Simulation and Analysis of Oleic Acid Pretreatment for Microwave-Assisted Biodiesel Production
Abstract
:1. Introduction
2. Methodology
2.1. Multiphysics Simulation
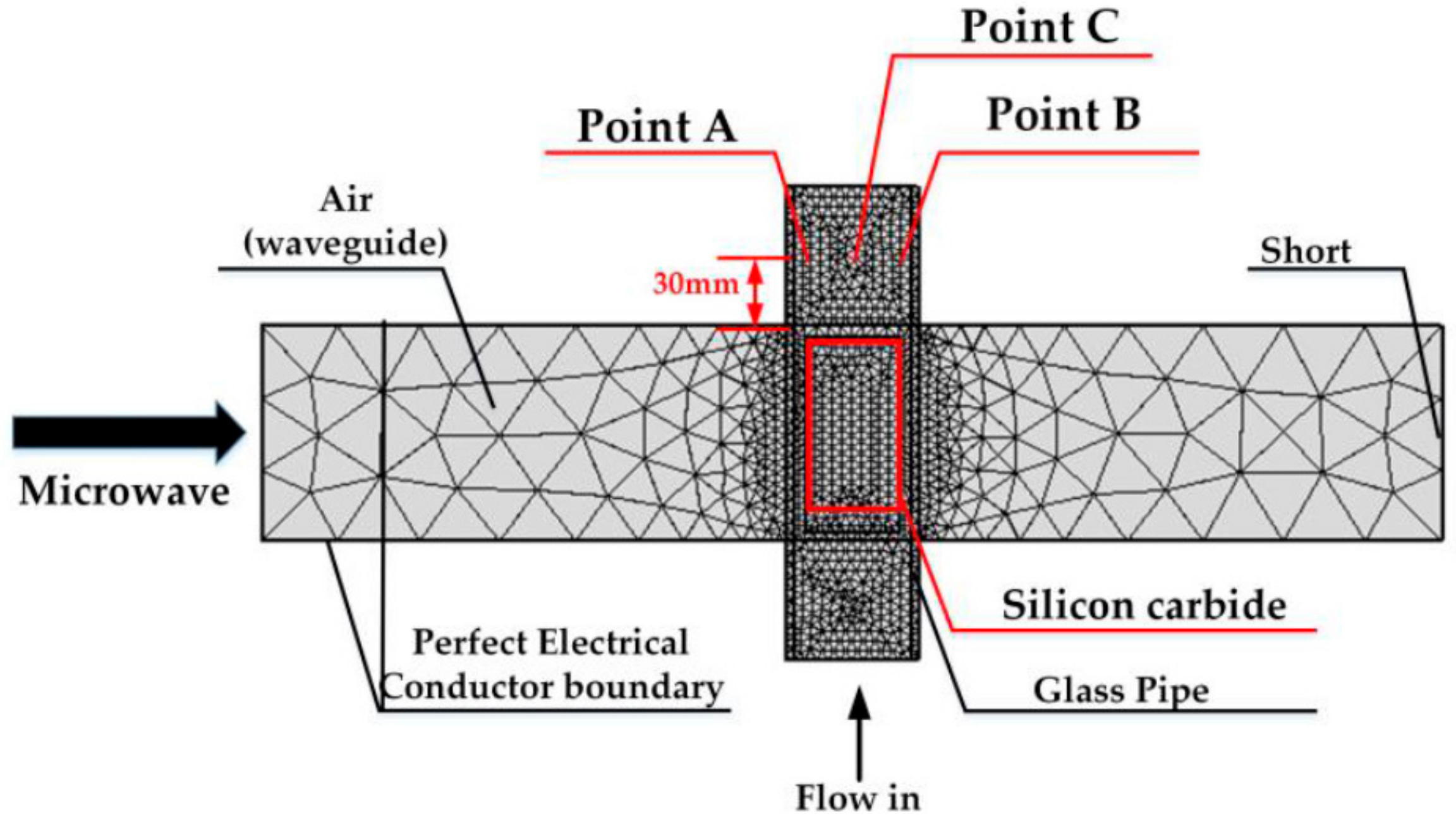
2.1.1. Geometry
2.1.2. Governing Equations
2.1.3. Input Parameters
2.1.4. Boundary Conditions
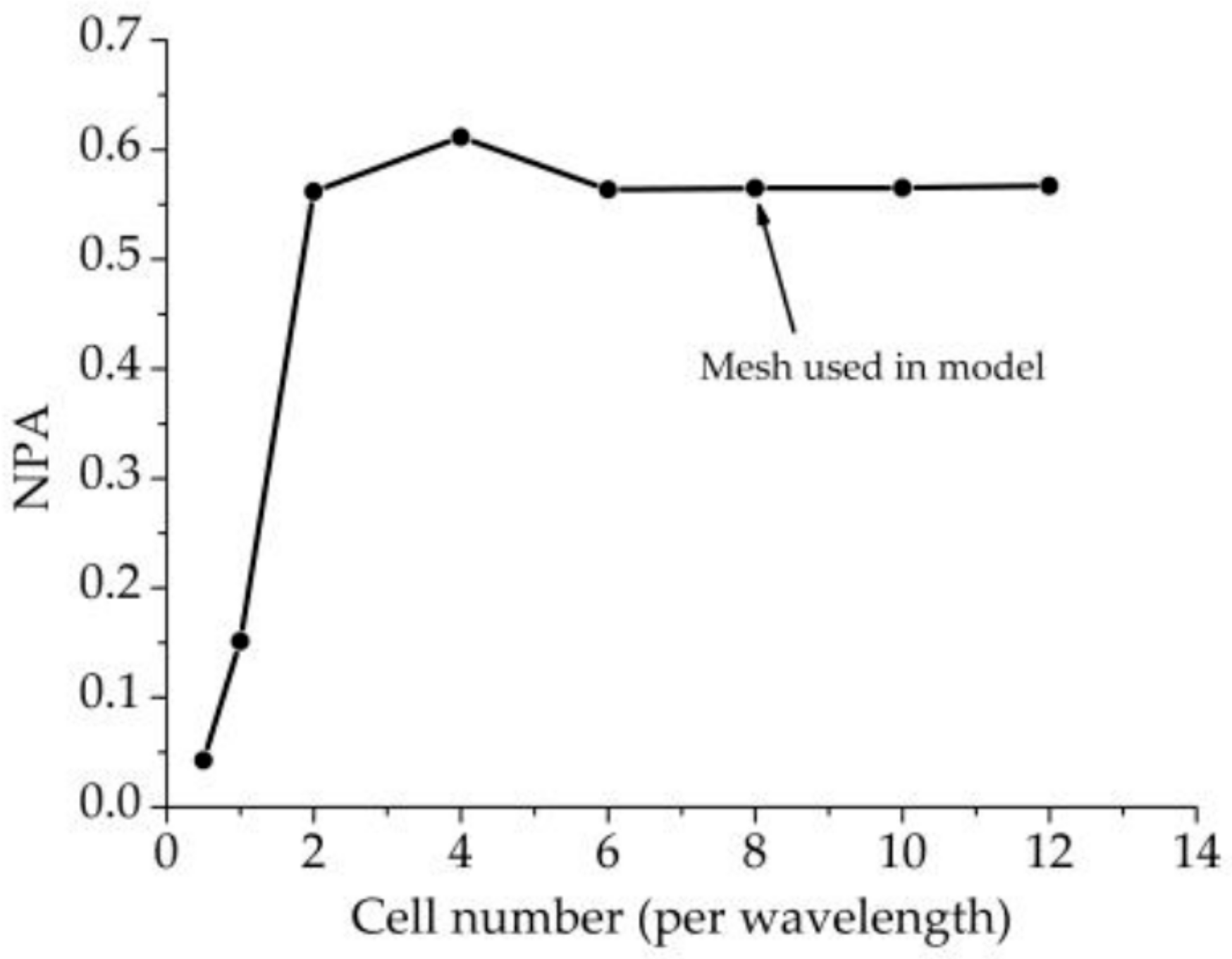
2.1.5. Mesh Size
2.2. Experimental Setup
3. Results and Discussion
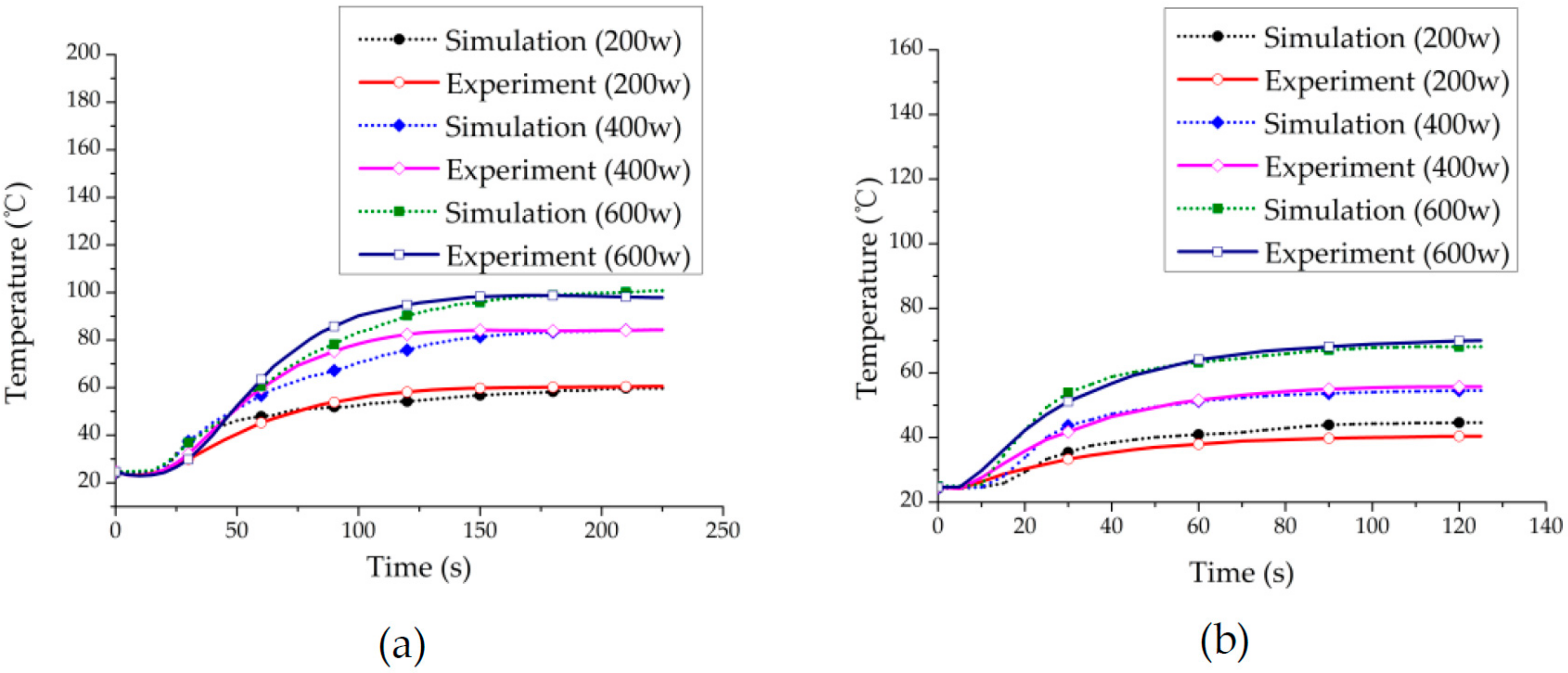
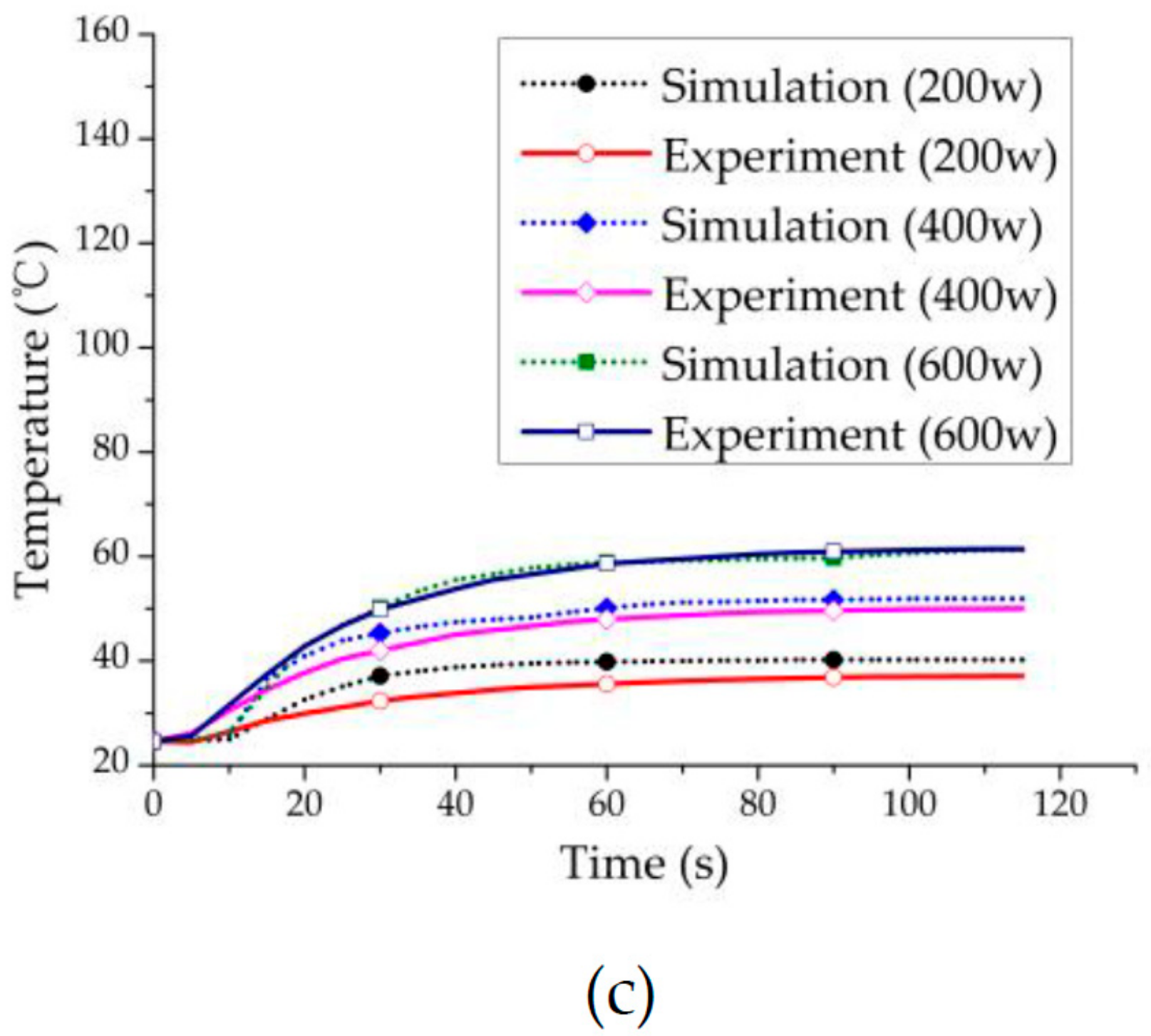
3.1. Experiment Validation
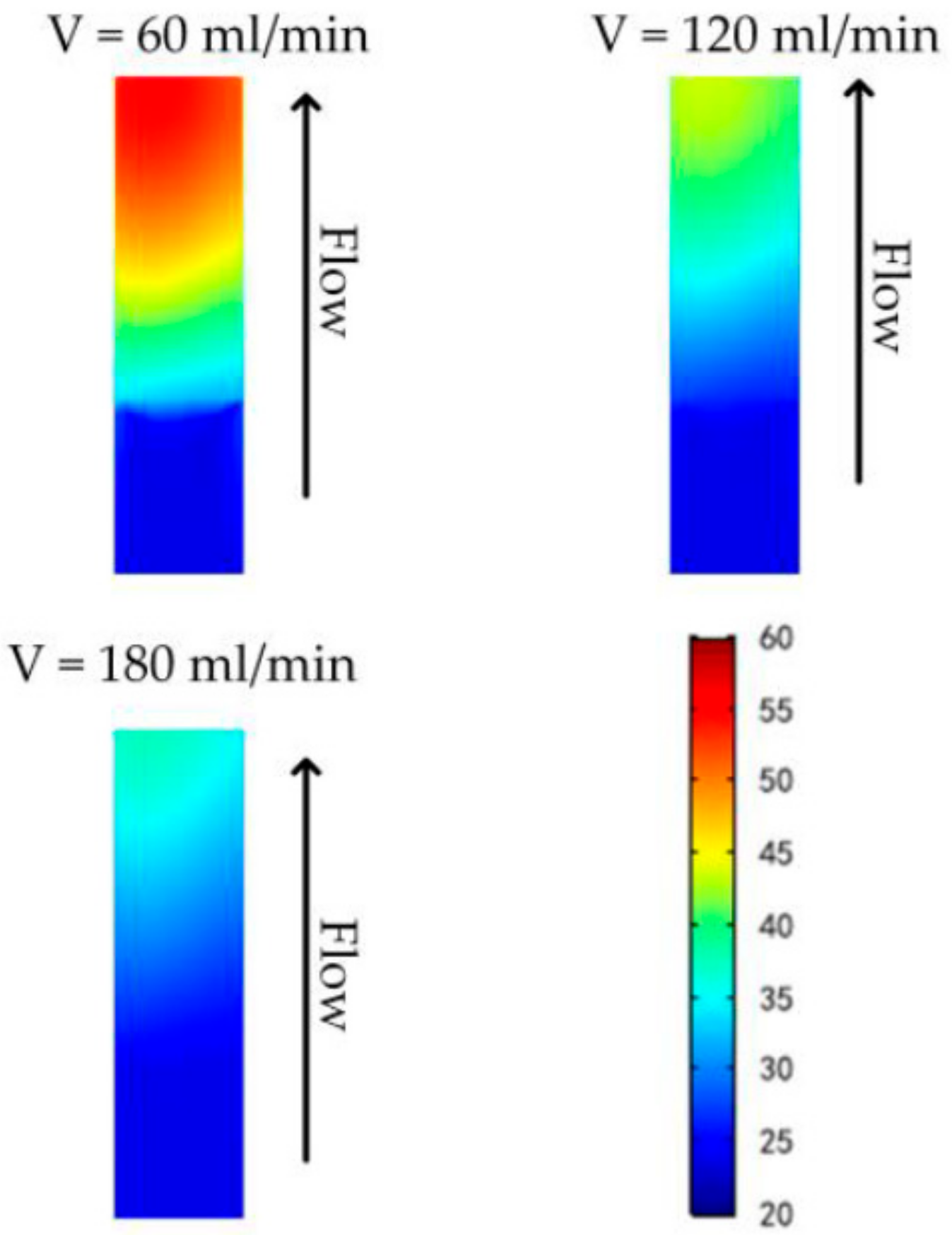
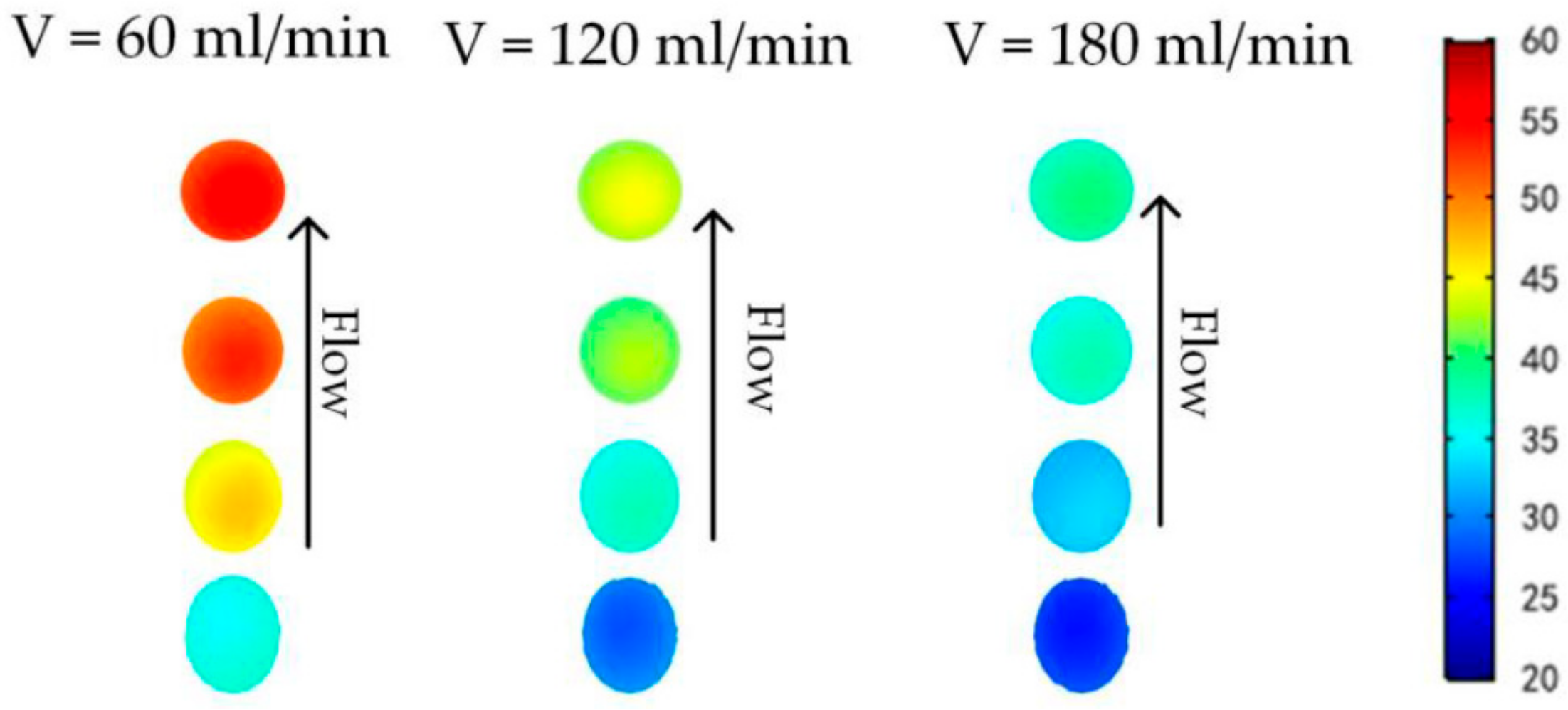
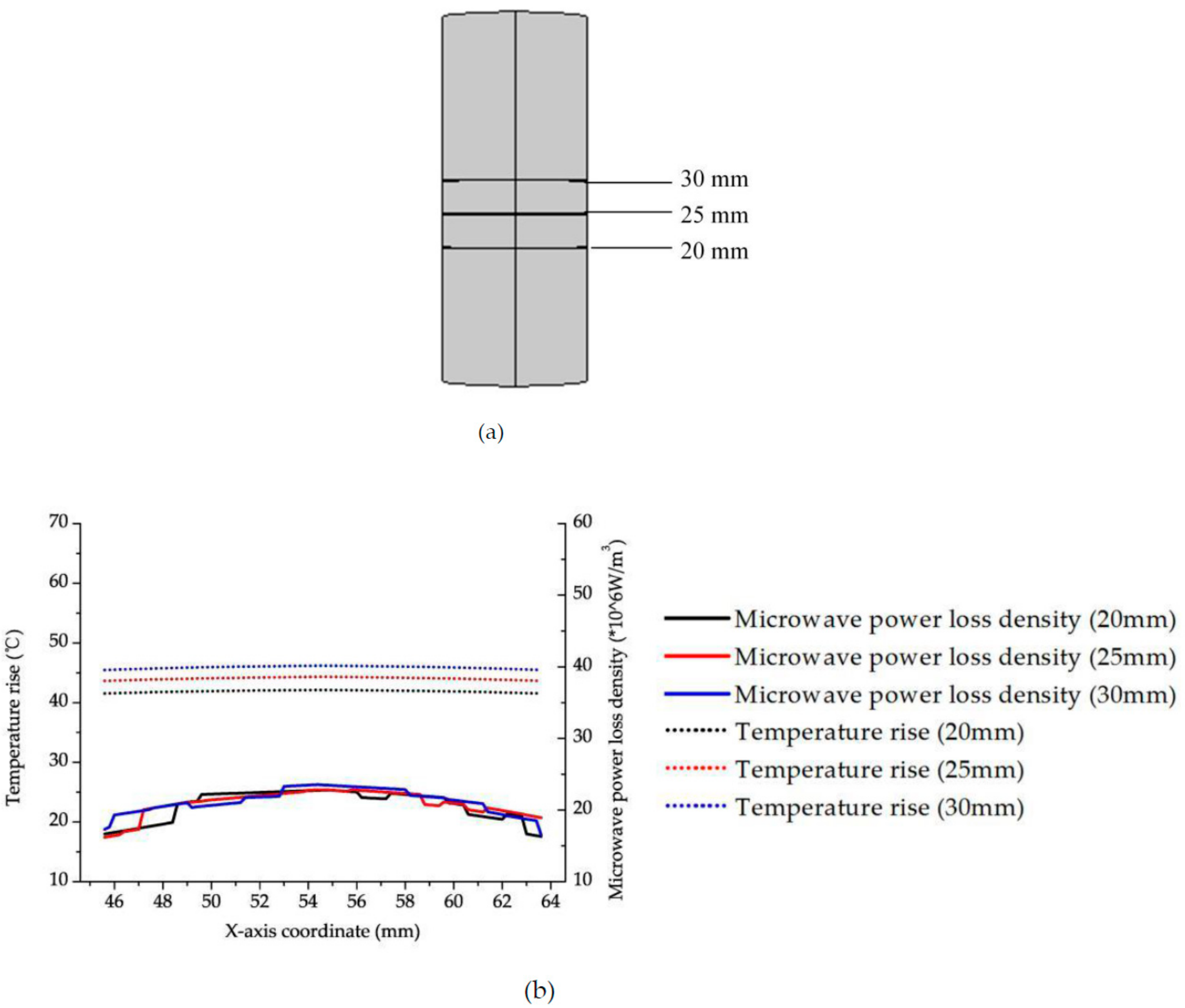
3.2. Efficient Heating Method Optimization and Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leadbeater, N.E.; Stencel, L.M. Fast, easy preparation of biodiesel using microwave heating. Energy Fuels 2006, 20, 2281–2283. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Deng, S.B.; Chen, P.; Liu, Y.H.; Wan, Y.Q.; Olson, A. Physical and chemical properties of bio-oils from microwave pyrolysis of corn stover. Appl. Biochem. Biotechnol. 2007, 137, 957–970. [Google Scholar] [PubMed]
