Dopamine Incorporated Forward Osmosis Membranes with High Structural Stability and Chlorine Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polysulfone Substrates
2.3. Preparation of Thin Film Composite (TFC) Membranes
2.4. Membrane Characterizations
2.5. FO Performance Tests
2.6. Evaluation of Membrane Structural Stability and Chlorine Resistance
3. Results and Discussion
3.1. Membrane Surface Characterization
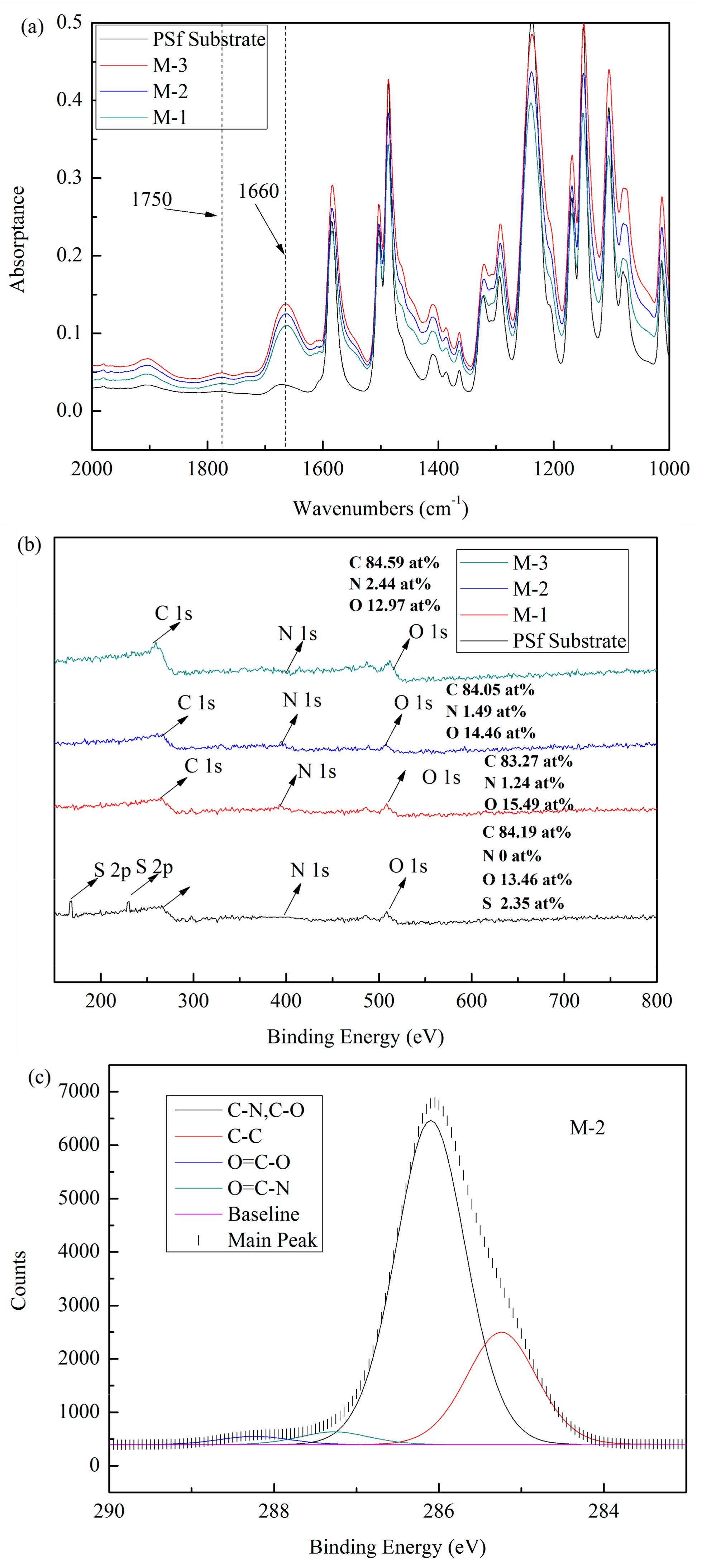
3.1.1. Membrane Surface Chemistry
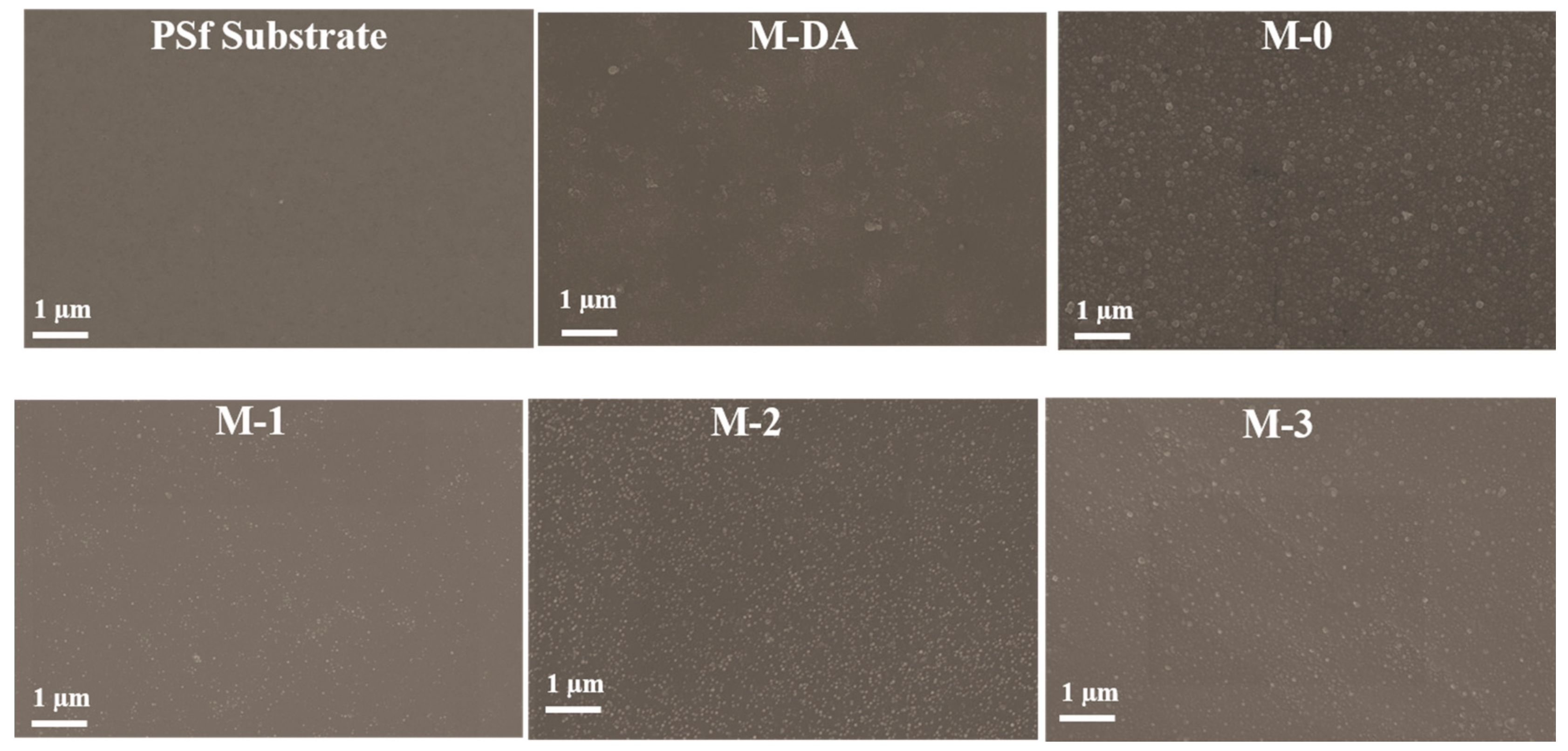
3.1.2. Membrane Surface Morphology
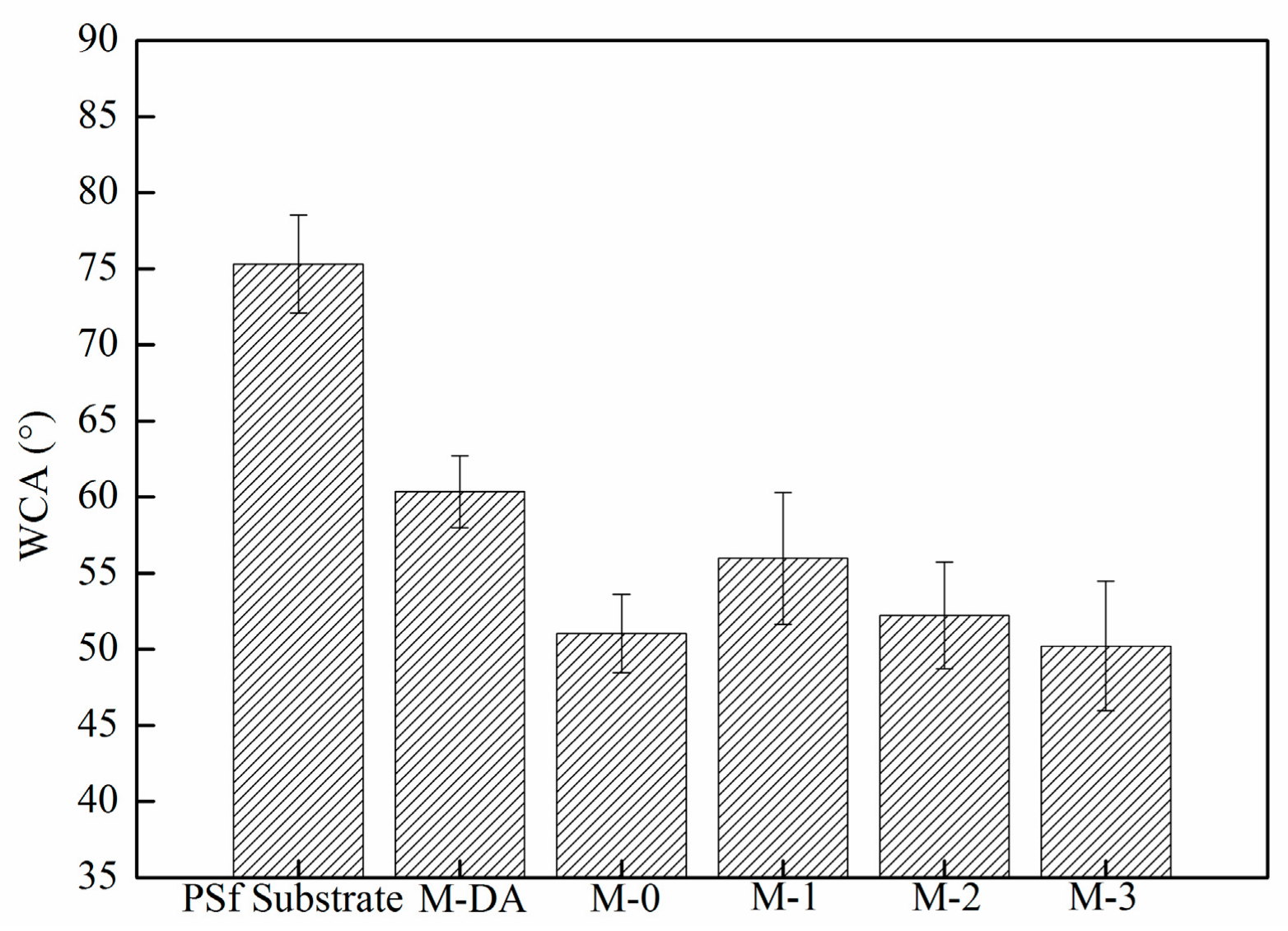
3.1.3. Membrane Surface Hydrophilicity
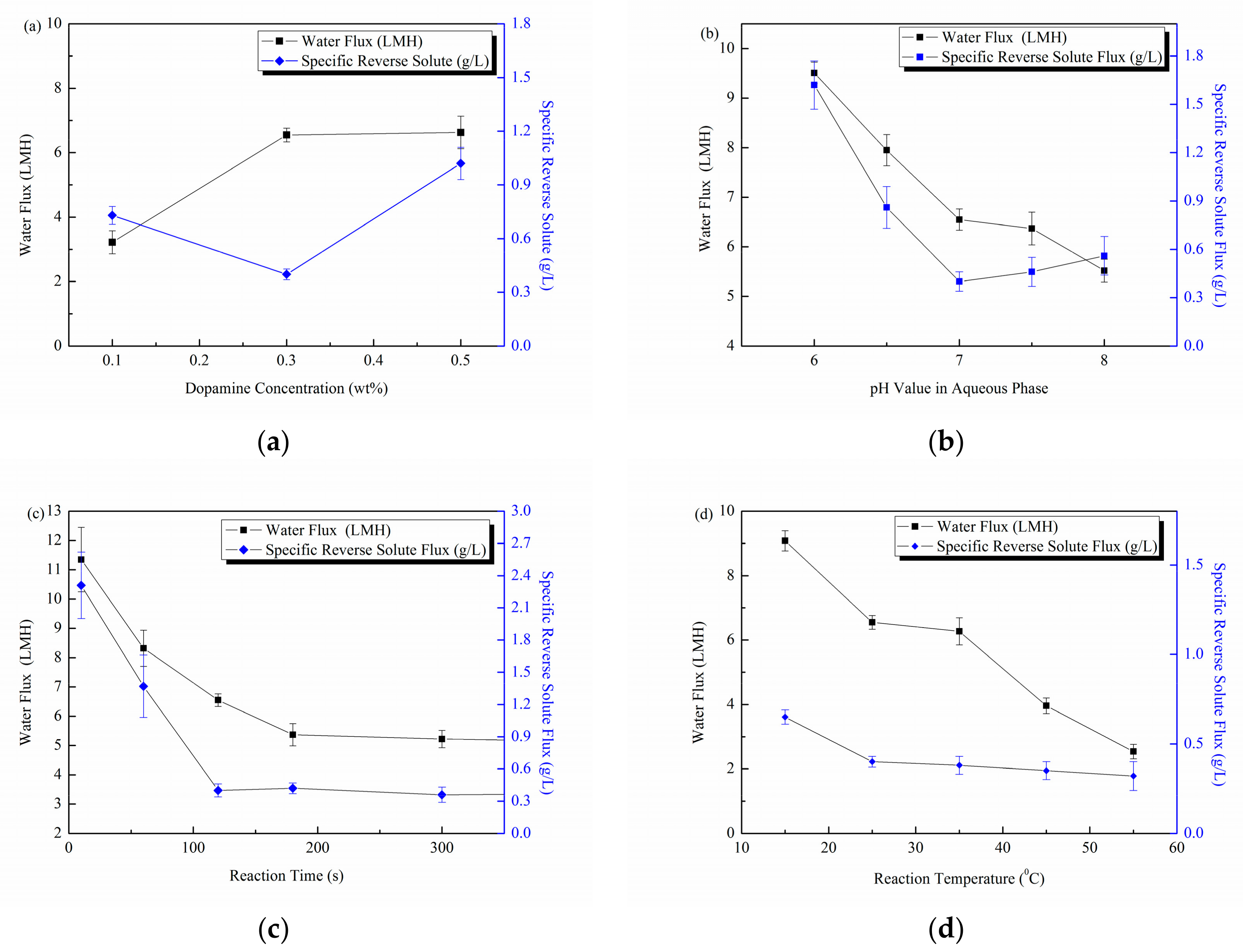
3.2. Membrane FO Performance and Preparation Parameter Optimization
3.3. Membrane Structural Stability and Chloride Resistance
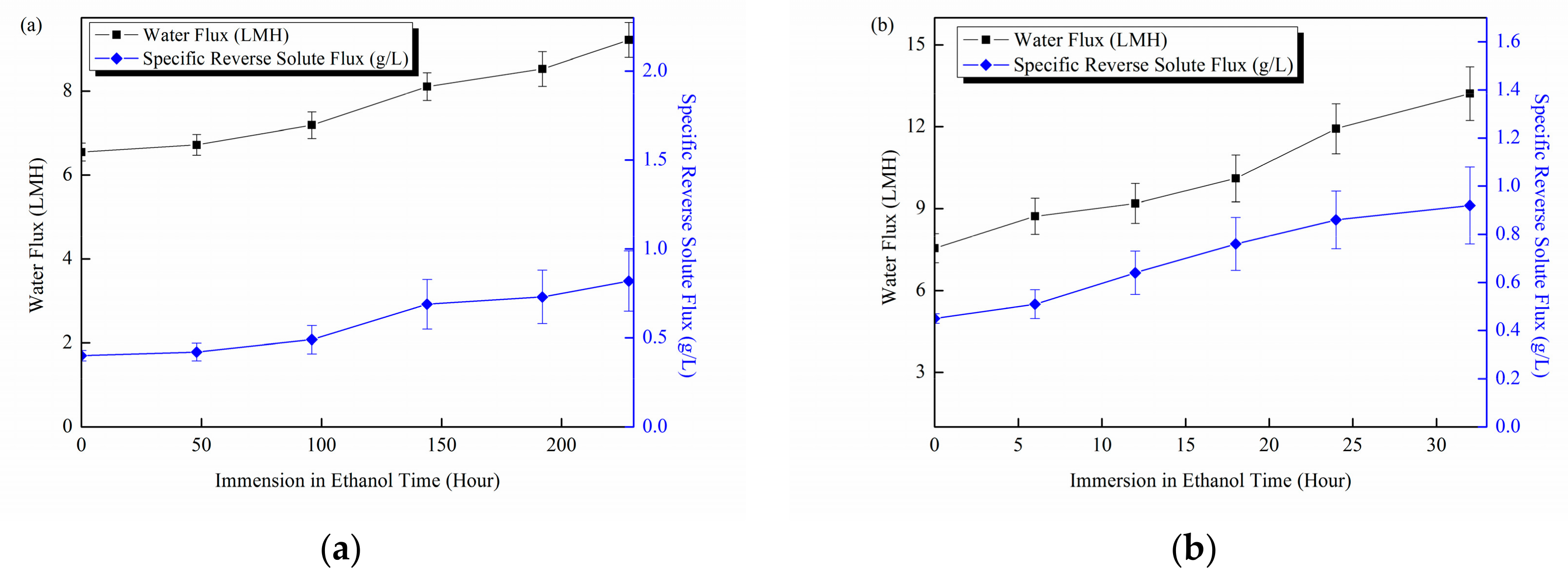
3.3.1. Membrane Structural Stability
3.3.2. Membrane Chlorine Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, S.; Zou, L.; Mulcahy, D. Brackish water desalination by a hybrid forward osmosis–nanofiltration system using divalent draw solute. Desalination 2012, 284, 175–181. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Choi, J.-S.; Oh, H.-J.; Lee, S.; Yang, D.R.; Kim, J.H. Toward a combined system of forward osmosis and reverse osmosis for seawater desalination. Desalination 2009, 247, 239–246. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L. Effects of working temperature on separation performance, membrane scaling and cleaning in forward osmosis desalination. Desalination 2011, 278, 157–164. [Google Scholar] [CrossRef]
- Korenak, J.; Basu, S.; Balakrishnan, M.; Helix-Nielsen, C.; Petrinic, I. Forward osmosis in wastewater treatment processes. Acta. Chim. Slov. 2017, 64, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yamamoto, K.; Tobino, T. Membrane fouling and long-term performance of seawater-driven forward osmosis for enrichment of nutrients in treated municipal wastewater. J. Membr. Sci. 2016, 499, 555–562. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Yuan, B.; Wang, Z.; Li, X.; Ren, Y. Comparison of biofouling mechanisms between cellulose triacetate (CTA) and thin-film composite (TFC) polyamide forward osmosis membranes in osmotic membrane bioreactors. Bioresour. Technol. 2016, 202, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, V.W.C.; Tang, C.Y. Osmotic membrane bioreactor (ombr) technology for wastewater treatment and reclamation: Advances, challenges, and prospects for the future. J. Membr. Sci. 2016, 504, 113–132. [Google Scholar] [CrossRef]
- Sahebi, S.; Phuntsho, S.; Eun Kim, J.; Hong, S.; Kyong Shon, H. Pressure assisted fertiliser drawn osmosis process to enhance final dilution of the fertiliser draw solution beyond osmotic equilibrium. J. Membr. Sci. 2015, 481, 63–72. [Google Scholar] [CrossRef]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S.; Kandasamy, J. Fertiliser drawn forward osmosis desalination: The concept, performance and limitations for fertigation. Rev. Environ. Sci. Bio/Technol. 2011, 11, 147–168. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A.; Zaragoza, G.; Ismail, A.F. Evaluation of fo-ro and pro-ro designs for power generation and seawater desalination using impaired water feeds. Desalination 2015, 368, 27–35. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Shaheed, M.H. Energy and thermodynamic analysis of power generation using a natural salinity gradient based pressure retarded osmosis process. Desalination 2014, 350, 86–94. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. Desalination by ammonia–carbon dioxide forward osmosis: Influence of draw and feed solution concentrations on process performance. J. Membr. Sci. 2006, 278, 114–123. [Google Scholar] [CrossRef]
