The Conversion of Pistachio and Walnut Shell Waste into Valuable Components with Subcritical Water
Abstract
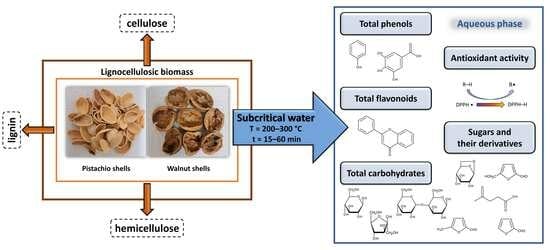
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The Characterization of Pistachio and Walnut Shells with TGA/DSC and Elemental Analysis
2.2.2. Biomass Waste Treatment with Subcritical Water
2.2.3. Conventional Extraction
2.2.4. The Determination of Antioxidant Activity of Dry Extract
2.2.5. The Determination of Total Phenolic Content in Dry Extract
2.2.6. The Determination of Total Flavonoids in Dry Extract
2.2.7. The Determination of Total Carbohydrates in Dry Extract
2.2.8. HPLC Analysis
2.2.9. The Assessment of the Energy Costs of the Laboratory Process
3. Results and Discussion
3.1. The Elemental Analysis of Raw Material
3.2. The TGA/DSC Properties of Raw Material
3.3. Extraction Yield
3.4. The Antioxidant Activity of Extracts
3.5. Total Phenols Content
3.6. Total Flavonoids Content
3.7. Total Carbohydrates Content
3.8. Sugars and Derivatives
3.9. The Electricity Costs of the Chemical Conversion of Waste Biomass into Valuable Products in Subcritical Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of Bioactive Compounds from Hazelnuts and Walnuts Shells: Quantitative-Qualitative Analysis and Chromatographic Purification. Biomolecules 2020, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Pistachios: Global Production 2022/23. Available online: https://www.statista.com/statistics/933073/pistachio-global-production/ (accessed on 18 August 2023).
- Walnut Production Worldwide 2022/23. Available online: https://www.statista.com/statistics/675967/walnut-production-worldwide/ (accessed on 18 August 2023).
- Han, H.; Wang, S.; Rakita, M.; Wang, Y.; Han, Q.; Xu, Q. Effect of Ultrasound-Assisted Extraction of Phenolic Compounds on the Characteristics of Walnut Shells. Food Nutr. Sci. 2018, 9, 1034–1045. [Google Scholar] [CrossRef]
- Yang, W.; Shimizu, I.; Ono, T.; Kimura, Y. Preparation of Biodegradable Foam from Walnut Shells Treated by Subcritical Water. J. Chem. Technol. Biotechnol. 2015, 90, 44–49. [Google Scholar] [CrossRef]
- Robles, E.; Izaguirre, N.; Martin, A.; Moschou, D.; Labidi, J. Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making. Molecules 2021, 26, 1371. [Google Scholar] [CrossRef]
- Marett, J.; Aning, A.; Foster, E.J. The Isolation of Cellulose Nanocrystals from Pistachio Shells via Acid Hydrolysis. Ind. Crops Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Wartelle, L.H.; Marshall, W.E.; Toles, C.A.; Johns, M.M. Comparison of Nutshell Granular Activated Carbons to Commercial Adsorbents for the Purge-and-Trap Gas Chromatographic Analysis of Volatile Organic Compounds. J. Chromatogr. A 2000, 879, 169–175. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Ghaemi, A. Evaluation of Pistachio Shells as Solid Wastes to Produce Activated Carbon for CO2 Capture: Isotherm, Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Modeling. Curr. Res. Green Sustain. Chem. 2022, 5, 100342. [Google Scholar] [CrossRef]
- Harini, K.; Chandra Mohan, C. Isolation and Characterization of Micro and Nanocrystalline Cellulose Fibers from the Walnut Shell, Corncob and Sugarcane Bagasse. Int. J. Biol. Macromol. 2020, 163, 1375–1383. [Google Scholar] [CrossRef]
