Modeling and Simulation Studies of a Novel Coupled Plug Flow Crystallizer for the Continuous Separation of Conglomerate-Forming Enantiomers
Abstract
:1. Introduction
2. Available Coupled Preferential Crystallizer Configurations
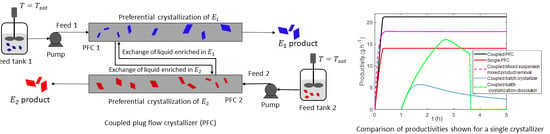
3. Proposed Coupled PFC Configuration
4. Model Development
5. Results and Discussion
5.1. Product CSD in Coupled PFC Configuration
5.2. Comparison of Productivity and Yield
5.3. Effect of Liquid Phase Exchange Location
5.4. Effect of the Amount of Liquid Phase Exchange
5.5. Effect of Seed Mass
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| COBC | Continuous oscillatory baffled crystallizer |
| CPC | Coupled preferential crystallization |
| CPC-D | Coupled preferential crystallization-dissolution |
| CPC-MSMPR | Coupled preferential crystallization in MSMPR |
| CSD | Crystal size distribution |
| MSMPR | Mixed suspension mixed product removal |
| PBE | Population balance equation |
| PFC | Plug flow crystallizer |
Appendix A. Model Parameters
| Parameter | Symbol | Value | Units |
|---|---|---|---|
| constant for density | |||
| constant for density | |||
| constant for density | |||
| volume shape factor | 0.1222 | - | |
| density of solid threonine | 1250 | kg | |
| ideal gas constant | 8.314 | J (K mol) | |
| activation energy for growth | J mol | ||
| activation energy for secondary nucleation | J mol | ||
| dissolution rate constant | m s | ||
| growth rate constant | m s | ||
| growth exponent | g | - | |
| growth parameter (size-dependent term) | m | ||
| growth exponent (size-dependent term) | −0.4 | - | |
| secondary nucleation rate constant | m s | ||
| exponent for third moment | 3.0258 | - | |
| secondary nucleation exponent | - | ||
| primary nucleation rate constant | 1000 | s | |
| primary nucleation exponent | 1 | - | |
| nucleation induction time | 7200 | ||
| slope of sigmoidal function | 0.01 | - | |
| solubility parameter | °C | ||
| solubility parameter | - | ||
| solubility parameter | - | ||
| solubility parameter | - | ||
| mean seed size of enantiomer (L-Thr) | 27,95 | m | |
| standard deviation of seed CSD | 0.3,0.34 | - | |
| mean size of racemate | 9 | m | |
| standard deviation of racemate CSD | 0.5 | - |
References
- Shen, Z.; Lv, C.; Zeng, S. Significance and challenges of stereoselectivity assessing methods in drug metabolism. J. Pharm. Anal. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kamarei, F.; Vajda, P.; Gritti, F.; Guiochon, G. The adsorption of naproxen enantiomers on the chiral stationary phase (R,R)-whelk-O1 under supercritical fluid conditions. J. Chromatogr. A 2014, 1345, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.; Seidel-Morgenstern, A. Processes To Separate Enantiomers. Angew. Chem. Int. Ed. 2014, 53, 1218–1250. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G. A Practical Approach to Chiral Separations by Liquid Chromatography; VCH Weinheim: Weinheim, Germany, 1994. [Google Scholar]
- Schurig, V. Separation of enantiomers by gas chromatography. J. Chromatogr. A 2001, 906, 275–299. [Google Scholar] [CrossRef]