- Faria, A.F.; Liu, C.H.; Xie, M.; Perreault, F.; Nghiem, L.D.; Ma, J.; Elimelech, M. Thin-film composite forward osmosis membranes functionalized with graphene oxide-silver nanocomposites for biofouling control. J. Membr. Sci. 2017, 525, 146–156. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, S.; Wang, Y. Graphene oxide incorporated thin-film composite membranes for forward osmosis applications. Chem. Eng. Sci. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Jia, Q.; Xu, Y.; Shen, J.; Yang, H.; Zhou, L. Effects of hydrophilic solvent and oxidation resistance post surface treatment on molecular structure and forward osmosis performance of polyamide thin-film composite (TFC) membranes. Appl. Surf. Sci. 2015, 356, 1105–1116. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X.; Widjojo, N.; Chung, T.-S. Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 2012, 80, 219–231. [Google Scholar] [CrossRef]
- Salter, R.J. Forward osmosis. Water Cond. Purif. 2005, 48, 36–38. [Google Scholar]
- Qin, J.; Chung, T.S. Effect of dope flow rate on the morphology, separation performance, thermal and mechanical properties of ultrafiltration hollow fibre membranes. J. Membr. Sci. 1999, 157, 35–51. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, L.-P.; Wang, Z.-H.; Hu, F.; Zhang, P.-B.; Zhu, B.-K. Improved chlorine resistance of polyamide thin-film composite membranes with a terpolymer coating. Sep. Purif. Technol. 