Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula var. ferrea in an Amazon Rehabilitating Mineland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction and Sequencing
2.3. Sequence Analyses
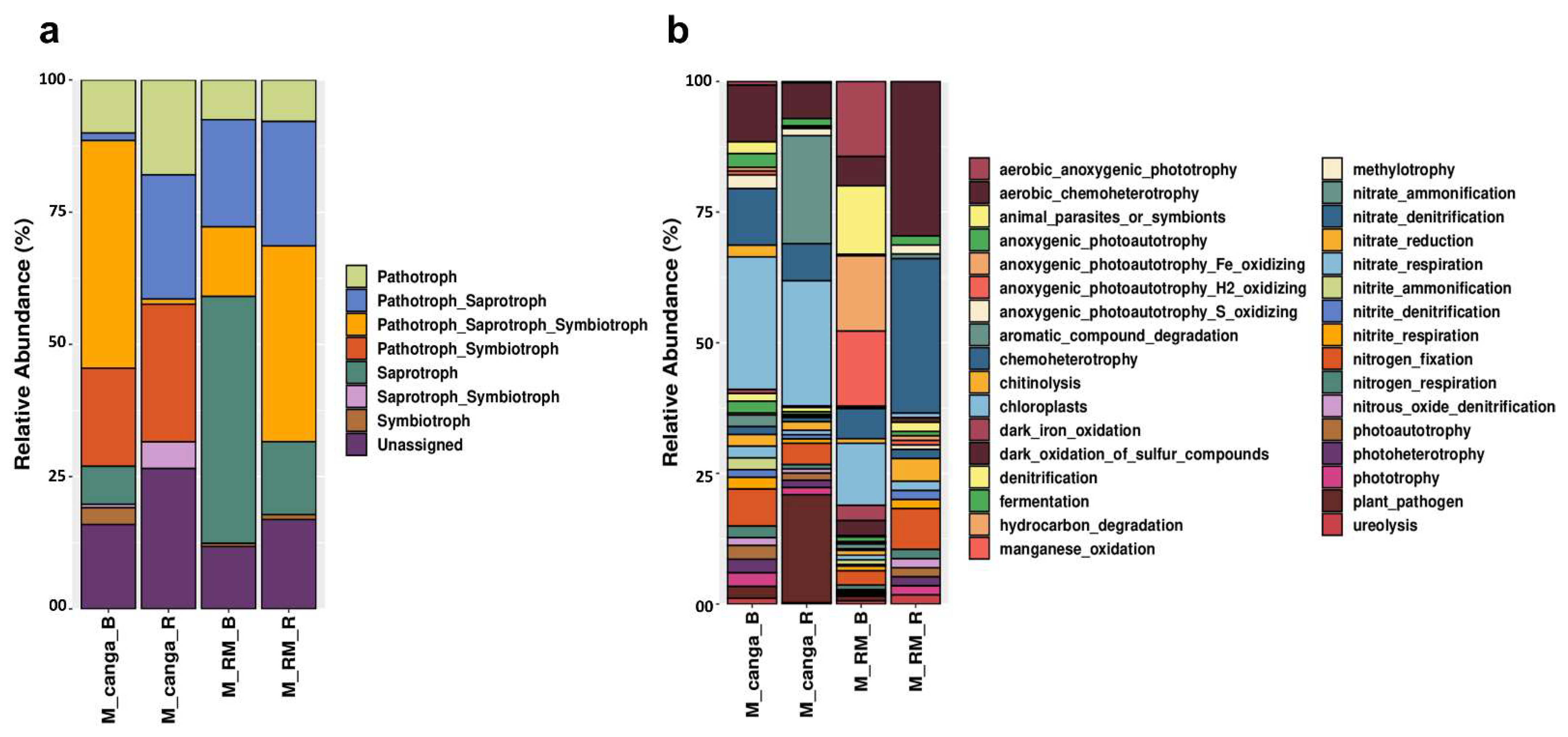
2.4. Functional Analysis of Rhizosphere-Associated Microbial Communities
2.5. Statistical Analyses
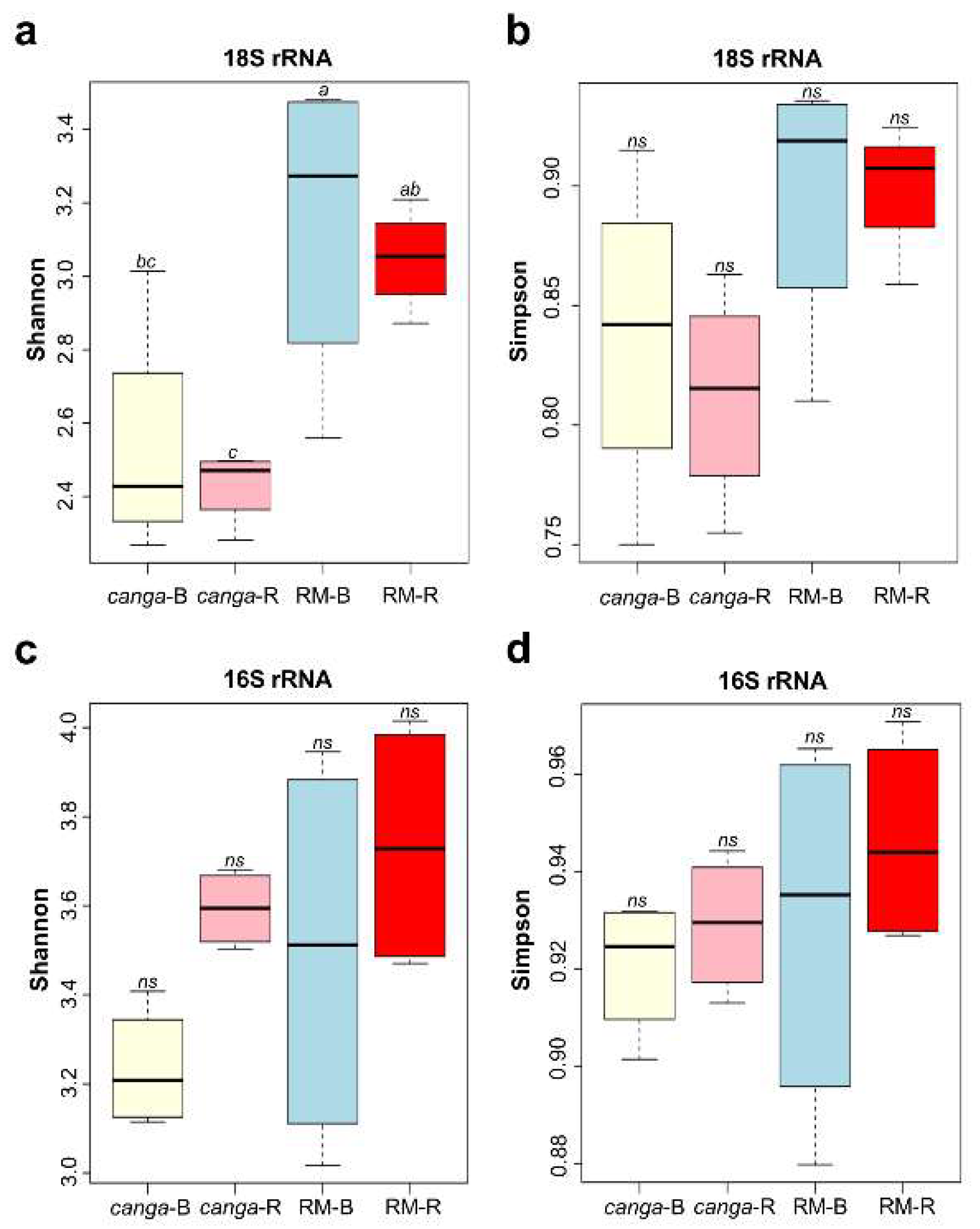
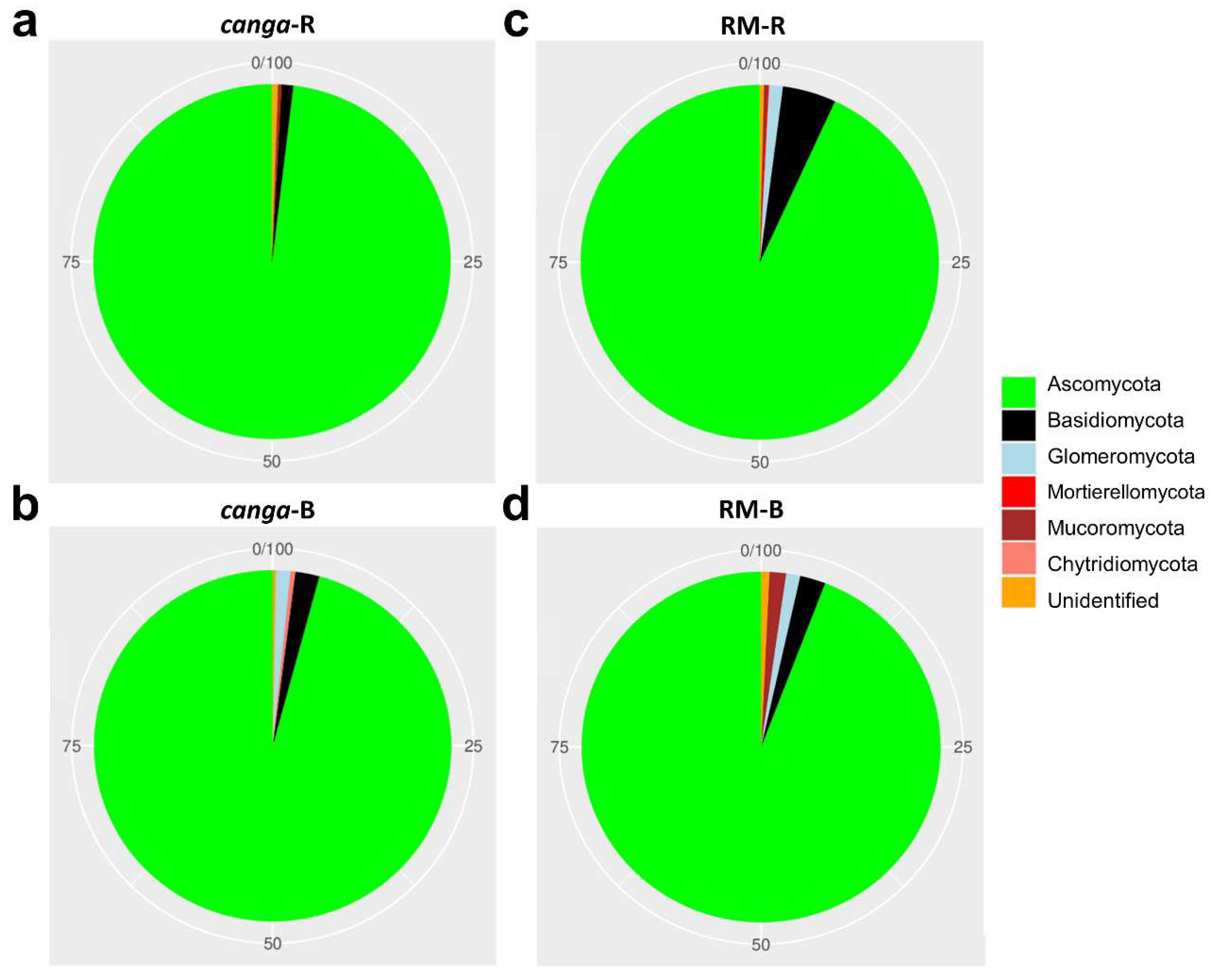
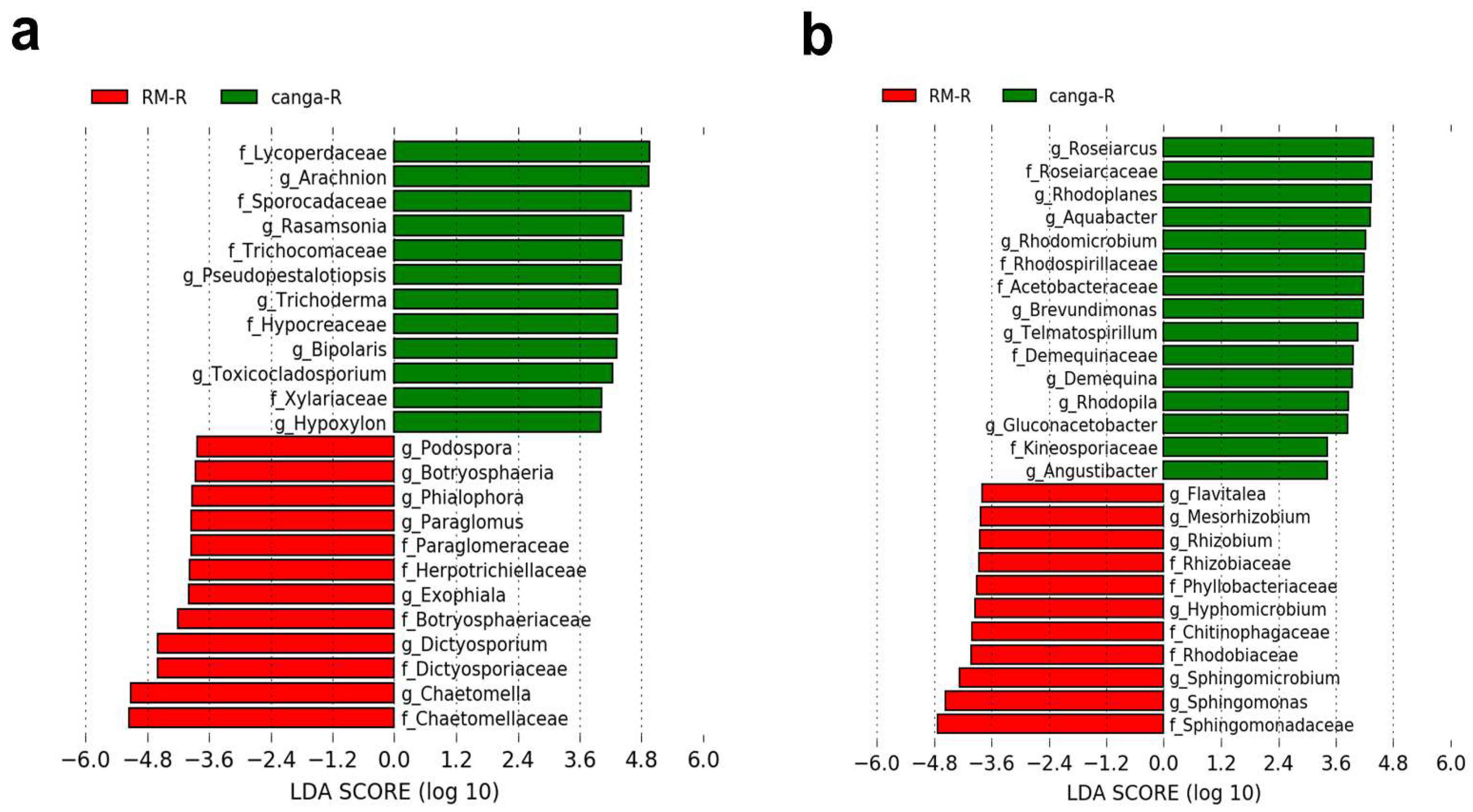
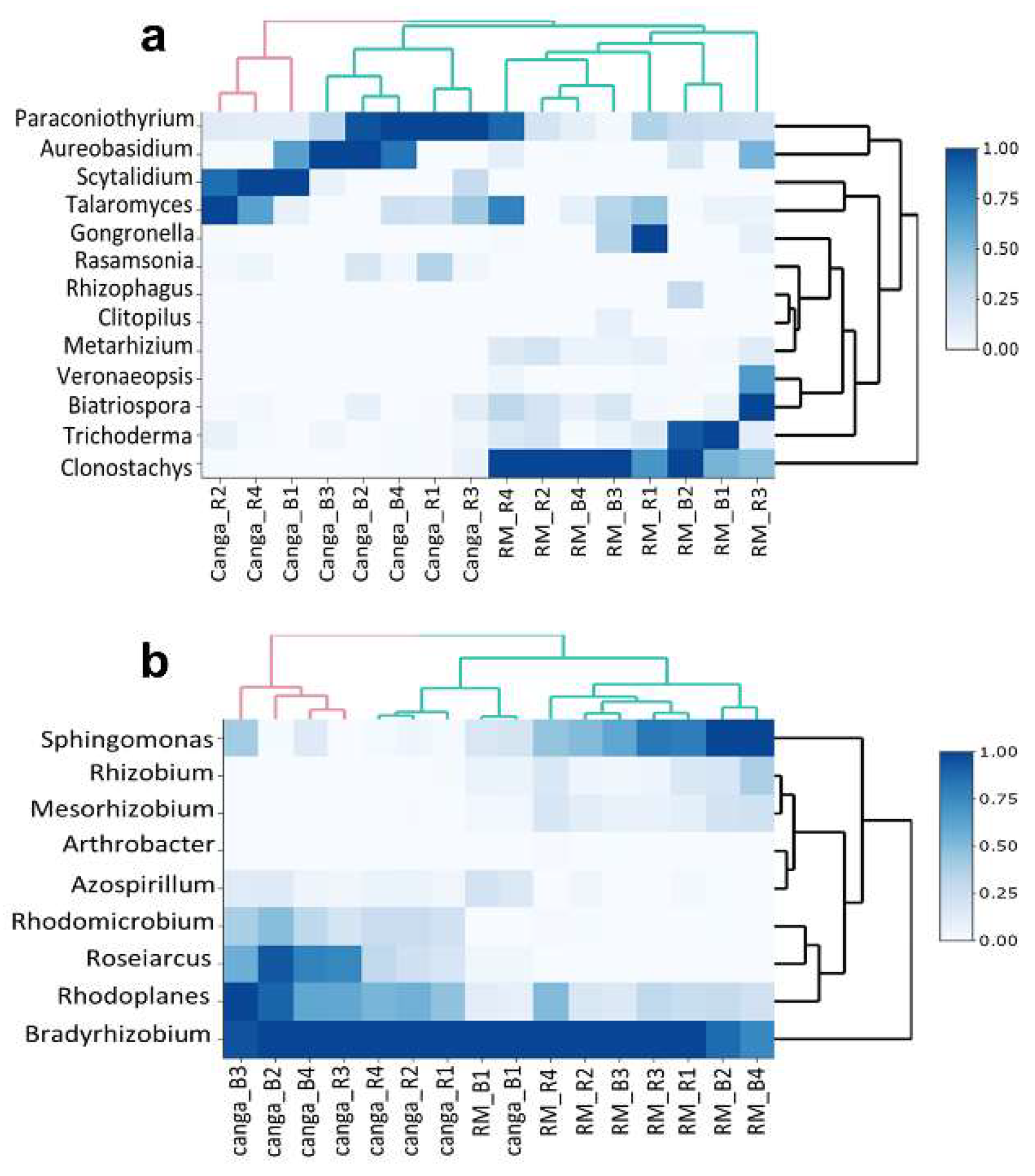
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salgado, A.A.R.; do Carmo, F.F. ‘Quadrilátero Ferrífero’: A Beautiful and Neglected Landscape between the Gold and Iron Ore Reservoirs. In Landscapes and Landforms of Brazil, 1st ed.; Carvalho, B., Rodrigues, A., Cordeiro, L., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 1, pp. 319–330. [Google Scholar]
- Santos, R.; Milanez, B. The Global Production Network for iron ore: Materiality, corporate strategies, and social contestation in Brazil. Extr. Ind. Soc. 2015, 2, 756–765. [Google Scholar] [CrossRef]
- Gastauer, M.; Vera, M.P.O.; De Souza, K.P.; Pires, E.S.; Alves, R.; Caldeira, C.F.; Ramos, S.J.; Oliveira, G. A metagenomic survey of soil microbial communities along a rehabilitation chronosequence after iron ore mining. Sci. Data 2019, 6, 190008. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.M.; Carmo, F.F.; Vincent, R.C.; Stehmann, J. Plant communities on ironstone outcrops: A diverse and endangered Brazilian ecosystem. Biodivers. Conserv. 2007, 16, 2185–2200. [Google Scholar] [CrossRef]
- Costa, W.F.; Ribeiro, M.; Saraiva, A.M.; Imperatriz-Fonseca, V.L.; Giannini, T. Bat diversity in Carajás National Forest (Eastern Amazon) and potential impacts on ecosystem services under climate change. Biol. Conserv. 2018, 218, 200–210. [Google Scholar] [CrossRef]