- Rajendran, A.; Paredes, G.; Mazzotti, M. Simulated moving bed chromatography for the separation of enantiomers. J. Chromatogr. A 2009, 1216, 709–738. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.C.; Wahab, M.F.; Armstrong, D.W.; Breitbach, Z.S. Advances in high-throughput and high-efficiency chiral liquid chromatographic separations. J. Chromatogr. A 2016, 1467, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Keurentjes, J.; Nabuurs, L.; Vegter, E. Liquid membrane technology for the separation of racemic mixtures. J. Membr. Sci. 1996, 113, 351–360. [Google Scholar] [CrossRef]
- Lee, S.B.; Mitchell, D.T.; Trofin, L.; Nevanen, T.K.; Söderlund, H.; Martin, C.R. Antibody-based bio-nanotube membranes for enantiomeric drug separations. Science 2002, 296, 2198–2200. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal—Organic framework membranes: from synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Köllges, T.; Vetter, T. Design and Performance Assessment of Continuous Crystallization Processes Resolving Racemic Conglomerates. Cryst. Growth Des. 2018, 18, 1686–1696. [Google Scholar] [CrossRef]
- Jacques, J.; Collet, A.; Wilen, S.H. Enantiomers, Racemates, and Resolutions; Wiley: Hoboken, NJ, USA, 1981. [Google Scholar]
- Elsner, M.P.; Ziomek, G.; Seidel-Morgenstern, A. Simultaneous preferential crystallization in a coupled, batch operation mode—Part I: Theoretical analysis and optimization. Chem. Eng. Sci. 2007, 62, 4760–4769. [Google Scholar] [CrossRef]
- Eicke, M.J.; Levilain, G.; Seidel-Morgenstern, A. Efficient Resolution of Enantiomers by Coupling Preferential Crystallization and Dissolution. Part 2: A Parametric Simulation Study to Identify Suitable Process Conditions. Cryst. Growth Des. 2013, 13, 1638–1648. [Google Scholar] [CrossRef]
- Galan, K.; Eicke, M.J.; Elsner, M.P.; Lorenz, H.; Seidel-Morgenstern, A. Continuous Preferential Crystallization of Chiral Molecules in Single and Coupled Mixed-Suspension Mixed-Product-Removal Crystallizers. Cryst. Growth Des. 2015, 15, 1808–1818. [Google Scholar] [CrossRef]
- Steendam, R.R.E.; ter Horst, J.H. Continuous Total Spontaneous Resolution. Cryst. Growth Des. 2017, 17, 4428–4436. [Google Scholar] [CrossRef]
- Viedma, C. Chiral Symmetry Breaking During Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef] [PubMed]
- Noorduin, W.L.; Izumi, T.; Millemaggi, A.; Leeman, M.; Meekes, H.; Van Enckevort, W.J.P.; Kellogg, R.M.; Kaptein, B.; Vlieg, E.; Blackmond, D.G. Emergence of a Single Solid Chiral State from a Nearly Racemic Amino Acid Derivative. J. Am. Chem. Soc. 2008, 130, 1158–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iggland, M.; Müller, R.; Mazzotti, M. On the Effect of Initial Conditions in Viedma Ripening. Cryst. Growth Des. 2014, 14, 2488–2493. [Google Scholar] [CrossRef]
- Xiouras, C.; Ter Horst, J.H.; Van Gerven, T.; Stefanidis, G.D. Coupling Viedma Ripening with Racemic Crystal Transformations: Mechanism of Deracemization. Cryst. Growth Des. 2017, 17, 4965–4976. [Google Scholar] [CrossRef]
- Suwannasang, K.; Flood, A.E.; Rougeot, C.; Coquerel, G. Using Programmed Heating–Cooling Cycles with Racemization in Solution for Complete Symmetry Breaking of a Conglomerate Forming System. Cryst. Growth Des. 2013, 13, 3498–3504. [Google Scholar] [CrossRef]
- Kittisak, S.; Gerard, C.; Celine, R.; Flood, A.E. Mathematical Modeling of Chiral Symmetry Breaking due to Differences in Crystal Growth Kinetics. Chem. Eng. Technol. 2014, 37, 1329–1339. [Google Scholar] [CrossRef]
- Li, W.W.; Spix, L.; de Reus, S.C.A.; Meekes, H.; Kramer, H.J.M.; Vlieg, E.; ter Horst, J.H. Deracemization of a Racemic Compound via Its Conglomerate-Forming Salt Using Temperature Cycling. Cryst. Growth Des. 2016, 16, 5563–5570. [Google Scholar] [CrossRef]