- Barczewski, M.; Sałasińska, K.; Szulc, J. Application of Sunflower Husk, Hazelnut Shell and Walnut Shell as Waste Agricultural Fillers for Epoxy-Based Composites: A Study into Mechanical Behavior Related to Structural and Rheological Properties. Polym. Test. 2019, 75, 1–11. [Google Scholar] [CrossRef]
- Thiagarajan, A.; Velmurugan, K.; Sangeeth, P.P. Synthesis and Mechanical Properties of Pistachio Shell Filler on Glass Fiber Polymer Composites by VARIM Process. Mater. Today Proc. 2021, 39, 610–614. [Google Scholar] [CrossRef]
- Aydin, H.; Baysal, G.; Bulut, Y. Utilization of Walnut Shells (Juglans regia) as an Adsorbent for the Removal of Acid Dyes. Desalination Water Treat. 2009, 2, 141–150. [Google Scholar] [CrossRef]
- Şentürk, İ.; Alzein, M. Adsorptive Removal of Basic Blue 41 Using Pistachio Shell Adsorbent—Performance in Batch and Column System. Sustain. Chem. Pharm. 2020, 16, 100254. [Google Scholar] [CrossRef]
- Kali, A.; Amar, A.; Loulidi, I.; Jabri, M.; Hadey, C.; Lgaz, H.; Alrashdi, A.A.; Boukhlifi, F. Characterization and Adsorption Capacity of Four Low-Cost Adsorbents Based on Coconut, Almond, Walnut, and Peanut Shells for Copper Removal. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Ahmad, R. Pistachio Shell Carbon (PSC)—An Agricultural Adsorbent for the Removal of Pb(II) from Aqueous Solution. Groundw. Sustain. Dev. 2017, 4, 42–48. [Google Scholar] [CrossRef]
- Pavlovič, I.; Knez, Ž.; Škerget, M. Hydrothermal Reactions of Agricultural and Food Processing Wastes in Sub- and Supercritical Water: A Review of Fundamentals, Mechanisms, and State of Research. J. Agric. Food Chem. 2013, 61, 8003–8025. [Google Scholar] [CrossRef] [PubMed]
- Knez, Ž.; Hrnčič, M.K.; Čolnik, M.; Škerget, M. Chemicals and Value Added Compounds from Biomass Using Sub- and Supercritical Water. J. Supercrit. Fluids 2018, 133, 591–602. [Google Scholar] [CrossRef]
- Jalili, A.; Heydari, R.; Sadeghzade, A.; Alipour, S. Reducing Power and Radical Scavenging Activities of Phenolic Extracts from Juglans regia Hulls and Shells. Afr. J. Biotechnol. 2012, 11, 9040–9047. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical Water Extraction of Phenolic and Antioxidant Constituents from Pistachio (Pistacia vera L.) Hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Mathew, M.; Singh, A.; Simha, P. Influence of Microwave and Ultrasound Pretreatment on Solvent Extraction of Bio-Components from Walnut (Julgans regia L.) Shells. Period. Polytech. Chem. Eng. 2016, 60, 40–48. [Google Scholar] [CrossRef]
- Ahorsu, R.; Cintorrino, G.; Medina, F.; Constantí, M. Microwave Processes: A Viable Technology for Obtaining Xylose from Walnut Shell to Produce Lactic Acid by Bacillus Coagulans. J. Clean. Prod. 2019, 231, 1171–1181. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent Advances in the Extraction of Bioactive Compounds with Subcritical Water: A Review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Zakaria, S.; Mustapa Kamal, S. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2015, 8, 23–34. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Akiya, N.; Savage, P.E. Roles of Water for Chemical Reactions in High-Temperature Water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef] [PubMed]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Ravber, M.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Fats, Carbohydrates and Proteins in Sunflower Seeds after Treatment with Subcritical Water. Chem. Biochem. Eng. Q. 2015, 29, 351–355. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal Liquefaction of Biomass: A Review of Subcritical Water Technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M. Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-662-43627-1. [Google Scholar]
