Photocatalytic Inactivation of Enterobacter cloacae and Escherichia coli Using Titanium Dioxide Supported on Two Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bacterial Strains, Preparation and Quantification
2.3. TiO2/SiO2-BGT
2.4. TiO2-LDPE Pellets
2.5. Characterization of Films
2.6. Solar CPC Photo-Reactor
2.7. Solar Photocatalytic Disinfection Experiments
3. Results and Discussion
3.1. Characterization of TiO2/SiO2-BGT
3.2. TiO2-LDPE Pellets
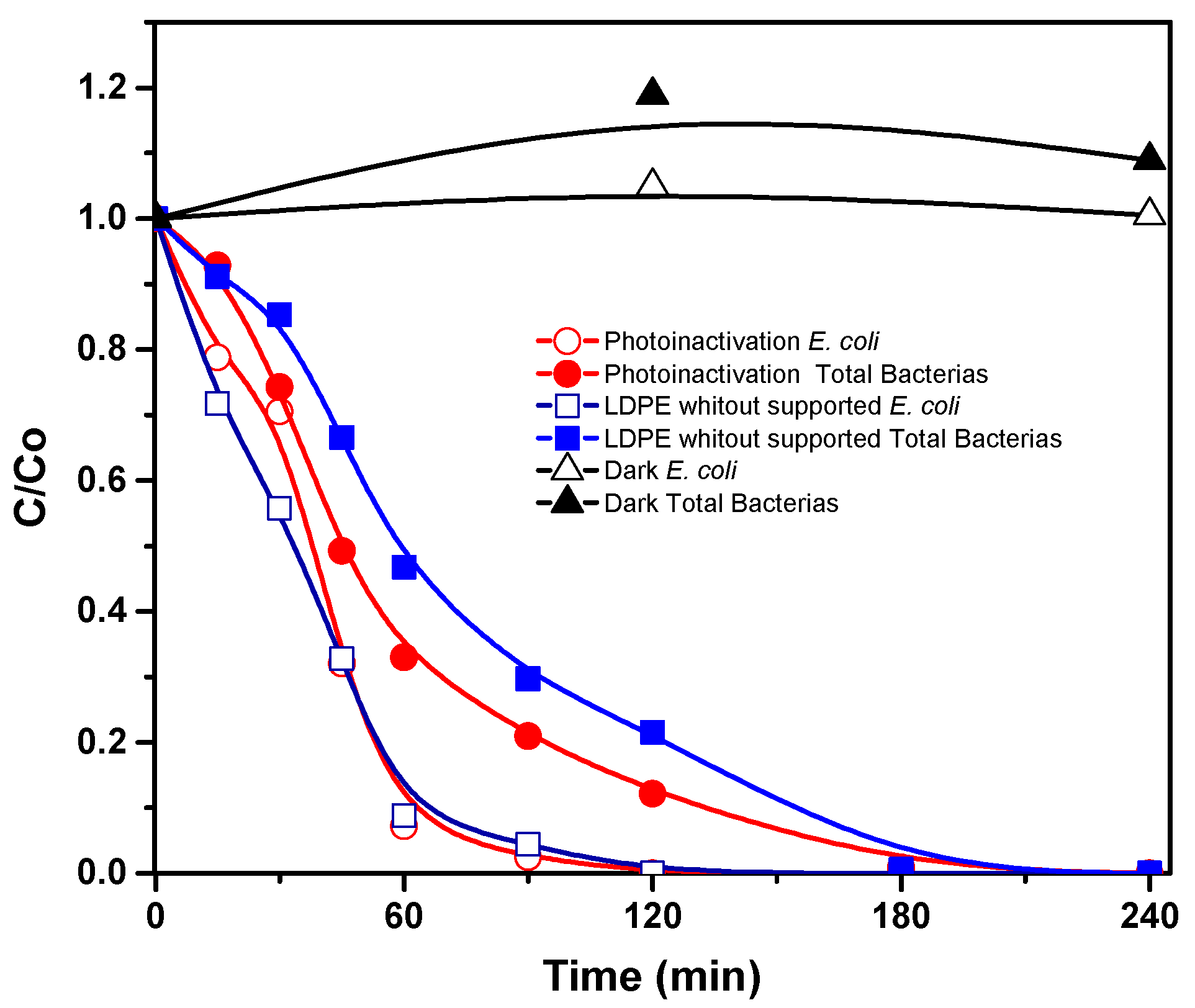
3.3. Effects of Solar Radiation and Mechanical Stress on E. coli and Total Bacteria
3.4. Photocatalytic Inactivation of Bacteria by Supported TiO2 Films
3.5. TiO2-LDPE Pellets Efficiency at Different Initial Bacterial Concentrations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lanao, M.; Ormad, M.P.; Mosteo, R.; Ovelleiro, J.L. Inactivation of Enterococcus sp. by photolysis and TiO2 photocatalysis with H2O2 in natural water. Sol. Energy 2012, 86, 619–625. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Fe3+ and TiO2 solar-light-assisted inactivation of E. coli at field scale. Catal. Today 2007, 122, 128–136. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Booshehri, A.Y.; Polo-Lopez, M.I.; Castro-Alférez, M.; Hea, P.; Xu, R.; Rong, W.; Malato, S.; Férnandez-Ibañez, P. Assessment of solar photocatalysis using Ag/BiVO4 at pilot solar Compound Parabolic Collector for inactivation of pathogens in well water and secondary effluents. Catal. Today 2017, 281, 124–134. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.J.; Pablos, C.; Díaz-Jiménez, L.; Cortés-Hernández, P.F.-I.D.; Sharma, P.K.; Castro-Alférez, M.; Férnandez-Ibañez, P.; Dunlop, P.S.M.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Castro-Alférez, M.; Polo-López, M.I.; Fernández-Ibáñez, P. Intracellular mechanisms of solar water disinfection. Sci. Rep. 2016, 6, 38145. [Google Scholar] [CrossRef] [Green Version]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Huang, Z.; Maness, P.