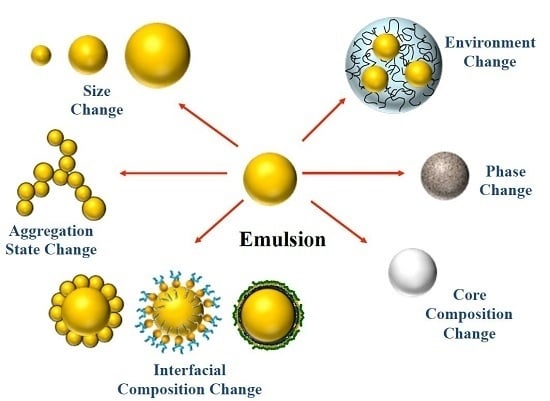
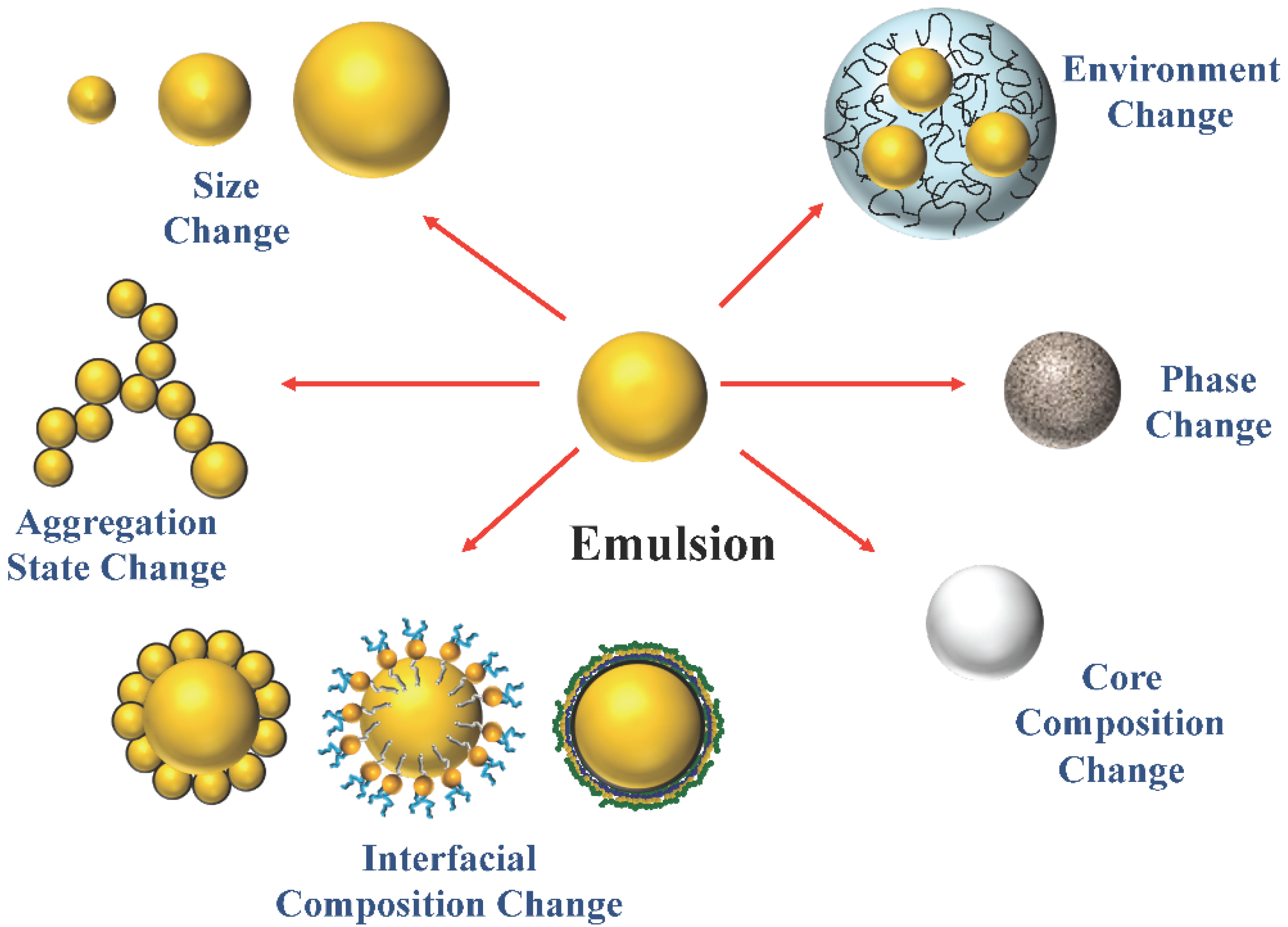
Extending Emulsion Functionality: Post-Homogenization Modification of Droplet Properties
Abstract
:1. Introduction
- Some homogenization methods are only capable of producing small droplets using certain types of oils and surfactants. However, these components may not have the desired functional attributes, e.g., the surfactant used to form the emulsion may not lead to droplets that are stable over the intended range of environmental conditions the emulsion will experience in a commercial application. Consequently, it may be necessary to form an emulsion using one type of surfactant, and then exchange the surfactant afterwards.