2016, 157, 112–119. [Google Scholar] [CrossRef]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Degradation of polyamide nanofiltration and reverse osmosis membranes by hypochlorite. Environ. Sci. Technol. 2012, 46, 852–859. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymer 2010, 51, 3472–3485. [Google Scholar] [CrossRef]
- Arena, J.T.; McCloskey, B.; Freeman, B.D.; McCutcheon, J.R. Surface modification of thin film composite membrane support layers with polydopamine: Enabling use of reverse osmosis membranes in pressure retarded osmosis. J. Membr. Sci. 2011, 375, 55–62. [Google Scholar] [CrossRef]
- Huang, Y.; Jin, H.; Li, H.; Yu, P.; Luo, Y. Synthesis and characterization of a polyamide thin film composite membrane based on a polydopamine coated support layer for forward osmosis. RSC Adv. 2015, 5, 106113–106121. [Google Scholar] [CrossRef]
- Guo, H.; Yao, Z.; Wang, J.; Yang, Z.; Ma, X.; Tang, C.Y. Polydopamine coating on a thin film composite forward osmosis membrane for enhanced mass transport and antifouling performance. J. Membr. Sci. 2018, 551, 234–242. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-covalent self-assembly and covalent polymerization co-contribute to polydopamine formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Z.; Zhao, S.; Ng, D.; Zhang, J.; Xie, Z. Dopamine incorporating forward osmosis membranes with enhanced selectivity and antifouling properties. RSC Adv. 2018, 8, 22469–22481. [Google Scholar] [CrossRef]