- Cravotto, C.; Grillo, G.; Binello, A.; Gallina, L.; Olivares-Vicente, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E.; Cravotto, G. Bioactive Antioxidant Compounds from Chestnut Peels through Semi-Industrial Subcritical Water Extraction. Antioxidants 2022, 11, 988. [Google Scholar] [CrossRef]
- Shitu, A.; Izhar, S.; Tahir, T.M. Sub-Critical Water as a Green Solvent for Production of Valuable Materials from Agricultural Waste Biomass: A Review of Recent Work. Glob. J. Environ. Sci. Manag. 2015, 1, 255–264. [Google Scholar] [CrossRef]
- Kapoor, S.; Sachdev, P. Drying Method Affects Bioactive Compounds and Antioxidant Activity of Carrot. Int. J. Veg. Sci. 2014, 21, 141217142840007. [Google Scholar] [CrossRef]
- Jokić, S.; Gagić, T.; Knez, Ž.; Šubarić, D.; Škerget, M. Separation of Active Compounds from Food By-Product (Cocoa Shell) Using Subcritical Water Extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the Extraction of Phenolic Antioxidants from Chestnut Shells by Subcritical Water Extraction Using Response Surface Methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef] [PubMed]
- Gagić, T.; Knez, Ž.; Škerget, M. Subcritical Water Extraction of Horse Chestnut (Aesculus hippocastanum) Tree Parts: Scientific Paper. J. Serbian Chem. Soc. 2021, 86, 603–613. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Xiao, Z.; Zhou, R.; Zhu, Y.; Wan, X. Kinetics of Peanut Shell Pyrolysis and Hydrolysis in Subcritical Water. J. Mater. Cycles Waste Manag. 2013, 16, 546–556. [Google Scholar] [CrossRef]
- Gur, C.S.; Dunford, N.T.; Gumus, Z.P. Cytotoxicity of Subcritical Water Extracts Obtained from Byproducts Generated at Commercial Pecan Shelling Operations on Cancer Cells. Bioresour. Bioprocess. 2023, 10, 47. [Google Scholar] [CrossRef]
- Liu, A.; Park, Y.; Huang, Z.; Wang, B.; Ankumah, R.O.; Biswas, P.K. Product Identification and Distribution from Hydrothermal Conversion of Walnut Shells. Energy Fuels 2006, 20, 446–454. [Google Scholar] [CrossRef]
- Safari, F.; Salimi, M.; Tavasoli, A.; Ataei, A. Non-Catalytic Conversion of Wheat Straw, Walnut Shell and Almond Shell into Hydrogen Rich Gas in Supercritical Water Media. Chin. J. Chem. Eng. 2016, 24, 1097–1103. [Google Scholar] [CrossRef]
- Safari, F.; Tavasoli, A.; Ataei, A. Gasification of Iranian Walnut Shell as a Bio-Renewable Resource for Hydrogen-Rich Gas Production Using Supercritical Water Technology. Int. J. Ind. Chem. 2017, 8, 29–36. [Google Scholar] [CrossRef]
- Demirbas, A. Hydrogen-Rich Gas from Fruit Shells via Supercritical Water Extraction. Int. J. Hydrogen Energy 2004, 29, 1237–1243. [Google Scholar] [CrossRef]
- Čolnik, M.; Knez, Ž.; Škerget, M. Sub- and Supercritical Water for Chemical Recycling of Polyethylene Terephthalate Waste. Chem. Eng. Sci. 2021, 233, 116389. [Google Scholar] [CrossRef]