- Miura, M.; Kaga, H.; Sakurai, A.; Kakuchi, T.; Takahashi, K. Rapid pyrolysis of wood block by microwave heating. J. Anal. Appl. Pyrolysis 2004, 71, 187–199. [Google Scholar] [CrossRef]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on fame production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Ismail, M.I.; Babi, D.K.; Simasatitkul, L.; Huusom, J.K.; Gani, R. Systematic sustainable process design and analysis of biodiesel processes. Processes 2013, 1, 167–202. [Google Scholar] [CrossRef]
- Lu, X.; Xi, B.; Zhang, Y.; Angelidaki, I. Microwave pretreatment of rape straw for bioethanol production: Focus on energy efficiency. Bioresour. Technol. 2011, 102, 7937–7940. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiong, Q.; Wang, K.; Liang, S.; Gao, M. Combining sliding mode neural network with Cuckoo Search to make a uniform microwave heating process. Int. J. Appl. Electron. 2015, 49, 61–77. [Google Scholar] [CrossRef]
- Mudgett, R.E. Microwave properties and heating characteristics of foods. Food Technol. 1986, 40, 84–87. [Google Scholar]
- Muley, P.D.; Boldor, D. Multiphysics numerical modeling of the continuous flow microwave-assisted transesterification process. J. Microw. Power Electromagn. Energy 2016, 46, 139–162. [Google Scholar] [CrossRef]
- Goldblith, S.A.; Wang, D.I.C. Effect of microwaves on Escherichia coli and Bacillus subtilis. Appl. Microbiol. 1967, 15, 1371–1375. [Google Scholar] [PubMed]