- Most homogenization methods require that the lipid phase be liquid during emulsion formation. However, for certain applications it is an advantage to have a lipid phase that is solid in the final product (e.g., to improve chemical stability or modulate release profiles). This may be achieved by using a lipid phase that is liquid at high temperatures, but that is solid at the intended temperature of utilization. The emulsion can then be homogenized at high temperatures, and then cooled afterwards.
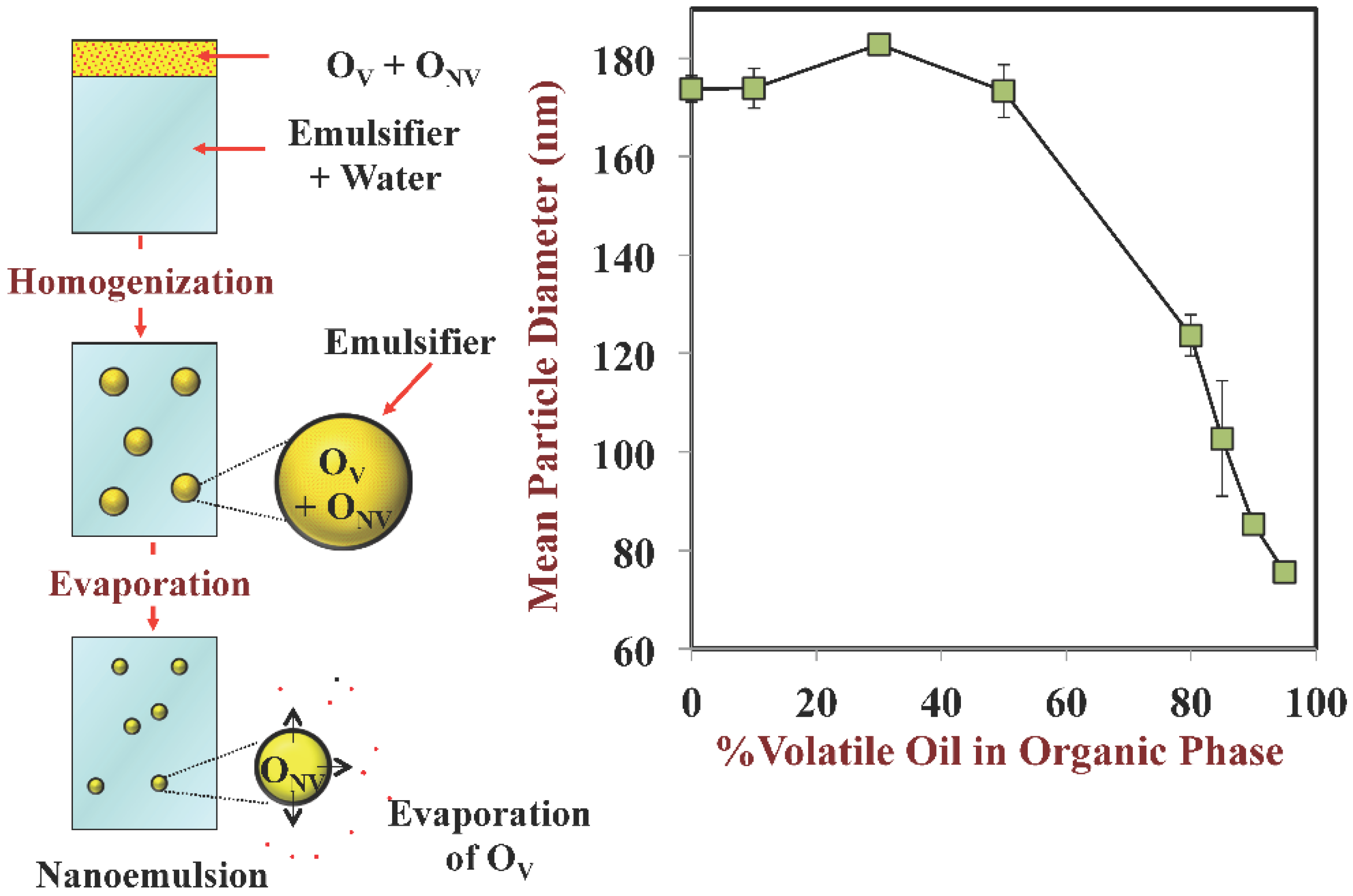
- Some homogenization methods are limited in the smallest size of droplets that they can produce due to mechanical limitations. For certain commercial applications it may be important to have very small droplets so as to create products that are optically transparent or that have high oral bioavailability. In this case, it may be possible to use a post-homogenization method to further reduce the size of the droplets in an emulsion after it has been produced.