- Artico, M.; Firpo, B.A.; Artico, L.L.; Tubino, R.M.C. Integrated use of sewage sludge and basalt mine waste as soil substitute for environmental restoration. REM—Int. Eng. J. 2020, 73, 225–232. [Google Scholar] [CrossRef]
- Maiti, S.K.; Ahirwal, J. Ecological Restoration of Coal Mine Degraded Lands:Topsoil Management, Pedogenesis, Carbon Sequestration, and Mine Pit Limnology. In Phytomanagement of Polluted Sites: Market Opportunities in Sustainable Phytoremediation, 1st ed.; Pandey, V., Bauddh, K., Eds.; Elsevier: Dhanbad, India, 2019; pp. 83–111. [Google Scholar]
- André, J.C.; Alvarez, F.L.; Rivero, J.F.L. Caracterização dos impactos ambientais e sociais na exploração de rochas e minerais industriais no desenvolvimento local no município de Sumbe (Angola)/Characterization of environmental and social impacts. Cadernos CIMEAC 2019, 9, 210–237. [Google Scholar] [CrossRef]
- Dong, L.; Tong, X.; Li, X.; Zhou, J.; Wang, S.; Liu, B. Some developments and new insights of environmental problems and deep mining strategy for cleaner production in mines. J. Clean. Prod. 2018, 210, 1562–1578. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Buta, M.; Blaga, G.; Paulette, L.; Păcurar, I.; Roșca, S.; Borsai, O.; Grecu, F.; Sînziana, P.E.; Negrușier, C. Soil Reclamation of Abandoned Mine Lands by Revegetation in Northwestern Part of Transylvania: A 40-Year Retrospective Study. Sustainability 2019, 11, 3393. [Google Scholar] [CrossRef] [Green Version]
- Berthelot, C.; Leyval, C.; Foulon, J.; Chalot, M.; Blaudez, D. Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal-polluted poplar phytomanagement sites. FEMS Microbiol. Ecol. 2016, 92, fiw144. [Google Scholar] [CrossRef] [Green Version]
- Farrar, K.; Bryant, D.; Cope-Selby, N. Understanding and engineering beneficial plant–microbe interactions: Plant growth promotion in energy crops. Plant Biotechnol. J. 2014, 12, 1193–1206. [Google Scholar] [CrossRef] [Green Version]