- Huang, K.M.; Liao, Y.H. Transient power loss density of electromagnetic pulse in debye media. IEEE Trans. Microw. Theory Tech. 2015, 63, 135–140. [Google Scholar] [CrossRef]
- Pandit, R.B.; Prasad, S. Finite element analysis of microwave heating of potato–Transient temperature profiles. J. Food Eng. 2003, 60, 193–202. [Google Scholar] [CrossRef]
- Pitchai, K.; Birla, S.L.; Subbiah, J.; Jones, D.; Thippareddi, H. Coupled electromagnetic and heat transfer model for microwave heating in domestic ovens. J. Food Eng. 2012, 112, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Pitchai, K.; Chen, J.; Birla, S.; Gonzalez, R.; Jones, D.; Subbiah, J. A microwave heat transfer model for a rotating multicomponent meal in a domestic oven: Development and validation. J. Food Eng. 2014, 128, 60–71. [Google Scholar] [CrossRef]
- Durlofsky, L.; Brady, J.F. Analysis of the brinkman equation as a model for flow in porous media. Phys. Fluids 1987, 30, 3329–3341. [Google Scholar] [CrossRef]
- Rhodes, M.E. Transport in Heterogeneous Porous Media. Ph.D. Thesis, Imperial College London, London, UK, 2006. [Google Scholar]
- Hwang, S.G.; ADvani, S.G. Numerical simulations of Stokes-Brinkman equations for permeability prediction of dual scale fibrous porous media. Phys. Fluids 2010, 22, 113101. [Google Scholar] [CrossRef]