- Čolnik, M.; Kotnik, P.; Knez, Ž.; Škerget, M. Hydrothermal Decomposition of Polyethylene Waste to Hydrocarbons Rich Oil. J. Supercrit. Fluids 2021, 169, 105136. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Ravber, M.; Volf, I.; Knez, Ž.; Popa, V.I. Isolation of Bioactive Compounds from Spruce Bark Waste Using Sub- and Supercritical Fluids. J. Supercrit. Fluids 2016, 117, 243–251. [Google Scholar] [CrossRef]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Treatment of Sugars to Obtain High-Value Products. J. Serbian Chem. Soc. 2020, 85, 97–109. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; El Hajjaji, S.; Szabó, T.; Trif, L.; Felhősi, I.; Ab, K.; Labjar, N.; Hermouche, L.; Shaban, A. Biomass Valorization of Walnut Shell into Biochar as a Resource for Electrochemical Simultaneous Detection of Heavy Metal Ions in Water and Soil Samples: Preparation, Characterization, and Applications. Arab. J. Chem. 2022, 15, 104252. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kaya, M.; Şahin, Ö.; Saka, C. Preparation and TG/DTG, FT-IR, SEM, BET Surface Area, Iodine Number and Methylene Blue Number Analysis of Activated Carbon from Pistachio Shells by Chemical Activation. Int. J. Chem. React. Eng. 2018, 16, 20170060. [Google Scholar] [CrossRef]
- Açıkalın, K. Thermogravimetric Analysis of Walnut Shell as Pyrolysis Feedstock. J. Therm. Anal. Calorim. 2011, 105, 145–150. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Hussain, S.Z.; Onyeaka, H.; Adeyanju, A.A.; Nwonuma, C.O.; Bashir, A.A.; Farooq, A.; Zhou, C.; Shittu, T.D. Lignin Polyphenol: From Biomass to Innovative Food Applications, and Influence on Gut Microflora. Ind. Crops Prod. 2023, 206, 117696. [Google Scholar] [CrossRef]
- Gagić, T.; Knez, Ž.; Škerget, M. Hydrothermal Hydrolysis of Sweet Chestnut (Castanea sativa) Tannins. J. Serbian Chem. Soc. 2019, 85, 108. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the Radical Scavenging Activity of Lignins—Natural Antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Xie, Y.; Zhuang, X.; Chen, H.; Qin, Y.; Cao, J.; Lan, T. Effect of High Antioxidant Activity on Bacteriostasis of Lignin from Sugarcane Bagasse. Biochem. Eng. J. 2022, 180, 108335. [Google Scholar] [CrossRef]
- Meng, W.; Shi, J.; Zhang, X.; Lian, H.; Wang, Q.; Peng, Y. Effects of Peanut Shell and Skin Extracts on the Antioxidant Ability, Physical and Structure Properties of Starch-Chitosan Active Packaging Films. Int. J. Biol. Macromol. 2020, 152, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Gani, A.; Ahmad, M.; Shah, A.; Masoodi, F.A.; Shah, Y.; Gani, A. Antioxidant and Antiproliferative Activity of Walnut Extract (Juglans regia L.) Processed by Different Methods and Identification of Compounds Using GC/MS and LC/MS Technique. J. Food Process. Preserv. 2017, 41, e12756. [Google Scholar] [CrossRef]
- Paysepar, H.; Venkateswara Rao, K.T.; Yuan, Z.; Shui, H.; Xu, C. Production of Phenolic Chemicals from Hydrolysis Lignin via Catalytic Fast Pyrolysis. J. Anal. Appl. Pyrolysis 2020, 149, 104842. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C.; Zhao, S.; Ge, F.; Liu, D. Effect of Solvents on the Antioxidant Activity of Walnut (Juglans regia L.) Shell Extracts. J. Food Nutr. Res. 2014, 2, 621–626. [Google Scholar] [CrossRef]
- Sultanova, M.; Dalabayev, A.; Saduakas, A.; Nurysh, A.; Akzhanov, N.; Yakiyayeva, M. The Potential of Non-Traditional Walnut Shells Waste for the Production of Antioxidant Reach Extracts Intended for the Food Industry. Potravinarstvo Slovak J. Food Sci. 2023, 17, 391–404. [Google Scholar] [CrossRef]
- Gagić, T.; Perva-Uzunalić, A.; Knez, Ž.; Škerget, M. Hydrothermal Degradation of Cellulose at Temperature from 200 to 300 °C. Ind. Eng. Chem. Res. 2018, 57, 6576–6584. [Google Scholar] [CrossRef]