- Gastauer, M.; Sarmento, P.S.D.M.; Santos, V.C.A.; Caldeira, C.F.; Ramos, S.J.; Teodoro, G.S.; Siqueira, J.O. Vegetative functional traits guide plant species selection for initial mineland rehabilitation. Ecol. Eng. 2020, 148, 105763. [Google Scholar] [CrossRef]
- Vincent, R.d.C.; Meguro, M. Influence of soil properties on the abundance of plant species in ferruginous rocky soils vegetation, southeastern Brazil. Rev. Bras. Bot. 2008, 31, 377–388. [Google Scholar] [CrossRef]
- Carvalho, C.; Forester, B.R.; Mitre, S.K.; Alves, R.; Imperatriz-Fonseca, V.L.; Ramos, S.J.; Resende-Moreira, L.C.; Siqueira, J.O.; Trevelin, L.C.; Caldeira, C.F. Combining genotype, phenotype, and environmental data to delineate site-adjusted provenance strategies for ecological restoration. Mol. Ecol. Resour. 2021, 21, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Chhabra, S.; Gill, S.S.; Singh, P.K.; Tuteja, N. The microbial symbionts: Potential for crop improvement in changing environments. In Advancement in Crop Improvement Techniques; Woodhead Publishing: Sawston, UK, 2020; pp. 233–240. [Google Scholar] [CrossRef]
- Jurburg, S.D.; Salles, J.F. Functional Redundancy and Ecosystem Function—The Soil Microbiota as a Case Study. In Biodiversity in Ecosystems—Linking Structure and Function, 1st ed.; Blanco, J., Lo, Y., Roy, S., Eds.; IntechOpen: London, UK, 2015; pp. 29–49. [Google Scholar]
- Fink, J.R.; Inda, A.V.; Bayer, C.; Torrent, J.; Barrón, V. Mineralogy and phosphorus adsorption in soils of south and central-west Brazil under conventional and no-tillage systems. Acta Sci. Agron. 2014, 36, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Cravo, M.d.S.; Vasconselos de Macêdo, J.L. Phosphorus management for perennial crops in central Amazonian upland soils. Plant Soil 2001, 237, 309–319. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Silva, R.L.; Nunes, G.L.; Oliveira, G. PIMBA: A PIpeline for MetaBarcoding Analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2013, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Abarenkov, K.; Nilsson, H.; Larsson, K.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Fish, J.A.; Chai, B.; McGarrell, D.M.; Sun, Y.; Brown, C.T.; Porras-Alfaro, A.; Kuske, C.R.; Tiedje, J.M. Ribosomal Database Project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2013, 42, D633–D642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.; Yang, G.; Ma, Y.; Yao, Q.; Ma, Y.; Ma, H.; Hu, Y.; Yang, Y.; Wang, S.; Pan, Y.; et al. Seasonal dynamics of microbial diversity in the rhizosphere of Ulmus pumila L. var. sabulosa in a steppe desert area of Northern China. PeerJ 2019, 7, e7526. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, R.A.; Giannini, T.C.; Gastauer, M.; Awade, M.; Siqueira, J.O. Topsoil application during the rehabilitation of a manganese tailing dam increases plant taxonomic, phylogenetic and functional diversity. J. Environ. Manag. 2018, 227, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, R.M.; Kemmelmeier, K.; Pedroso, D.D.F.; Pinto, F.A.; dos Santos, J.V.; Gastauer, M.; Caldeira, C.F.; Ramos, S.J.; Siqueira, J.O.; Carneiro, M.A.C. Native arbuscular mycorrhizal fungi respond to rehabilitation in iron ore mining areas from the Eastern Brazilian Amazon. Pedobiologia 2021, 89, 150768. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.M.; Guimarães, A.A.; de Castro, J.L.; Siqueira, J.O.; Carneiro, M.A.C.; Moreira, F.M.D.S. Rhizobia and endophytic bacteria isolated from rainforest fragments within an iron ore mining site of the Eastern Brazilian Amazon. Braz. J. Microbiol. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.K.; Borges, L.G.D.A.; Marconatto, L.; Giongo, A.; Stürmer, S.L. Microbiome of a revegetated iron-mining site and pristine ecosystems from the Brazilian Cerrado. Appl. Soil Ecol. 2018, 131, 55–65. [Google Scholar] [CrossRef]