-C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A Chem. 2000, 130, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Gelover, S.; Gómez, L.A.; Reyes, K.; Teresa Leal, M. A practical demonstration of water disinfection using TiO2 films and sunlight. Water Res. 2006, 40, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Gelover, S.; Mondragón, P.; Jiménez, A. Titanium dioxide sol–gel deposited over glass and its application as a photocatalyst for water decontamination. J. Photochem. Photobiol. A Chem. 2004, 16, 241–246. [Google Scholar] [CrossRef]
- Pozzo, R.L.; Baltanás, M.A.; Cassano, A.E. Supported titanium oxide as photocatalyst in water decontamination: State of the art. Catal. Today 1997, 39, 219–231. [Google Scholar] [CrossRef]
- Portela, R.; Sánchez, B.; Coronado, J.M.; Candal, R.; Suárez, S. Selection of TiO2-support: UV-transparent alternatives and long-term use limitations for H2S removal. Catal. Today 2007, 129, 223–230. [Google Scholar] [CrossRef]
- Turki, A.; Kochkar, H.; García-Fernández, I.; Polo-López, M.I.; Ghorbel, A.; Guillard, C.; Berhault, G.; Fernández-Ibáñez, P. Solar photocatalytic inactivation of Fusarium Solani over TiO2 nanomaterials with controlled morphology—Formic acid effect. Catal. Today 2013, 209, 147–152. [Google Scholar] [CrossRef]
- Mejía, M.I.; Marín, J.M.; Restrepo, G.; Rios, L.A.; Pulgarín, C.; Kiwi, J. Preparation, testing and performance of a TiO2/polyester photocatalyst for the degradation of gaseous methanol. Appl. Catal. B Environ. 2010, 94, 166–172. [Google Scholar] [CrossRef]
- Grieken, R.V.; Marugán, J.; Sordo, C.; Pablos, C. Comparison of the photocatalytic disinfection of E. coli suspensions in slurry, wall and fixed-bed reactors. Catal. Today 2009, 144, 48–54. [Google Scholar] [CrossRef]
- Alrousan, D.M.A.; Polo-López, M.I.; Dunlop, P.S.M.; Fernández-Ibáñez, P.; Byrne, J.A. Solar photocatalytic disinfection of water with immobilised titanium dioxide in re-circulating flow CPC reactors. Appl. Catal. B Environ. 2012, 128, 126–134. [Google Scholar] [CrossRef]
- Mallak, M.; Bockmeyer, M.; Löbmann, P. Liquid phase deposition of TiO2 on glass: Systematic comparison to films prepared by sol–gel processing. Thin Solid Films 2007, 515, 8072–8077. [Google Scholar] [CrossRef]