- Steendam, R.R.E.; ter Horst, J.H. Scaling Up Temperature Cycling-Induced Deracemization by Suppressing Nonstereoselective Processes. Cryst. Growth Des. 2018, 18, 3008–3015. [Google Scholar] [CrossRef]
- Suwannasang, K.; Flood, A.E.; Coquerel, G. A Novel Design Approach to Scale-Up of the Temperature Cycle Enhanced-Deracemization Process: Coupled Mixed-Suspension Vessels. Cryst. Growth Des. 2016, 11, 6461–6467. [Google Scholar] [CrossRef]
- McGlone, T.; Briggs, N.E.B.; Clark, C.A.; Brown, C.J.; Sefcik, J.; Florence, A.J. Oscillatory Flow Reactors (OFRs) for Continuous Manufacturing and Crystallization. Ph.D. Thesis, University of Strathclyde, Glasgow, UK, 2015. [Google Scholar]
- Alvarez, A.J.; Myerson, A.S. Continuous Plug Flow Crystallization of Pharmaceutical Compounds. Cryst. Growth Des. 2010, 10, 2219–2228. [Google Scholar] [CrossRef]
- Ridder, B.J.; Majumder, A.; Nagy, Z.K. Population Balance Model-Based Multiobjective Optimization of a Multisegment Multiaddition (MSMA) Continuous Plug-Flow Antisolvent Crystallizer. Ind. Eng. Chem. Res. 2014, 53, 4387–4397. [Google Scholar] [CrossRef]
- Neugebauer, P.; Khinast, J.G. Continuous Crystallization of Proteins in a Tubular Plug-Flow Crystallizer. Cryst. Growth Des. 2015, 15, 1089–1095. [Google Scholar] [CrossRef]
- Zhao, Y.; Kamaraju, V.K.; Hou, G.; Power, G.; Donnellan, P.; Glennon, B. Kinetic identification and experimental validation of continuous plug flow crystallisation. Chem. Eng. Sci. 2015, 133, 106–115. [Google Scholar] [CrossRef]
- Su, Q.; Benyahia, B.; Nagy, Z.K.; Rielly, C.D. Mathematical Modeling, Design, and Optimization of a Multisegment Multiaddition Plug-Flow Crystallizer for Antisolvent Crystallizations. Org. Process Res. Dev. 2015, 12, 1859–1870. [Google Scholar] [CrossRef]
- Lawton, S.; Steele, G.; Shering, P.; Zhao, L.; Laird, I.; Ni, X.W. Continuous Crystallization of Pharmaceuticals Using a Continuous Oscillatory Baffled Crystallizer. Org. Process Res. Dev. 2009, 13, 1357–1363. [Google Scholar] [CrossRef]
- Brown, C.J.; Adelakun, J.A.; Ni, X.w. Characterization and modelling of antisolvent crystallization of salicylic acid in a continuous oscillatory baffled crystallizer. Chem. Eng. Process. Process Intensifi. 2015, 97, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Ramkrishna, D. Population Balances: Theory and Applications to Particulate Systems in Engineering; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Majumder, A.; Nagy, Z. A Comparative Study of Coupled Preferential Crystallizers for the Efficient Resolution of Conglomerate-Forming Enantiomers. Pharmaceutics 2017, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.; Galan, K.; Peter Elsner, M.; Hussain, I.; Seidel-Morgenstern, A. Theoretical investigation of simultaneous continuous preferential crystallization in a coupled mode. Chem. Eng. Sci. 2013, 98, 25–39. [Google Scholar] [CrossRef]
- Elsner, M.P.; Ziomek, G.; Seidel-Morgenstern, A. Efficient separation of enantiomers by preferential crystallization in two coupled vessels. AIChE J. 2009, 55, 640–649. [Google Scholar] [CrossRef]
- Elsner, M.P.; Ziomek, G.; Seidel-Morgenstern, A. Simultaneous preferential crystallization in a coupled batch operation mode. Part II: Experimental study and model refinement. Chem. Eng. Sci. 2011, 66, 1269–1284. [Google Scholar] [CrossRef]