- Deng, J.; Yin, Y.; Zhu, W.; Zhou, Y. Response of soil environment factors and microbial communities to phytoremediation with Robinia pseudoacacia in an open-cut magnesite mine. Land Degrad. Dev. 2020, 31, 2340–2355. [Google Scholar] [CrossRef]
- Goh, Y.K.; Marzuki, N.F.; Pa, T.N.F.T.; Goh, T.-K.; Kee, Z.S.; Goh, Y.K.; Yusof, M.T.; Vujanovic, V.; Goh, K.J. Biocontrol and Plant-Growth-Promoting Traits of Talaromyces apiculatus and Clonostachys rosea Consortium against Ganoderma Basal Stem Rot Disease of Oil Palm. Microorganisms 2020, 8, 1138. [Google Scholar] [CrossRef]
- Shu, W.; Pablo, G.P.; Jun, Y.; Danfeng, H. Abundance and diversity of nitrogen-fixing bacteria in rhizosphere and bulk paddy soil under different duration of organic management. World J. Microbiol. Biotechnol. 2011, 28, 493–503. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masse, J.; Prescott, C.E.; Renaut, S.; Terrat, Y.; Grayston, S.J. Plant Community and Nitrogen Deposition as Drivers of Alpha and Beta Diversities of Prokaryotes in Reconstructed Oil Sand Soils and Natural Boreal Forest Soils. Appl. Environ. Microbiol. 2017, 83, e03319-16. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Leung, P.M.; Wood, J.L.; Bay, S.K.; Hugenholtz, P.; Kessler, A.J.; Shelley, G.; Waite, D.W.; Franks, A.E.; Cook, P.L.M.; et al. Metabolic flexibility allows bacterial habitat generalists to become dominant in a frequently disturbed ecosystem. ISME J. 2021, 15, 2986–3004. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Castilho, A.; Chaparro, C.; Carvalho, N.; Tzotzos, G.; Siqueira, J.O. Canga biodiversity, A matter of mining. Front. Plant Sci. 2014, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Li, T.; Cui, H.; Wang, J.; Yu, X.; Ding, Y.; Wang, C.; Yang, Z.; Zhao, Z. Diversity and characterization of Cd-tolerant dark septate endophytes (DSEs) associated with the roots of Nepal alder (Alnus nepalensis) in a metal mine tailing of southwest China. Appl. Soil Ecol. 2015, 93, 11–18. [Google Scholar] [CrossRef]