- Song, M.Y.; Park, Y.K.; Jurng, J. Direct coating of V2O5/TiO2 nanoparticles onto glass beads by chemical vapor deposition. Power Technol. 2012, 231, 135–140. [Google Scholar] [CrossRef]
- Velásquez, J.; Valencia, S.; Rios, L.; Restrepo, G.; Marín, J. Characterization and photocatalytic evaluation of polypropylene and polyethylene pellets coated with P25 TiO2 using the controlled-temperature embedding method. Chem. Eng. J. 2012, 203, 398–405. [Google Scholar] [CrossRef]
- Giovannetti, R.; D’Amato, C.A.; Zannotti, M.; Rommozzi, E.; Gunnella, R.; Miniucci, M.; Di Cicco, A. Visible light photoactivity of polypropylene coated Nano-TiO2 for dyes degradation in water. Sci. Rep. 2015, 5, 17801. [Google Scholar] [CrossRef] [PubMed]
- Rubio, D.; Casanueva, J.F.; Nebot, E. Improving UV seawater disinfection with immobilized TiO2: Study of the viability of photocatalysis (UV254/ TiO2) as seawater disinfection technology. J. Photochem. Photobiol. A Chem. 2013, 271, 16–23. [Google Scholar] [CrossRef]
- Yu, H.; Song, L.; Hao, Y.; Lu, N.; Quan, X.; Chen, S.; Zhang, Y.; Feng, Y. Fabrication of pilot-scale photocatalytic disinfection device by installing TiO2 coated helical support into UV annular reactor for strengthening sterilization. Chem. Eng. J. 2016, 283, 1506–1513. [Google Scholar] [CrossRef]
- Ratova, M.; Mills, A. Antibacterial titania-based photocatalytic extruded plastic films. J. Photochem. Photobiol. A Chem. 2015, 299, 159–165. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Andrzejczuk, M.; Pulgarin, C.; Kulik, A.; Kiwi, J. Innovative transparent non-scattering TiO2 bactericide thin films inducing increased E. coli cell wall fluidity. Surf. Coat. Technol. 2014, 254, 333–343. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Hung, Y.C. Photocatalytic TiO2 coating of plastic cutting board to prevent microbial cross-contamination. Food Control 2017, 77, 88–95. [Google Scholar] [CrossRef]
- Yañez, D.; Guerrero, S.; Lieberwirth, I.; Ulloa, M.T.; Gomez, T.; Rabagliati, F.M.; Zapata, P.A. Photocatalytic inhibition of bacteria by TiO2 nanotubes-doped polyethylene composites. Appl. Catal. A Gen. 2015, 489, 255–261. [Google Scholar] [CrossRef]
