | Glycine, N,N-dimethyl-, methyl ester | 8.066N,N-dimethylglycinate | 5.708 | 117.079 | C5H11NO2 |
| 3 | 1,2-Cyclohexanedione | 2.888 | 8.17 | 112.052 | C6H8O2 |
| 4 | Dimethylaminomethyl-isopropyl-sulfide | 7.391 | 11.294 | 133.093 | C6H15NS |
| 5 | Hydroquinone | 2.859 | 13.75 | 110.037 | C6H6O2 |
| 6 | Phenol, 2-methoxy-4-(1-propenyl)-, (Z)- | 1.022 | 15.238 | 164.084 | C10H12O2 |
| 7 | p-Menth-4(8)-en-9-ol | 0.780 | 15.785 | 154.136 | C10H18O |
| 8 | Phenol, 2,6-dimethoxy-4-(2-propenyl)- | 1.142 | 17.809 | 194.094 | C11H14O3 |
| 9 | 1H-2-Indenone,2,4,5,6,7,7a-hexahydro-3-(1-methylethyl)-7a-methyl | 2.629 | 22.745 | 192.151 | C13H20O |
| Compound Name | % | RT (mn) | MW (g/mol) | Chemical Formula | |||
|---|---|---|---|---|---|---|---|
| Methanol | Methanol 80% | Water | |||||
| 1 | 1-Pyrrolidinylacetonitrile | 0.27 | 2.023 | - | 7.566 | 110.084 | C6H10N2 |
| 2 | cis-2,6-Dimethyl-2,6-octadiene | 3.01 | 1.839 | 3.885 | 7.852 | 138.141 | C10H18 |
| 3 | Limonene | 2.98 | 3.246 | - | 8.577 | 136.125 | C10H16 |
| 4 | 1,4-Dioxane-2,5-dione, 3,6-dimethyl- | - | 10.486 | 10.989 | 144.042 | 144.042 | C6H8O4 |
| 5 | 2-Methoxy-4-vinylphenol | 29.79 | 29.647 | 40.390 | 13.005 | 150.068 | C9H10O2 |
| 6 | Phenol, 2,6-dimethoxy- | - | 0.493 | 3.061 | 13.521 | 154.063 | C8H10O3 |
| 7 | 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-, methyl ester | 1.19 | 2.223 | - | 21.002 | 208.074 | C11H12O4 |
| 8 | Hexadecanoic acid, methyl ester | 5.95 | 2.338 | - | 21.772 | 270.256 | C17H34O2 |
| 9 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 4.15 | 2.283 | - | 23.515 | 294.256 | C19H34O2 |
| 10 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | 11.16 | 5.476 | - | 23.585 | 292.24 | C19H32O2 |
| 11 | Phytol | 21.69 | 2.541 | - | 23.706 | 296.308 | C20H40O |
| 12 | 9,12-Octadecadienoic acid (Z,Z)- | 1.76 | 3.265 | - | 23.948 | 280.24 | C18H32O2 |
| 13 | Methyl 8,11,14-heptadecatrienoate | 0.42 | 5.222 | - | 24.018 | 278.225 | C18H30O2 |
| Microorganisms | Mean Diameter of Growth Inhibition Zone (GIZ±SD) Expressed in (mm) | |||
|---|---|---|---|---|
| R. epapposum Oliv. Extracts | Ampicillin (10 mg mL−1) | |||
| Water | Methanol | Methanol 80% | ||
| Gram + and Gram – bacteria | ||||
| S. aureus (ATCC 29213) | 44.67 ± 1.00 | 44.67 ± 1.15 | 39.33 ± 1.15 | 28.00 ± 1.00 |
| S. epidermidis (ATCC 12228) | 23.33 ± 1.15 | 22.33 ± 1.15 | 24.00 ± 0 | 22.67 ± 1.15 |
| Methicillin Resistant-S. aureus (MRSA-136) | 33.00 ± 0.58 | 42.00 ± 1.00 | 41.67 ± 0.58 | 31.67 ± 0.58 |
| P. aeruginosa (ATCC 27853) | 42.67 ± 1.15 | 37.33 ± 1.15 | 37.33 ± 1.15 | 8.67 ± 1.15 |
| S. paucimobilis (clinical strain, 144) | 16.00 ± 0.58 | 14.67 ± 1.00 | 18.33 ± 1.15 | 37.67 ± 0.58 |
| E. cloacae (clinical strain, 155) | 38.67 ± 0.58 | 35.00 ± 2.08 | 36.67 ± 1.15 | 7.67 ± 0.58 |
| K. pneumoniae (clinical strain, 220) | 41.33 ± 1.53 | 25.67 ± 1.53 | 40.67 ± 1.15 | 32.67 ± 1.53 |