- Hou, J.; Liu, W.; Wu, L.; Ge, Y.; Hu, P.; Li, Z.; Christie, P. Rhodococcus sp. NSX2 modulates the phytoremediation efficiency of a trace metal-contaminated soil by reshaping the rhizosphere microbiome. Appl. Soil Ecol. 2018, 133, 62–69. [Google Scholar] [CrossRef]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Kim, Y.-H.; Imran, M.; Khan, M.J.; Al-Harrasi, A.; Kim, T.H.; Lee, I.-J. Mechanisms of Cr(VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr(VI) phytotoxic mitigating effects in soybean (Glycine max L.). Ecotoxicol. Environ. Saf. 2018, 164, 648–658. [Google Scholar] [CrossRef]
- Li, Y.; Chen, F.; Dong, K.; Wang, G. Actinotalea ferrariae sp. nov., isolated from an iron mine, and emended description of the genus Actinotalea. Int. J. Syst. Evol. Microbiol. 2013, 63, 3398–3403. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, M.; Seneviratne, G.; Madawala, H.; Vithanage, M. Role of Rhizospheric Microbes in Heavy Metal Uptake by Plants. In Agro-Environmental Sustainability, 1st ed.; Singh, J., Seneviratne, G., Eds.; Springer: Singapore, 2017; pp. 147–163. [Google Scholar]
- Checcucci, A.; Bazzicalupo, M.; Mengoni, A. Exploiting Nitrogen-Fixing Rhizobial Symbionts Genetic Resources for Improving Phytoremediation of Contaminated Soils. In Enhancing Cleanup of Environmental Pollutants, 1st ed.; Anjum, N., Singh, S., Tuteja, N., Eds.; Springer: Fiorentino, Italy, 2017; pp. 275–288. [Google Scholar]
- Oliveira, A.; Azarias Guimarães, A.; da Costa, A.M.; Louzada Rodrigues, T.; de Soares Carvalho, T.; Reis Sales, F.; de Souza Moreira, F.M. Plant growth-promoting rhizobacterial communities from an area under the influence of iron mining and from the adjacent phytophysiognomies which have high genetic diversity. Land Degrad. Dev. 2020, 31, 2237–2254. [Google Scholar] [CrossRef]