- Bahloul, W.; Mélis, F.; Bounor-Legaré, V.; Cassagnau, P. Structural characterisation and antibacterial activity of PP/TiO2 nanocomposites prepared by an in situ sol–gel method. Mater. Chem. Phys. 2012, 134, 399–406. [Google Scholar] [CrossRef]
- Marín, J.M.; Fidelgranda, C.; Galeano, L.; Rios, L.A.; Restrepo, G. Impregnación de TiO2 sobre borosilicato por el método sol-gel usando inmersión a velocidad controlada. Sci. Tech. 2007, 2, 441–446. [Google Scholar]
- García-Fernández, I.; Polo-López, M.I.; Oller, I.; Fernández-Ibáñez, P. Bacteria and fungi inactivation using Fe3+/sunlight, H2O2/sunlight and near neutral photo-Fenton: A comparative study. Appl. Catal. B Environ. 2012, 121–122, 20–29. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Gao, X.; Ding, Y.; Zhang, S.; Zhao, J.; Liu, S.; Yang, M. Immobilization of TiO2 nanoparticles in polymeric substrates by chemical bonding for multi-cycle photodegradation of organic pollutants. J. Hazard. Mater. 2012, 227–228, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Safajou, H.; Khojasteh, H.; Salavati-Niasari, M.; Mortazavi-Derazkola, S. Enhanced photocatalytic degradation of dyes over graphene/Pd/ TiO2 nanocomposites: TiO2 nanowires versus TiO2 nanoparticles. J. Colloid Interface Sci. 2017, 498, 423–432. [Google Scholar] [CrossRef] [PubMed]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and raman characterization of TiO2 nanoparticles coated with Polyethylene Glycol as carrier for 2-Methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Fischer, E.W. Effect of annealing and temperature on the morphological structure of polymers. Pure Appl. Chem. 1972, 31, 113–132. [Google Scholar] [CrossRef]
- Matsuzawa, S.; Maneerat, C.; Hayata, Y.; Hirakawa, T.; Negishi, N.; Sano, T. Immobilization of TiO2 nanoparticles on polymeric substrates by using electrostatic interaction in the aqueous phase. Appl. Catal. B Environ. 2008, 83, 39–45. [Google Scholar] [CrossRef]