- Polyanin, A.D.; Aristov, S.N. A new method for constructing exact solutions to three-dimensional Navier-Stokes and Euler equations. Theor. Found. Chem. Eng. 2011, 45, 885–890. [Google Scholar] [CrossRef]
- Jacimovic, N.; Hosoda, T.; Kishida, K.; Ivetic, M. Numerical solution of the Navier-Stokes equations for incompressible flow in porous media with free surface boundary. J. Appl. Mech. 2005, 8, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Drazin, P.G.; Riley, N. The Navier-Stokes equations. A classification of flows and exact solutions, Moduli Spaces. J. Fluid Mech. 2006, 38, 217–220. [Google Scholar]
- Noureddini, H.; Teoh, B.C.; Clements, L.D. Viscosities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 1184–1188. [Google Scholar] [CrossRef]
- Baroncini, C.; Filippo, P.D.; Latini, G.; Pacetti, M. Organic liquid thermal conductivity: A prediction method in the reduced temperature range 0.3 to 0.8. Int. J. Thermophys. 1981, 2, 21–38. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, M. Dieletric properties modeling studies of silicon nitride ceramic in high temperature. Piezoelectr. Acoustoopt. 2014, 36, 857–860. [Google Scholar]
- Sun, X.; Liu, X.; Huang, L. Effects of TiN addition on mechanic properties and electroconductive of Si3N4 matrix materials. J. Ceram. 1999, 20, 196–189. [Google Scholar]