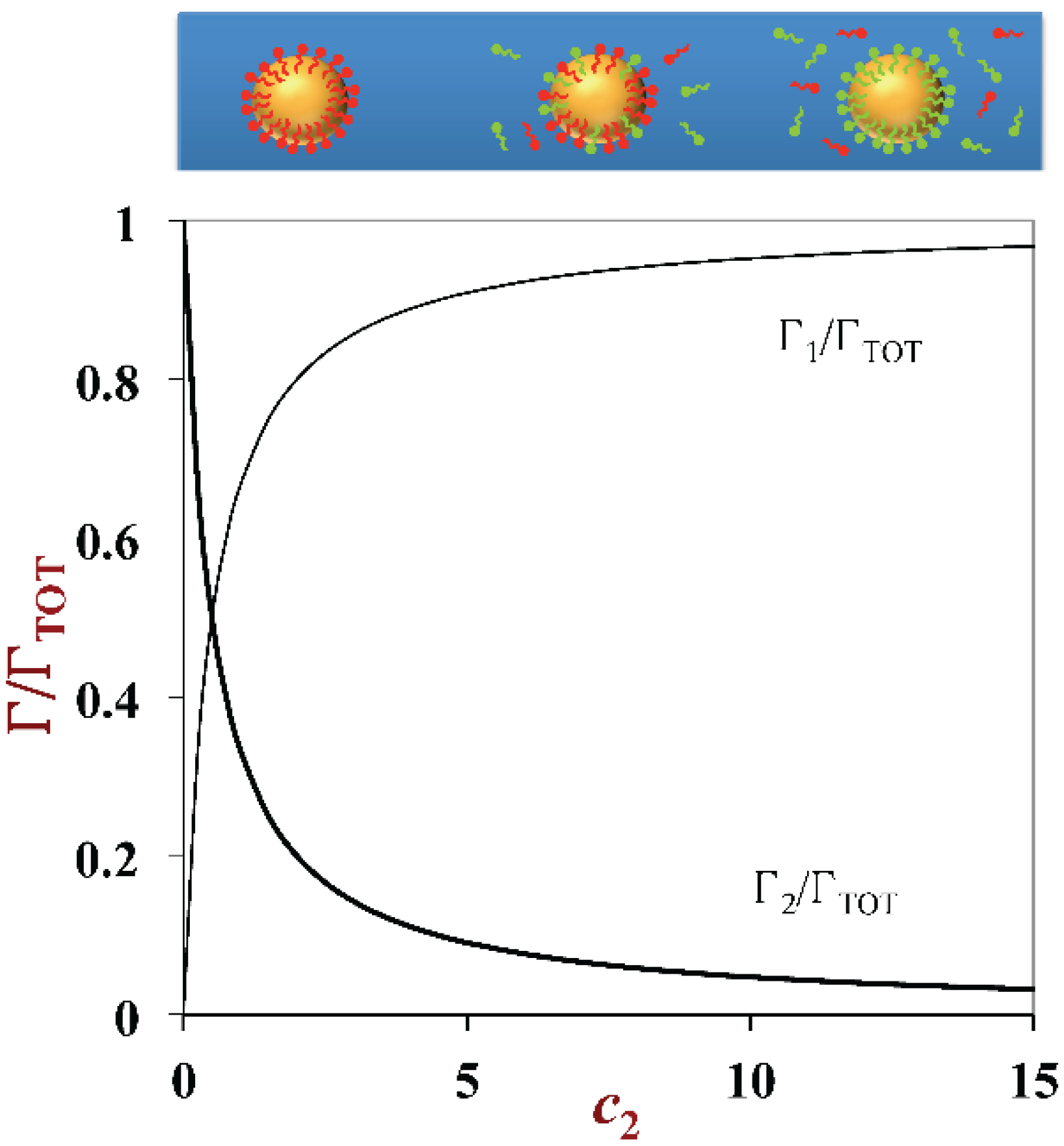
- In some research applications it is useful to have emulsions that all have the same droplet size distribution, but that have different interfacial properties, such as charge. This can be achieved by creating one type of oil-in-water emulsion using a certain type of emulsifier, and then exchanging the original emulsifiers from the droplet surfaces afterwards.
2. Impact of Droplet Properties on Emulsion Functionality
2.1. Droplet Concentration
2.2. Droplet Size
2.3. Droplet Charge and Other Interfacial Properties
2.4. Disperse Phase Composition