- Zhao, J.; Su, Y.; He, X.; Zhao, X.; Li, Y.; Zhang, R.; Jiang, Z. Dopamine composite nanofiltration membranes prepared by self-polymerization and interfacial polymerization. J. Membr. Sci. 2014, 465, 41–48. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Freger, V. Nanoscale heterogeneity of polyamide membranes formed by interfacial polymerization. Langmuir 2003, 19, 4791–4797. [Google Scholar] [CrossRef]
- Dong, L.-X.; Huang, X.-C.; Wang, Z.; Yang, Z.; Wang, X.-M.; Tang, C.Y. A thin-film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles. Sep. Purif. Technol. 2016, 166, 230–239. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Rahimpour, A.; Peyravi, M. Developing thin film composite poly(piperazine-amide) and poly(vinyl-alcohol) nanofiltration membranes. Desalination 2010, 257, 129–136. [Google Scholar] [CrossRef]
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Wang, Y.; Zhu, B.-K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(dopa) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Liang, S.; Zhou, T.; Xue, T.; Mei, X.; Wang, Q. Layered double hydroxide nanoparticle modified forward osmosis membranes via polydopamine immobilization with significantly enhanced chlorine and fouling resistance. Desalination 2017, 421, 99–109. [Google Scholar] [CrossRef]