- Wang, S. Fabrication and Properties of Si3N4/SiC Composites Ceramic. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, China, 2012. [Google Scholar]
- Wu, M. Porous Dielectric Material Thermal Behavior Research. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2016. [Google Scholar]
- Motasemi, F.; Salema, A.A.; Afzal, M.T. Dielectric characterization of corn stover for microwave processing technology. Fuel Process. Technol. 2015, 131, 370–375. [Google Scholar] [CrossRef]
- Alexandra, K.; Jan, D.; Pavol, S. Thermal shock resistance and fracture toughness of liquid-phase-sintered SiC-based ceramics. J. Eur. Ceram. Soc. 2009, 29, 2387–2394. [Google Scholar]
- Liu, X.; Li, H.; Gao, X. Research progress of foam SiC ceramic materials. Chem. Ind. Eng. Prog. 2012, 30, 2520–2524. [Google Scholar]
- Zhu, H.; Gulati, T.; Datta, A.K.; Huang, K. Microwave drying of spheres: Coupled electromagnetics-multiphase transport modeling with experimentation. Part I: Model development and experimental methodology. Food. Bioprod. Process. 2015, 96, 314–325. [Google Scholar] [CrossRef]
- Zhu, J.; Kuznetsov, A.V.; Sandeep, K.P. Numerical simulation of forced convection in a duct subjected to microwave heating. Heat Mass Transf. 2006, 43, 255–264. [Google Scholar] [CrossRef]