2.5. Physical State
2.6. Local Environment
2.7. Droplet Spatial Distribution
3. Post-Homogenization Droplet Modification Methods
3.1. Droplet Concentration: Dilution and Concentration Methods
3.2. Droplet Size: Disperse Phase Evaporation and Ripening Methods
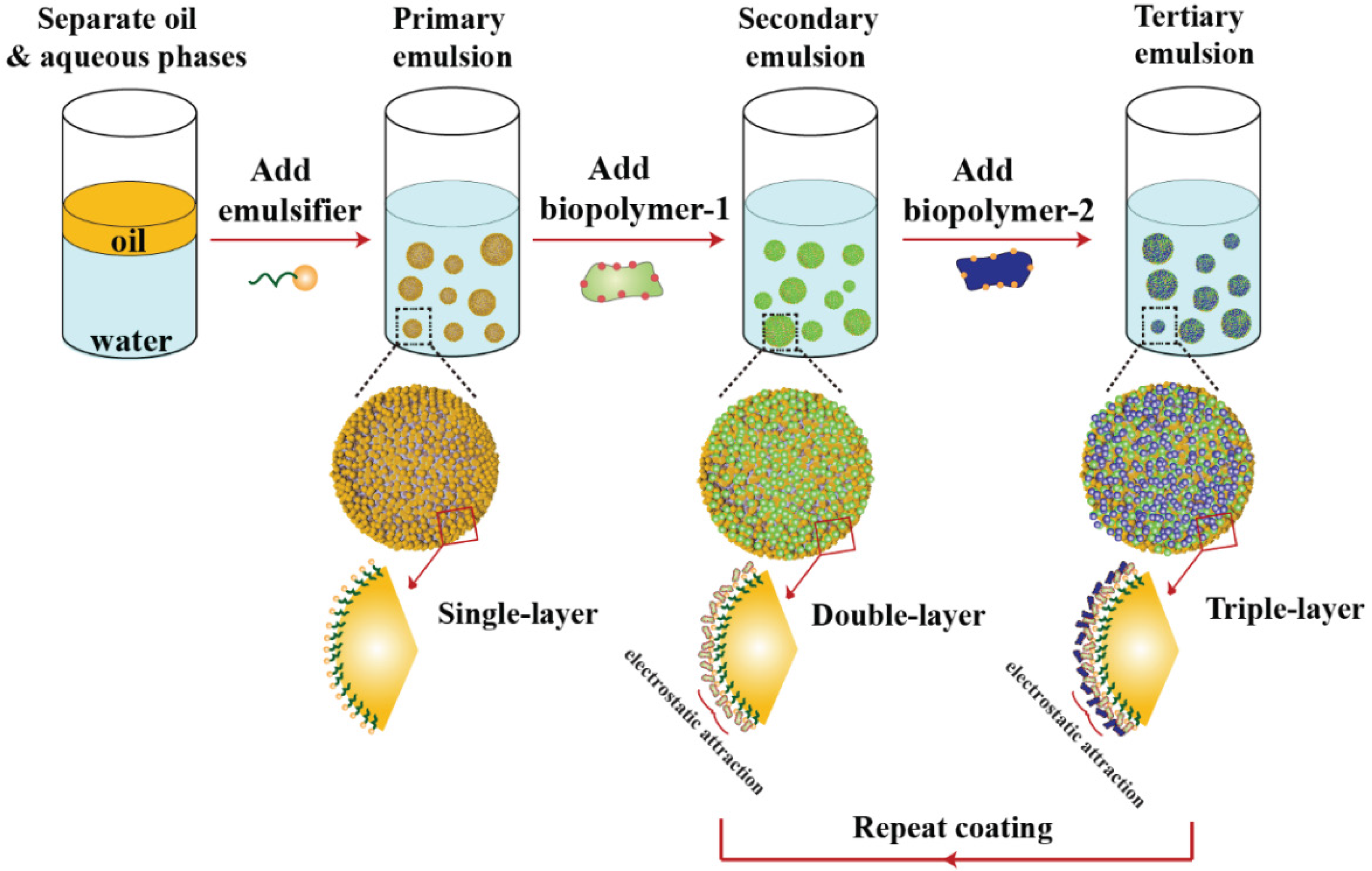
3.3. Droplet Charge and other Interfacial Properties: Surfactant Exchange and Coating Methods
3.4. Disperse Phase Composition: Compositional Ripening Methods
3.5. Physical State: Controlling Temperature or Solvent Quality
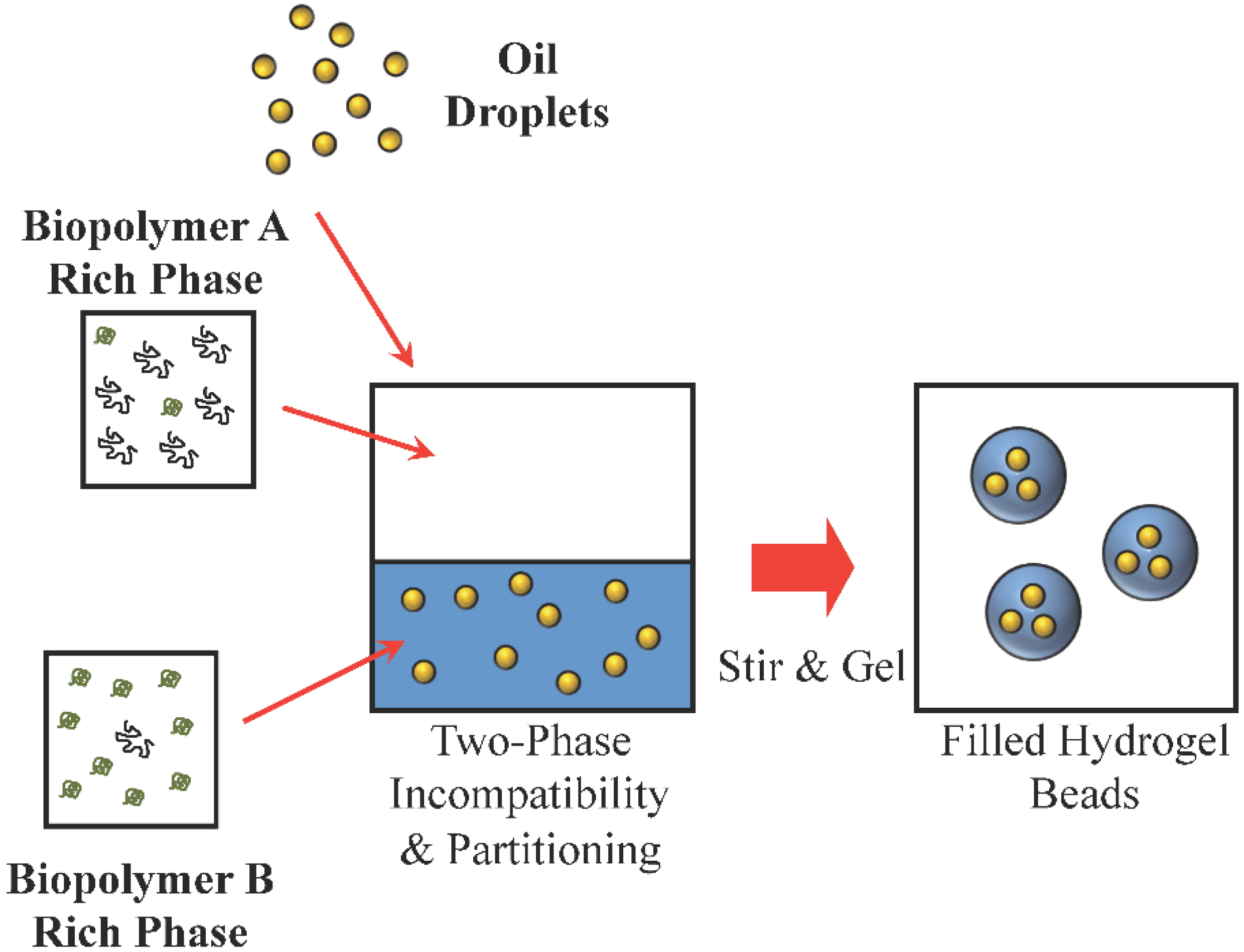