- Binev, D.; Seidel-Morgenstern, A.; Lorenz, H. Continuous Separation of Isomers in Fluidized Bed Crystallizers. Cryst. Growth Des. 2016, 16, 1409–1419. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.; Gao, T.; Nagy, Z.K. Integrated Upstream and Downstream Application of Wet Milling with Continuous Mixed Suspension Mixed Product Removal Crystallization. Cryst. Growth Des. 2015, 15, 5879–5885. [Google Scholar] [CrossRef]
- Manninen, M.; Gorshkova, E.; Immonen, K.; Ni, X.W. Evaluation of axial dispersion and mixing performance in oscillatory baffled reactors using CFD. J. Chem. Technol. Biotechnol. 2013, 88, 553–562. [Google Scholar] [CrossRef]
- Kacker, R.; Regensburg, S.I.; Kramer, H.J.M. Residence time distribution of dispersed liquid and solid phase in a continuous oscillatory flow baffled crystallizer. Chem. Eng. J. 2017, 317, 413–423. [Google Scholar] [CrossRef]
- Gunawan, R.; Fusman, I.; Braatz, R.D. High resolution algorithms for multidimensional population balance equations. AIChE J. 2004, 50, 2738–2749. [Google Scholar] [CrossRef]
- Qamar, S.; Elsner, M.P.; Angelov, I.A.; Warnecke, G.; Seidel-Morgenstern, A. A comparative study of high resolution schemes for solving population balances in crystallization. Comput. Chem. Eng. 2006, 30, 1119–1131. [Google Scholar] [CrossRef]
- Majumder, A.; Nagy, Z.K. Dynamic Modeling of Encrust Formation and Mitigation Strategy in a Continuous Plug Flow Crystallizer. Cryst. Growth Des. 2015, 15, 1–22. [Google Scholar] [CrossRef]
- Qamar, S.; Elsner, M.P.; Hussain, I.; Seidel-Morgenstern, A. Seeding strategies and residence time characteristics of continuous preferential crystallization. Chem. Eng. Sci. 2012, 71, 5–17. [Google Scholar] [CrossRef]
- Ferguson, S.; Ortner, F.; Quon, J.; Peeva, L.; Livingston, A.; Trout, B.L.; Myerson, A.S. Use of Continuous MSMPR Crystallization with Integrated Nanofiltration Membrane Recycle for Enhanced Yield and Purity in API Crystallization. Cryst. Growth Des. 2014, 14, 617–627. [Google Scholar] [CrossRef]









| CPC-MSMPR | CPC | CPC-D | |||||
|---|---|---|---|---|---|---|---|
| Variable | Tank 1 | Tank 2 | Tank 1 | Tank 2 | Tank 1 | Tank 2 | |
| Liquid phase | g | g | g | g | g | g | |
| g | g | g | g | g | g | ||
| Solid phase | (L-Thr) | g | − | g | − | g | g |
| (D-Thr) | − | g | − | g | − | g | |
| Temperatures | 36 °C | 36 °C | 36 °C | 36 °C | 36 °C | 45 °C | |
| Exchange rate | 80 mL min−1 | 80 mL min−1 | 80 mL min−1 | ||||
| Feed rate | q | 80 mL min−1 | 80 mL min−1 | − | − | − | − |
| Removal rate | q | 80 mL min−1 | 80 mL min−1 | − | − | − | − |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majumder, A. Modeling and Simulation Studies of a Novel Coupled Plug Flow Crystallizer for the Continuous Separation of Conglomerate-Forming Enantiomers. Processes 2018, 6, 247. https://doi.org/10.3390/pr6120247
Majumder A. Modeling and Simulation Studies of a Novel Coupled Plug Flow Crystallizer for the Continuous Separation of Conglomerate-Forming Enantiomers. Processes. 2018; 6(12):247. https://doi.org/10.3390/pr6120247
Chicago/Turabian StyleMajumder, Aniruddha. 2018. "Modeling and Simulation Studies of a Novel Coupled Plug Flow Crystallizer for the Continuous Separation of Conglomerate-Forming Enantiomers" Processes 6, no. 12: 247. https://doi.org/10.3390/pr6120247