- Rylander, T.; Bondeson, A. Stable FEM-FDTD hybrid method for Maxwell’s equations. Comput Phys Commun. 2000, 125, 75–82. [Google Scholar] [CrossRef]
- Pathak, S.K.; Liu, F.; Tang, J. Finite difference time domain (FDTD) characterization of a single mode applicator. J. Microw. Power Electromagn. Energy 2016, 38, 37–48. [Google Scholar] [CrossRef]
- Hong, Y.; Lin, B.; Li, H.; Dai, H.; Zhu, C.; Yao, H. Three-dimensional simulation of microwave heating coal sample with varying parameters. Appl. Therm. Eng. 2016, 93, 1145–1154. [Google Scholar] [CrossRef]
- Qin, Q.; Zhang, Y.; Zhang, X. Research progress of microwave absorption materials. Electron. Compon. Mater. 2009, 28, 78–81. [Google Scholar]
- Li, Y.; Yang, L.; Hong, L.; Yang, C.; Gao, J. Thermal stability of oxygen containing silicon nitride ceramic fibers in inert atmosphere. Bull. Chin. Ceram. Soc. 2015, 34, 1798–1802. [Google Scholar]






| Property | Relative Permittivity | Dynamic Viscosities (Pa·s) | Conductivity (S/m) | Heat Conductivity Coefficient (W/m∙K) |
|---|---|---|---|---|
| Oleic acid | 2.563 − j × 0.0074 | 0.00249 + 1663.122 × exp (−T/26.876) [22] | 3 × 10−13 | 0.3292 − 0.000331 × T [23] |