3.6. Environment: Embedding Methods
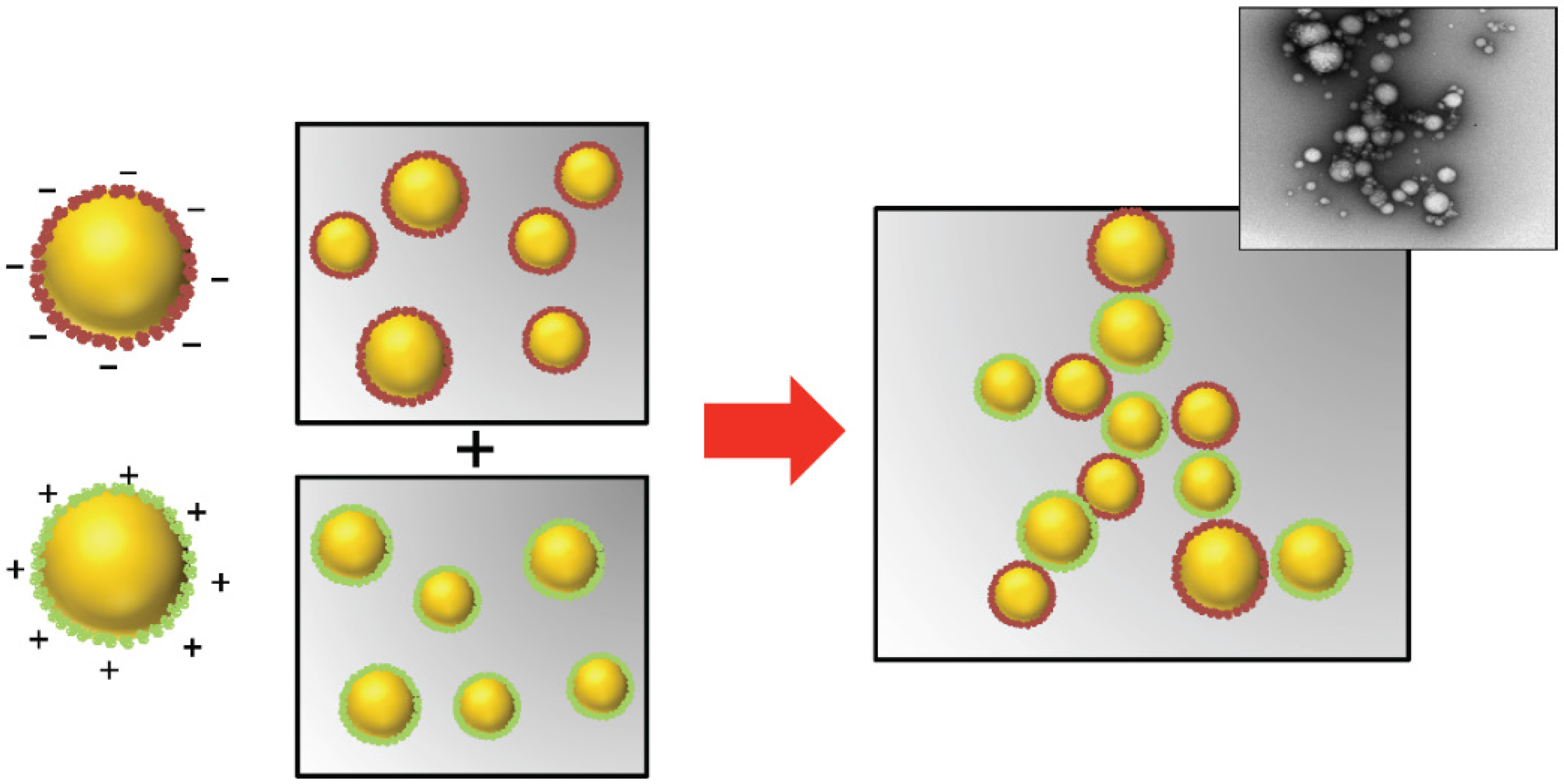
3.7. Droplet Spatial Distribution: Controlled Droplet Aggregation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Donsi, F.; Sessa, M.; Mediouni, H.; Mgaidi, A.; Ferrari, G. Encapsulation of bioactive compounds in nanoemulsion-based delivery systems. In Proceedings of the 11th International Congress on Engineering and Food, Athens, Greece, 22–26 May 2011; Saravacos, G., Taoukis, P., Krokida, M., Karathanos, V., Lazarides, H., Stoforos, N., Tzia, C., Yanniotis, S., Eds.; Volume 1, pp. 1666–1671.