- Sordo, C.; Van Grieken, R.; Marugán, J.; Fernández-Ibáñez, P. Solar photocatalytic disinfection with immobilised TiO2 at pilot-plant scale. Water Sci. Technol. 2010, 61, 507. [Google Scholar] [CrossRef] [PubMed]
- Hincapié, M.; Balaguera, A.; Botero, L.; Sánchez, C.; Restrepo, G.; Marín, J. Purificación del agua por fotocatálisis. In Even. X Jornadas Investig. Univ. Medellín INNOVACIÓN Y Transf. Conoc. EN Ing.; Sello Editorial Universidad de MedellSín: Medellín, Colombia, 2014; pp. 75–92. [Google Scholar]
- Marugán, J.; Grieken, R.V.; Sordo, C.; Cruz, C. Kinetics of the photocatalytic disinfection of Escherichia coli suspensions. Appl. Catal. B Environ. 2008, 82, 27–36. [Google Scholar] [CrossRef]
- Helali, S.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ohtani, B.; Amano, F.; Malato, S.; Guillard, C. Solar photocatalysis: A green technology for E. coli contaminated water disinfection. Effect of concentration and different types of suspended catalyst. J. Photochem. Photobiol. A Chem. 2014, 276, 31–40. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Bactericidal action of illuminated TiO2 on pure Escherichia coli and natural bacterial consortia: post-irradiation events in the dark and assessment of the effective disinfection time. Appl. Catal. B Environ. 2004, 49, 99–112. [Google Scholar] [CrossRef]
- Craik, S.A.; Weldon, D.; Finch, G.R.; Bolton, J.R.; Belosevic, M. Inactivation of cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 2001, 35, 1387–1398. [Google Scholar] [CrossRef]


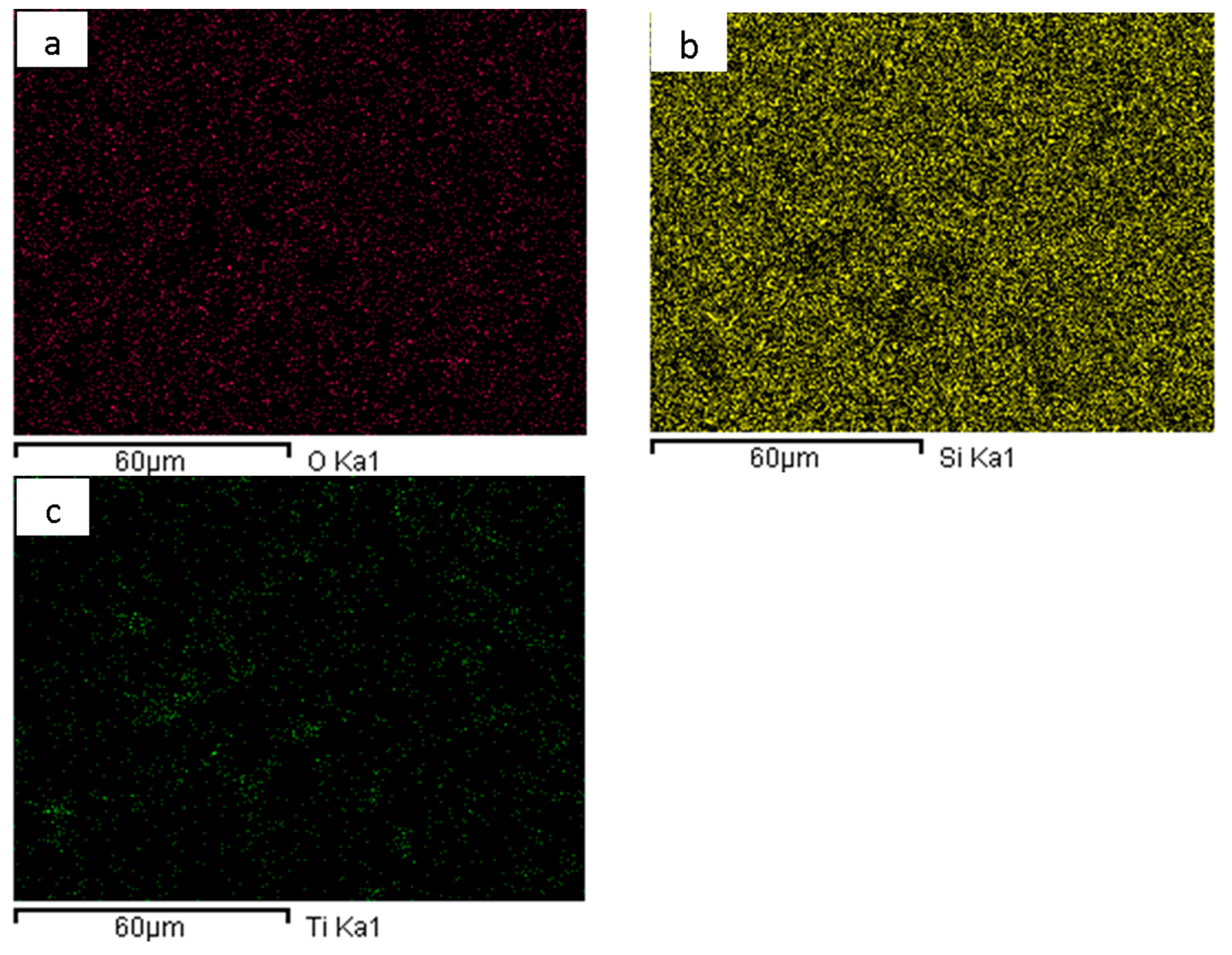


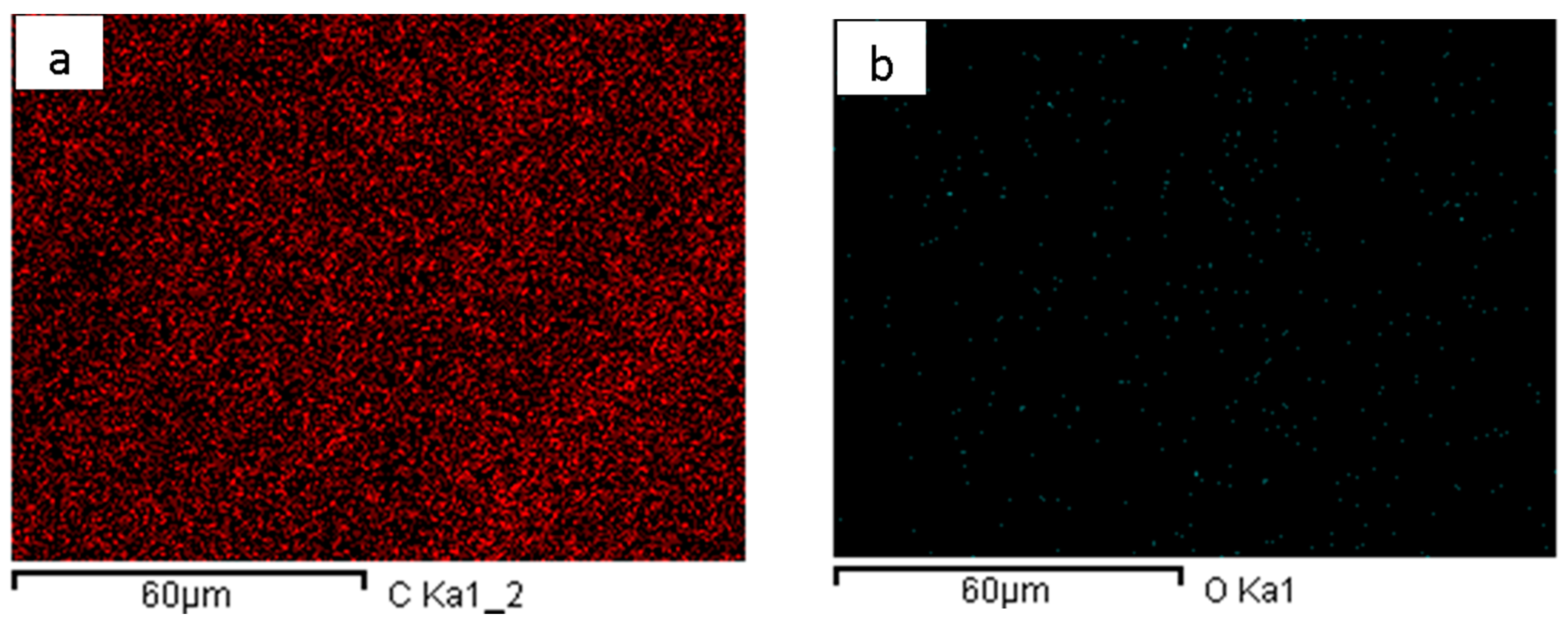



| Hours of Matrix Silica Aging | Atomic % Ti | Atomic % Si |
|---|---|---|
| 24 | 1.08 | 41.49 |
| 36 | 1.49 | 32.39 |
| 48 | 1.13 | 39.77 |
| 60 | 1.10 | 40.40 |
| TiO2-LDPE Pellets | % Weight Loss | mg TiO2/g LDPE Pellets |
|---|---|---|
| 1 layer | 0.1836 | 0.2374 |
| 2 layers | 0.1974 | 0.1495 |
| 3 layers | 0.2253 | 0.1412 |
| 4 layers | 0.2679 | 0.1080 |
| 5 layers | 0.2884 | 0.0914 |
| Treatment | TiO2 (mg·L−1) | Conc. (CFU·mL−1) | k (L·kJ−1) | Shoulder Length (kJ·L−1) | Log (Nres) | R2 | Kinetic Model |
|---|---|---|---|---|---|---|---|
| Total Bacteria | |||||||