- Mok, W.S.L.; Antal, M.J., Jr. Uncatalyzed Solvolysis of Whole Biomass Hemicellulose by Hot Compressed Liquid Water. Ind. Eng. Chem. Res. 1992, 31, 1157–1161. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of Degradation Compounds from Lignocellulosic Biomass in the Biorefinery: Sugar Reaction Mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef] [PubMed]







| Elemental Composition (wt.%) | |||||
|---|---|---|---|---|---|
| Biomass Waste | C | H | N | S | O 1 |
| Pistachio shells | 44.98 | 6.34 | 0.20 | 0.45 | 48.03 |
| Walnut shells | 47.72 | 6.41 | 0.56 | 0.30 | 45.01 |
| Conditions | Cellobiose | Glucose | Fructose | Lactose | 1,6-AG * | 5-HMF * | F * | MF * | LA * |
|---|---|---|---|---|---|---|---|---|---|
| Walnut shells | |||||||||
| 150 °C, 60 min | 11.21 | 8.69 | 12.75 | / | / | 2.15 | 9.32 | 0.74 | 3.15 |
| 200 °C, 15 min | 83.39 | 10.32 | 92.08 | / | / | 4.28 | 36.90 | 2.49 | 1.85 |
| 200 °C, 30 min | 14.34 | 24.13 | 86.62 | / | 4.18 | 8.69 | 42.18 | 5.14 | 5.40 |
| 200 °C, 60 min | 2.76 | 25.76 | 60.39 | / | 5.32 | 12.07 | 59.02 | 8.11 | 15.15 |
| 250 °C, 15 min | 13.66 | 29.9 | 117.10 | / | 8.1 | 19.99 | 30.62 | 5.28 | 22.25 |
| 250 °C, 30 min | 3.69 | 44.03 | 94.5 | / | 37.68 | 18.7 | 11.6 | 7.46 | 29.15 |
| 250 °C, 60 min | 3.7 | 51.3 | 54.16 | / | 52.11 | 16.9 | 9.5 | 2.95 | 37.33 |
| 300 °C, 15 min | / | 14.94 | 7.1 | / | 55.01 | 5.6 | 5.4 | 1.79 | 53.61 |
| 300 °C, 30 min | / | 6.09 | 3.04 | / | 13.6 | 2.68 | 1.25 | 1.74 | 40.54 |
| 300 °C, 60 min | / | 1.18 | 1.9 | / | 5.9 | 1.15 | 0.99 | 1.96 | 56.39 |
| ACE | / | / | 1.52 | / | / | / | / | / | / |
| ACE/H2O | / | 5.6 | 11.84 | 1.61 | / | / | / | / | / |
| EtOH | / | 2.8 | 3.6 | / | / | / | / | / | / |
| Pistachio shells | |||||||||
| 150 °C, 60 min | / | 0.39 | 49.30 | 0.18 | / | 1.17 | 6.13 | 0.08 | / |
| 200 °C, 15 min | 0.49 | / | 21.28 | 2.65 | / | 3.55 | 71.8 | 4.44 | 3.15 |
| 200 °C, 30 min | 1.06 | 1.72 | 42.12 | / | / | 10.25 | 167.6 | 3.85 | 9.31 |
| 200 °C, 60 min | 1.71 | 17.9 | 61.26 | / | 3.24 | 60.6 | 430.2 | 13.59 | 25.83 |
| 250 °C, 15 min | 0.48 | 10.21 | 4.45 | / | 4.24 | 101.4 | 167.8 | 6.03 | 48.17 |
| 250 °C, 30 min | / | 7.70 | 4.31 | / | 16.36 | 124.7 | 126.8 | 9.74 | 53.19 |
| 250 °C, 60 min | / | 5.71 | 2.13 | / | 66.07 | 47.7 | 67.7 | 8.89 | 56.1 |
| 300 °C, 15 min | / | / | / | / | 39.2 | 28.3 | 63.5 | 12.13 | 25.39 |
| 300 °C, 30 min | / | / | / | / | 12.9 | 6.7 | 57.6 | 6.92 | 48.63 |
| 300 °C, 60 min | / | / | / | / | 4.2 | 0.06 | 37.68 | 5.10 | 65.26 |
| ACE | / | 1.68 | 4.11 | / | / | / | / | / | / |
| ACE/H2O | / | 6.3 | 8.7 | / | / | / | / | / | / |
| EtOH | / | 2.37 | 3.22 | 2.3 | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čolnik, M.; Irgolič, M.; Perva, A.; Škerget, M. The Conversion of Pistachio and Walnut Shell Waste into Valuable Components with Subcritical Water. Processes 2024, 12, 195. https://doi.org/10.3390/pr12010195
Čolnik M, Irgolič M, Perva A, Škerget M. The Conversion of Pistachio and Walnut Shell Waste into Valuable Components with Subcritical Water. Processes. 2024; 12(1):195. https://doi.org/10.3390/pr12010195
Chicago/Turabian StyleČolnik, Maja, Mihael Irgolič, Amra Perva, and Mojca Škerget. 2024. "The Conversion of Pistachio and Walnut Shell Waste into Valuable Components with Subcritical Water" Processes 12, no. 1: 195. https://doi.org/10.3390/pr12010195