- McClements, D.J. Crystals and crystallization in oil-in-water emulsions: Implications for emulsion-based delivery systems. Adv. Colloid Interface Sci. 2012, 174, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Nanoemulsions for food applications: Development and characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Velikov, K.P.; Pelan, E. Colloidal delivery systems for micronutrients and nutraceuticals. Soft Matter 2008, 4, 1964–1980. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Maindarkar, S.N.; Hoogland, H.; Henson, M.A. Predicting the combined effects of oil and surfactant concentrations on the drop size distributions of homogenized emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 467, 18–30. [Google Scholar] [CrossRef]
- Raikar, N.B.; Bhatia, S.R.; Malone, M.F.; Henson, M.A. Experimental studies and population balance equation models for breakage prediction of emulsion drop size distributions. Chem. Eng. Sci. 2009, 64, 2433–2447. [Google Scholar] [CrossRef]
- Hakansson, A.; Innings, F.; Tragardh, C.; Bergenstahl, B. A high-pressure homogenization emulsification model-improved emulsifier transport and hydrodynamic coupling. Chem. Eng. Sci. 2013, 91, 44–53. [Google Scholar] [CrossRef]
- Galooyak, S.S.; Dabir, B.; Zolfaghari, M. An innovative numerical approach for simulation of emulsion formation in a microfluidizer. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 487, 169–179. [Google Scholar] [CrossRef]
- Bai, L.; McClements, D.J. Development of microfluidization methods for efficient production of concentrated nanoemulsions: Comparison of single- and dual-channel microfluidizers. J. Colloid Interface Sci. 2016, 466, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R. Rheology of emulsions. Adv. Colloid Interface Sci. 2009, 151, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Le Reverend, B.J.D.; Norton, I.T.; Cox, P.W.; Spyropoulos, F. Colloidal aspects of eating. Curr. Opin. Colloid Interface Sci. 2010, 15, 84–89. [Google Scholar] [CrossRef]
- McClements, D.J. Theoretical prediction of emulsion color. Adv. Colloid Interface Sci. 2002, 97, 63–89. [Google Scholar] [CrossRef]
- Robins, M.M. Emulsions—Creaming phenomena. Curr. Opin. Colloid Interface Sci. 2000, 5, 265–272. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, 3rd ed.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Pal, R. Rheology of high internal phase ratio emulsions. Food Hydrocoll. 2006, 20, 997–1005. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Sek, J.; Jozwiak, B. Application of the continuity theory for the prediction of creaming phenomena in emulsions. J. Dispers. Sci. Technol. 2015, 36, 991–999. [Google Scholar] [CrossRef]
- Rahn-Chique, K.; Urbina-Villalba, G. Use of the lifshitz-slyosov-wagner theory for the prediction of the drop size of an emulsion subject to flocculation and coalescence. Interciencia 2015, 40, 847–853. [Google Scholar]
- Van Aken, G.A.; Vingerhoeds, M.H.; de Wijk, R.A. Textural perception of liquid emulsions: Role of oil content, oil viscosity and emulsion viscosity. Food Hydrocoll. 2011, 25, 789–796. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Dan, N. Transport and release in nano-carriers for food applications. J. Food Eng. 2016, 175, 136–144. [Google Scholar] [CrossRef]
- Salcedo-Sandoval, L.; Cofrades, S.; Ruiz-Capillas, C.; Matalanis, A.; McClements, D.J.; Decker, E.A.; Jimenez-Colmenero, F. Oxidative stability of n-3 fatty acids encapsulated in filled hydrogel particles and of pork meat systems containing them. Food Chem. 2015, 184, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; McClements, D.J. Influence of interfacial characteristics on ostwald ripening in hydrocarbon oil-in-water emulsions. Langmuir 2006, 22, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Ropers, M.H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Boon, C.S.; Xu, Z.; Yue, X.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors affecting lycopene oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on beta-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.C.; Perrechil, F.A.; Cunha, R.L. High- and low-energy emulsifications for food applications: A focus on process parameters. Food Eng. Rev. 2013, 5, 107–122. [Google Scholar] [CrossRef]
- Helgason, T.; Salminen, H.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Formation of transparent solid lipid nanoparticles by microfluidization: Influence of lipid physical state on appearance. J. Colloid Interface Sci. 2015, 448, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Schafer-Korting, M.; Gohla, S. Vitamin a-loaded solid lipid nanoparticles for topical use drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Drusch, S.; Serfert, Y.; Scampicchio, M.; Schmidt-Hansberg, B.; Schwarz, K. Impact of physicochemical characteristics on the oxidative stability of fish oil microencapsulated by spray-drying. J. Agric. Food Chem. 2007, 55, 11044–11051. [Google Scholar] [CrossRef] [PubMed]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, S.J.; Li, Y.; Decker, E.A.; McClements, D.J. Protein-stabilized nanoemulsions and emulsions: Comparison of physicochemical stability, lipid oxidation, and lipase digestibility. J. Agric. Food Chem. 2011, 59, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; McClements, D.J. Fabrication of protein-stabilized nanoemulsions using a combined homogenization and amphiphilic solvent dissolution/evaporation approach. Food Hydrocoll. 2010, 24, 560–569. [Google Scholar] [CrossRef]