| Photo-inactivation | 0 | 105 | 0.22 ± 0.03 | 1.56 ± 0.01 | – | 0.966 | Shoulder + Log Linear |
| TiO2-suspended | 50 | 105 | 0.47 ± 0.03 | – | – | 0.967 | Log Linear |
| TiO2/SiO2-BGT | 59 | 105 | 0.37 ± 0.01 | 3.27 ± 0.02 | 1.17 ± 0.02 | 0.997 | Shoulder + Log Lineal + Tail |
| TiO2-LDPE pellets | 52 | 105 | 0.49 ± 0.02 | – | – | 0.970 | Log Linear |
| E. coli | |||||||
| Photo-inactivation | 0 | 105 | 0.40 ± 0.10 | 1.56 ± 0.01 | – | 0.946 | Shoulder + Log Linear |
| TiO2-suspended | 50 | 105 | 1.50 ± 0.10 | – | – | 0.923 | Log Linear |
| TiO2/SiO2-BGT | 59 | 105 | 0.46 ± 0.05 | – | – | 0.954 | Log Linear |
| TiO2-LDPE pellets | 52 | 105 | 0.90 ± 0.04 | – | – | 0.910 | Log Linear |
| Treatment | TiO2 (mg·L−1) | Concentration (CFU·mL−1) | k (L·kJ−1) | R2 | Kinetic Model |
|---|---|---|---|---|---|
| Total Bacteria | |||||
| TiO2-LDPE pellets | 52 | 105 | 0.49 ± 0.02 | 0.970 | Log Linear |
| TiO2-LDPE pellets | 52 | 103 | 0.39 ± 0.03 | 0.974 | Log Linear |
| TiO2-LDPE pellets | 52 | 101 | 0.22 ± 0.01 | 0.977 | Log Linear |
| E. coli | |||||
| TiO2-LDPE pellets | 52 | 105 | 0.90 ± 0.04 | 0.891 | Log Linear |
| TiO2-LDPE pellets | 52 | 103 | 0.89 ± 0.08 | 0.962 | Log Linear |
| TiO2-LDPE pellets | 52 | 101 | 0.38 ± 0.10 | 0.936 | Log linear |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguas, Y.; Hincapié, M.; Sánchez, C.; Botero, L.; Fernández-Ibañez, P. Photocatalytic Inactivation of Enterobacter cloacae and Escherichia coli Using Titanium Dioxide Supported on Two Substrates. Processes 2018, 6, 137. https://doi.org/10.3390/pr6090137
Aguas Y, Hincapié M, Sánchez C, Botero L, Fernández-Ibañez P. Photocatalytic Inactivation of Enterobacter cloacae and Escherichia coli Using Titanium Dioxide Supported on Two Substrates. Processes. 2018; 6(9):137. https://doi.org/10.3390/pr6090137
Chicago/Turabian StyleAguas, Yelitza, Margarita Hincapié, Camilo Sánchez, Liliana Botero, and Pilar Fernández-Ibañez. 2018. "Photocatalytic Inactivation of Enterobacter cloacae and Escherichia coli Using Titanium Dioxide Supported on Two Substrates" Processes 6, no. 9: 137. https://doi.org/10.3390/pr6090137