- Ortiz, J.; Soto, J.; Fuentes, A.; Herrera, H.; Meneses, C.; Arriagada, C. The Endophytic Fungus Chaetomium cupreum Regulates Expression of Genes Involved in the Tolerance to Metals and Plant Growth Promotion in Eucalyptus globulus Roots. Microorganisms 2019, 7, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Tekaya, M.; Mechri, B.; Mbarki, N.; Cheheb, H.; Hammami, M.; Attia, F. Arbuscular mycorrhizal fungus Rhizophagus irregularis influences key physiological parameters of olive trees (Olea europaea L.) and mineral nutrient profile. Photosynthetica 2017, 55, 308–316. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Hou, L.; Ren, Y.; Wang, S.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naraghi, L.; Heydari, A.; Rezaee, S.; Razavi, M. Biocontrol Agent Talaromyces flavus Stimulates the Growth of Cotton and Potato. J. Plant Growth Regul. 2012, 31, 471–477. [Google Scholar] [CrossRef]
- Ramos, S.J.; Gastauer, M.; Mitre, S.K.; Caldeira, C.F.; Silva, J.R.; Neto, A.E.F.; Oliveira, G.; Filho, P.W.M.S.; Siqueira, J.O. Plant growth and nutrient use efficiency of two native Fabaceae species for mineland revegetation in the eastern Amazon. J. For. Res. 2019, 31, 2287–2293. [Google Scholar] [CrossRef]
- Burghardt, L.T. Evolving together, evolving apart: Measuring the fitness of rhizobial bacteria in and out of symbiosis with leguminous plants. New Phytol. 2019, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.-Y.; Fan, X.-L.; Zhou, L.-R.; Shao, S.-C.; Liu, Q.; Selosse, M.-A.; Gao, J.-Y. Symbiotic fungi undergo a taxonomic and functional bottleneck during orchid seeds germination: A case study on Dendrobium moniliforme. Symbiosis 2019, 79, 205–212. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Jiang, L.; Luo, Y. Trends in soil microbial communities during secondary succession. Soil Biol. Biochem. 2017, 115, 92–99. [Google Scholar] [CrossRef]