| E. coli (ATCC 10536) | 35.33 ± 1.15 | 41.00 ± 0.58 | 37.33 ± 1.15 | 25.67 ± 1.15 |
| Yeasts | Amphotericin B (10 mg mL−1) | |||
| C. albicans (ATCC 20402) | 39.33 ± 1.15 | 36.33 ± 0.58 | 39.33 ± 1.15 | 13.67 ± 0.58 |
| C. guillermondii (ATCC 6260) | 37.67 ± 0.58 | 43.33 ± 1.15 | 43.33 ± 1.15 | 9.67 ± 0.58 |
| C. vaginalis (clinical strain, 136) | 36.33 ± 1.15 | 35.00 ± 1.00 | 42.67 ± 1.53 | 11.33 ± 0.58 |
| C. utilis (ATCC 9255) | 37.33 ± 1.15 | 37.67 ± 0.58 | 43.33 ± 1.15 | 12.67 ± 0.58 |
| C. tropicalis (ATCC 1362) | 37.33 ± 0.58 | 34.67 ± 1.15 | 39.33 ± 1.15 | 12.00 ± 0 |
| Gram + and Gram – Bacteria | Aqueous Extract (mg mL−1) | Methanolic Extract (mg mL−1) | Methanol 80% Extract (mg mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC Ratio | MIC | MBC | MBC/MIC Ratio | MIC | MBC | MBC/MIC Ratio | |
| S. aureus (ATCC 29213) | 12.5 | >50 | >4 | 12.5 | >50 | >4 | 25 | 50 | 2 |
| S. epidermidis (ATCC 12228) | 12.5 | 25 | 2 | 12.5 | 50 | 4 | 12.5 | 50 | 2 |
| Methicillin Resistant-S. aureus (MRSA-136) | 12.5 | 50 | 4 | 12.5 | >50 | >4 | 12.5 | >50 | >4 |
| P. aeruginosa (ATCC 27853) | 12.5 | 25 | 2 | 12.5 | >50 | >4 | 12.5 | >50 | >4 |
| S. paucimobilis (clinical strain, 144) | 12.5 | >50 | >4 | 12.5 | >50 | >4 | 25 | >50 | >4 |
| E. cloacae (clinical strain, 155) | 12.5 | >50 | >4 | 12.5 | >50 | >4 | 12.5 | >50 | >4 |
| K. pneumoniae (clinical strain, 220) | 12.5 | 50 | 4 | 12.5 | >50 | >4 | 25 | >50 | >2 |
| E. coli (ATCC 10536) | 12.5 | 50 | 4 | 12.5 | 50 | 4 | 25 | >50 | >2 |
| Yeasts | MIC | MFC | MFC/MIC ratio | MIC | MFC | MFC/MIC ratio | MIC | MFC | MFC/MIC ratio |
| C. albicans (ATCC 20402) | 12.5 | >50 | >4 | 6.25 | 25 | 4 | 12.5 | >50 | >4 |
| C. guillermondii (ATCC 6260) | 12.5 | 50 | 4 | 12.5 | 50 | 4 | 12.5 | >50 | >4 |
| C. vaginalis (clinical strain, 136) | 3.125 | 6.25 | 2 | 12.5 | 50 | 4 | 6.25 | 25 | 4 |
| C. utilis (ATCC 9255) | 25 | 50 | 2 | 12.5 | 50 | 4 | 12.5 | >50 | >4 |
| C. tropicalis (ATCC 1362) | 25 | 50 | 2 | 6.25 | 25 | 4 | 6.25 | 25 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendrasozhan, S.; Moll, H.E.; Snoussi, M.; Romeilah, R.M.; Shalaby, E.A.; Younes, K.M.; El-Beltagi, H.S. Phytochemical Screening and Antimicrobial Activity of Various Extracts of Aerial Parts of Rhanterium epapposum. Processes 2021, 9, 1351. https://doi.org/10.3390/pr9081351
Rajendrasozhan S, Moll HE, Snoussi M, Romeilah RM, Shalaby EA, Younes KM, El-Beltagi HS. Phytochemical Screening and Antimicrobial Activity of Various Extracts of Aerial Parts of Rhanterium epapposum. Processes. 2021; 9(8):1351. https://doi.org/10.3390/pr9081351
Chicago/Turabian StyleRajendrasozhan, Saravanan, Hani El Moll, Mejdi Snoussi, Ramy M. Romeilah, Emad A. Shalaby, Kareem M. Younes, and Hossam S. El-Beltagi. 2021. "Phytochemical Screening and Antimicrobial Activity of Various Extracts of Aerial Parts of Rhanterium epapposum" Processes 9, no. 8: 1351. https://doi.org/10.3390/pr9081351