| Silicon carbide | 20.08 − j × 1.5 (in this study) | - | 0.16 (in this study) | 1280 (in this study) |
| Silicon nitride | 7.8 − j × 0.05 [24] | - | 0.11 [25] | 18.42 [26] |
| Air | 1 | - | 0 | 2.524 |
| Quartz | 4.2 | - | 1 × 10−14 | 10 |
| Property | Heat Capacity at Constant Pressure (J/kg∙K) | Factor of Porosity | Density (kg/m3) | Heat Capacity at Constant Pressure (J/kg∙K) |
| Oleic acid | 2670.852 + 4.147 × T | - | 1100.15 − 0.698 × T [22] | 2670.852 + 4.147 × T |
| Silicon carbide | 1600 (in this study) | 0.9 (by measurement) | 580 (in this study) | 1600 (in this study) |
| Silicon nitride | 710 [26] | - | 3198 [27] | 710 [26] |
| Air | 1005 | - | 1.205 | 1005 |
| Quartz | 260 | - | 2600 | 260 |
| Temperature | ||
|---|---|---|
| 27 °C | 2.563416 | 0.007422231 |
| 37 °C | 2.572715 | 0.008652959 |
| 43 °C | 2.577733 | 0.009912491 |
| 54 °C | 2.564682 | 0.005191663 |
| 70 °C | 2.566721 | 0.005631242 |
| Quartz Tube’s Radius (mm) | 13 | 12 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Silicon Carbide’s Radius (mm) | 11 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| S11 (dB) | −6.03 | −6.48 | −6.61 | −6.96 | −7.12 | −6.95 | −6.85 | −6.62 | −6.36 |
| Stable Temperature (°C) | 51.97 | 38.07 | 50.54 | 50.78 | 49.34 | 47.76 | 47.76 | 45.78 | 48.74 |
| Quartz Tube’s Radius (mm) | 17 | 18 | 19 | 20 | 17 | 18 | 19 |