- Troncoso, E.; Aguilera, J.M.; McClements, D.J. Influence of particle size on the in vitro digestibility of protein-coated lipid nanoparticles. J. Colloid Interface Sci. 2012, 382, 110–116. [Google Scholar] [PubMed]
- Kabalnov, A.S.; Shchukin, E.D. Ostwald ripening theory—Applications to fluorocarbon emulsion stability. Adv. Colloid Interface Sci. 1992, 38, 69–97. [Google Scholar]
- Kabalnov, A.S.; Pertzov, A.V.; Shchukin, E.D. Ostwald ripening in 2-component disperse phase systems—Application to emulsion stability. Colloids Surfaces 1987, 24, 19–32. [Google Scholar] [CrossRef]
- Pena, A.A.; Miller, C.A. Solubilization rates of oils in surfactant solutions and their relationship to mass transport in emulsions. Adv. Colloid Interface Sci. 2006, 123, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.Y.; McClements, D.J.; Decker, E.A. Lipid oxidation in emulsions as affected by charge status of antioxidants and emulsion droplets. J. Agric. Food Chem. 1999, 47, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Razumovsky, L.; Damodaran, S. Incompatibility of mixing of proteins in adsorbed binary protein films at the air-water interface. J. Agric. Food Chem. 2001, 49, 3080–3086. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.J.; McClements, D.J. Stabilization of phase inversion temperature nanoemulsions by surfactant displacement. J. Agric. Food Chem. 2010, 58, 7059–7066. [Google Scholar] [CrossRef] [PubMed]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Stabilization of vitamin e-enriched nanoemulsions: Influence of post-homogenization cosurfactant addition. J. Agric. Food Chem. 2014, 62, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Ziani, K.; Chang, Y.H.; McLandsborough, L.; McClements, D.J. Influence of surfactant charge on antimicrobial efficacy of surfactant-stabilized thyme oil nanoemulsions. J. Agric. Food Chem. 2011, 59, 6247–6255. [Google Scholar] [CrossRef] [PubMed]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128, 227–248. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Design of nano-laminated coatings to control bioavailability of lipophilic food components. J. Food Sci. 2010, 75, R30–R42. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Thongkaew, C.; Weiss, J. Theoretical and practical considerations in electrostatic depositioning of charged polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Formation of colloidosomes by adsorption of small charged oil droplets onto the surface of large oppositely charged oil droplets. Food Hydrocoll. 2007, 21, 516–526. [Google Scholar] [CrossRef]
- McClements, D.J.; Dungan, S.R. Factors that affect the rate of oil exchange between oil-in-water emulsion droplets stabilized by a nonionic surfactant—Droplet size, surfactant concentration, and ionic-strength. J. Phys. Chem. 1993, 97, 7304–7308. [Google Scholar] [CrossRef]
- McClements, D.J.; Dungan, S.R.; German, J.B.; Kinsella, J.E. Oil exchange between oil-in-water emulsion droplets stabilized with a nonionic surfactant. Food Hydrocoll. 1992, 6, 415–422. [Google Scholar] [CrossRef]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Clausse, D. Thermal behaviour of emulsions studied by differential scanning calorimetry. J. Therm. Anal. Calorim. 1998, 51, 191–201. [Google Scholar] [CrossRef]
- Coupland, J.N. Crystallization in emulsions. Curr. Opin. Colloid Interface Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Denkov, N.; Tcholakova, S.; Lesov, I.; Cholakova, D.; Smoukov, S.K. Self-shaping of oil droplets via the formation of intermediate rotator phases upon cooling. Nature 2015, 528, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Influence of polymorphic transformations on gelation of tripalmitin solid lipid nanoparticle suspensions. J. Am. Oil Chem. Soc. 2008, 85, 501–511. [Google Scholar] [CrossRef]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, R.J.; Chen, L.; Tong, Q.Y.; McClements, D.J. Designing hydrogel particles for controlled or targeted release of lipophilic bioactive agents in the gastrointestinal tract. Eur. Polym. J. 2015, 72, 698–716. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; Du, Y.; Xiao, H.; McClements, D.J. Control of lipase digestibility of emulsified lipids by encapsulation within calcium alginate beads. Food Hydrocoll. 2011, 25, 122–130. [Google Scholar] [CrossRef]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Retention and release of oil-in-water emulsions from filled hydrogel beads composed of calcium alginate: Impact of emulsifier type and ph. Soft Matter 2015, 11, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Tello, F.; Falfan-Cortes, R.N.; Martinez-Bustos, F.; da Silva, V.M.; Hubinger, M.D.; Grosso, C. Alginate and pectin-based particles coated with globular proteins: Production, characterization and anti-oxidative properties. Food Hydrocoll. 2015, 43, 670–678. [Google Scholar] [CrossRef]