- Barfknecht, D.F.; Li, G.; Martinez, K.A.; Gibson, D.J. Interactive disturbances drive community composition, heterogeneity, and the niches of invasive exotic plant species during secondary succession. Plant Ecol. Divers. 2021, 1–13. [Google Scholar] [CrossRef]
- Silva, J.R.; Gastauer, M.; Ramos, S.J.; Mitre, S.K.; Neto, A.E.F.; Siqueira, J.O.; Caldeira, C.F. Initial growth of Fabaceae species: Combined effects of topsoil and fertilizer application for mineland revegetation. Flora 2018, 246–247, 109–117. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, P.H.d.O.; Nascimento, S.V.d.; Herrera, H.; Gastauer, M.; Ramos, S.J.; Caldeira, C.F.; Oliveira, G.; Valadares, R.B.d.S. Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula var. ferrea in an Amazon Rehabilitating Mineland. Processes 2021, 9, 2079. https://doi.org/10.3390/pr9112079
Costa PHdO, Nascimento SVd, Herrera H, Gastauer M, Ramos SJ, Caldeira CF, Oliveira G, Valadares RBdS. Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula var. ferrea in an Amazon Rehabilitating Mineland. Processes. 2021; 9(11):2079. https://doi.org/10.3390/pr9112079
Chicago/Turabian StyleCosta, Paulo Henrique de Oliveira, Sidney Vasconcelos do Nascimento, Hector Herrera, Markus Gastauer, Silvio Junio Ramos, Cecílio Frois Caldeira, Guilherme Oliveira, and Rafael Borges da Silva Valadares. 2021. "Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula var. ferrea in an Amazon Rehabilitating Mineland" Processes 9, no. 11: 2079. https://doi.org/10.3390/pr9112079
APA StyleCosta, P. H. d. O., Nascimento, S. V. d., Herrera, H., Gastauer, M., Ramos, S. J., Caldeira, C. F., Oliveira, G., & Valadares, R. B. d. S. (2021). Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula var. ferrea in an Amazon Rehabilitating Mineland. Processes, 9(11), 2079. https://doi.org/10.3390/pr9112079