| Quartz Tube’s Inner Radius (mm) | 15 | 16 | 17 | 18 | 16 | 16 | 16 |
| Silicon Carbide’s Radius (mm) | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| S11 (dB) | −7.78 | −7.11 | −6.35 | −5.82 | −7.93 | −7.12 | −6.18 |
| Stable Temperature (°C) | 38.79 | 49.34 | 55.56 | 54.98 | 52.36 | 49.34 | 44.87 |
| Porosity | 0.45 | 0.6 | 0.75 | 0.9 |
| S11 (dB) | −2.8 | −3.54 | −5.76 | −7.24 |
| Stable temperature (°C) | 34.81 | 37.65 | 44.20 | 54.36 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Hong, T.; Xie, T.; Wang, F.; Luo, B.; Zhou, J.; Yang, Y.; Zhu, H.; Huang, K. Simulation and Analysis of Oleic Acid Pretreatment for Microwave-Assisted Biodiesel Production. Processes 2018, 6, 142. https://doi.org/10.3390/pr6090142
Ma W, Hong T, Xie T, Wang F, Luo B, Zhou J, Yang Y, Zhu H, Huang K. Simulation and Analysis of Oleic Acid Pretreatment for Microwave-Assisted Biodiesel Production. Processes. 2018; 6(9):142. https://doi.org/10.3390/pr6090142
Chicago/Turabian StyleMa, Weiquan, Tao Hong, Tian Xie, Fengxia Wang, Bin Luo, Jie Zhou, Yang Yang, Huacheng Zhu, and Kama Huang. 2018. "Simulation and Analysis of Oleic Acid Pretreatment for Microwave-Assisted Biodiesel Production" Processes 6, no. 9: 142. https://doi.org/10.3390/pr6090142