- Matalanis, A.; Decker, E.A.; McClements, D.J. Inhibition of lipid oxidation by encapsulation of emulsion droplets within hydrogel microspheres. Food Chem. 2012, 132, 766–772. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Decker, E.A.; McClements, D.J. Encapsulation, protection, and release of polyunsaturated lipids using biopolymer-based hydrogel particles. Food Res. Int. 2014, 64, 520–526. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J.; Park, Y.; McClements, D.J. Modulation of lipid digestibility using structured emulsion-based delivery systems: Comparison of in vivo and in vitro measurements. Food Funct. 2012, 3, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Thakur, G.; Naqvi, M.A.; Rousseau, D.; Pal, K.; Mitra, A.; Basak, A. Gelatin-based emulsion gels for diffusion-controlled release applications. J. Biomater. Sci. Polym. Ed. 2012, 23, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; McClements, D.J. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends Food Sci. Technol. 2013, 34, 109–123. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.M.; Velikov, K.P. Sodium caseinate stabilized zein colloidal particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- De Paz, E.; Martin, A.; Estrella, A.; Rodriguez-Rojo, S.; Matias, A.A.; Duarte, C.M.M.; Cocero, M.J. Formulation of beta-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant. Food Hydrocoll. 2012, 26, 17–27. [Google Scholar] [CrossRef]
- De Paz, E.; Martin, A.; Bartolome, A.; Largo, M.; Cocero, M.J. Development of water-soluble beta-carotene formulations by high-temperature, high-pressure emulsification and antisolvent precipitation. Food Hydrocoll. 2014, 37, 14–24. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, R.J.; Tong, Q.Y.; Decker, E.A.; McClements, D.J. Food-grade filled hydrogels for oral delivery of lipophilic active ingredients: Temperature-triggered release microgels. Food Res. Int. 2015, 69, 274–280. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, R.J.; Decker, E.A.; McClements, D.J. Development of food-grade filled hydrogels for oral delivery of lipophilic active ingredients: Ph-triggered release. Food Hydrocoll. 2015, 44, 345–352. [Google Scholar] [CrossRef]
- Piacentini, E.; Giorno, L.; Dragosavac, M.M.; Vladisavljevic, G.T.; Holdich, R.G. Microencapsulation of oil droplets using cold water fish gelatine/gum arabic complex coacervation by membrane emulsification. Food Res. Int. 2013, 53, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Fustier, P.; Achouri, A.; Taherian, A.R.; Britten, M.; Pelletier, M.; Sabik, H.; Villeneuve, S.; Mondor, M. Protein-protein multilayer oil-in-water emulsions for the microencapsulation of flaxseed oil: Effect of whey and fish gelatin concentration. J. Agric. Food Chem. 2015, 63, 9239–9250. [Google Scholar] [CrossRef] [PubMed]
- Matalanis, A.; Lesmes, U.; Decker, E.A.; McClements, D.J. Fabrication and characterization of filled hydrogel particles based on sequential segregative and aggregative biopolymer phase separation. Food Hydrocoll. 2010, 24, 689–701. [Google Scholar] [CrossRef]
- Matalanis, A.; McClements, D.J. Impact of encapsulation within hydrogel microspheres on lipid digestion: An in vitro study. Food Biophys. 2012, 7, 145–154. [Google Scholar] [CrossRef]
- Firoozmand, H.; Rousseau, D. Microstructure and elastic modulus of phase-separated gelatin-starch hydrogels containing dispersed oil droplets. Food Hydrocoll. 2013, 30, 333–342. [Google Scholar] [CrossRef]
- Dickinson, E. Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf. B Biointerfaces 2010, 81, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.Y.; McClements, D.J. Fabrication of functional micro-clusters by heteroaggregation of oppositely charged protein-coated lipid droplets. Food Hydrocoll. 2012, 27, 80–90. [Google Scholar] [CrossRef]
- Mao, Y.Y.; McClements, D.J. Modulation of food texture using controlled heteroaggregation of lipid droplets: Principles and applications. J. Appl. Polym. Sci. 2013, 130, 3833–3841. [Google Scholar] [CrossRef]
- Mao, Y.Y.; McClements, D.J. Modification of emulsion properties by heteroaggregation of oppositely charged starch-coated and protein-coated fat droplets. Food Hydrocoll. 2013, 33, 320–326. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; McClements, D.J. Extending Emulsion Functionality: Post-Homogenization Modification of Droplet Properties. Processes 2016, 4, 17. https://doi.org/10.3390/pr4020017
Bai L, McClements DJ. Extending Emulsion Functionality: Post-Homogenization Modification of Droplet Properties. Processes. 2016; 4(2):17. https://doi.org/10.3390/pr4020017
Chicago/Turabian StyleBai, Long, and David Julian McClements. 2016. "Extending Emulsion Functionality: Post-Homogenization Modification of Droplet Properties" Processes 4, no. 2: 17. https://doi.org/10.3390/pr4020017