- Li, B.; Liu, W.; Jiang, Z.; Dong, X.; Wang, B.; Zhong, Y. Ultrathin and stable active layer of dense composite membrane enabled by poly(dopamine). Langmuir 2009, 25, 7368–7374. [Google Scholar] [CrossRef] [PubMed]

| Membranes | DA (wt%) in Aqueous Phase | PIP (wt%) in Aqueous Phase | TMC (wt%) in Organic Phase |
|---|---|---|---|
| M-0 | 0 | 1.0 | 0.15 |
| M-DA | 0.50 | 0 | 0 |
| M-1 | 0.10 | 0 | 0.15 |
| M-2 | 0.30 | 0 | 0.15 |
| M-3 | 0.50 | 0 | 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Fang, Z.; Xie, C.; Zhao, S.; Ng, D.; Xie, Z. Dopamine Incorporated Forward Osmosis Membranes with High Structural Stability and Chlorine Resistance. Processes 2018, 6, 151. https://doi.org/10.3390/pr6090151
Wang Y, Fang Z, Xie C, Zhao S, Ng D, Xie Z. Dopamine Incorporated Forward Osmosis Membranes with High Structural Stability and Chlorine Resistance. Processes. 2018; 6(9):151. https://doi.org/10.3390/pr6090151
Chicago/Turabian StyleWang, Yi, Zhendong Fang, Chaoxin Xie, Shuaifei Zhao, Derrick Ng, and Zongli Xie. 2018. "Dopamine Incorporated Forward Osmosis Membranes with High Structural Stability and Chlorine Resistance" Processes 6, no. 9: 151. https://doi.org/10.3390/pr6090151






