Graphene Oxide from Graphite of Spent Batteries as Support of Nanocatalysts for Fuel Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Obtaining and Processing of Raw Material
2.3. Synthesis of Graphene Oxide
2.4. Synthesis of Metallic Nanoparticles Decorated on GO (NPs-M/GO)
2.5. Characterization of Materials
2.6. Hydrogen Evolution from Borohydride
2.7. Reaction Parameters Evaluation
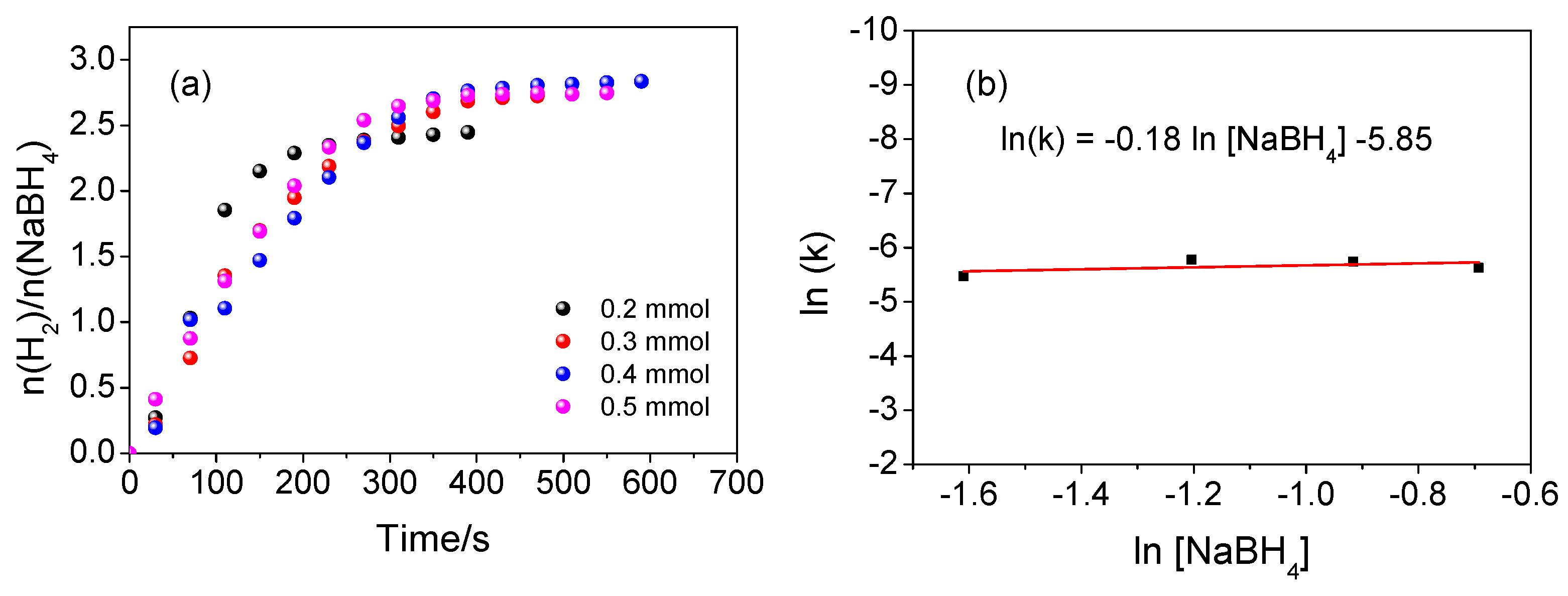
2.7.1. Evaluation of the Influence of NaBH4 Concentration
2.7.2. Evaluation of the Influence of Catalyst Dose
2.7.3. Temperature
2.7.4. NaOH Influence
2.7.5. Reuse of the Material
2.7.6. Activation Energy
3. Results and Discussion
3.1. Material Characterization
3.2. Hydrogen Evolution from NaBH4
3.3. Evaluation of the Influence of NaBH4 Concentration
3.4. Evaluation of the Influence of Catalyst Dose
3.5. Evaluation of NaOH Presence in the Hydrogen Evolution
3.6. Evaluation of Temperature in the Hydrogen Evolution
3.7. Reuse of the Material
3.8. Performance of Ni/Co Supported on GO Derived from Graphite in Zn-C Batteries and Analytical Grade
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karthik, P.E.; Rajan, H.; Jothi, V.R.; Sang, B.I.; Yi, S.C. Electronic Wastes: A near Inexhaustible and an Unimaginably Wealthy Resource for Water Splitting Electrocatalysts. J. Hazard. Mater. 2022, 421, 126687. [Google Scholar] [PubMed]
- Albright, G.; Edie AllCell Technologies, J.; Crossley, P.; Vassallo, A. Battery Storage for Renewables: Market Status and Technology Outlook; International Renewable Energy Agency: Masdar City, United Arab Emirates, 2015. [Google Scholar]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of Batteries: A Review of Current Processes and Technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Alcaraz, L.; García-Díaz, I.; González, L.; Rabanal, M.E.; Urbieta, A.; Fernández, P.; López, F.A. New Photocatalytic Materials Obtained from the Recycling of Alkaline and Zn/C Spent Batteries. J. Mater. Res. Technol. 2019, 8, 2809–2818. [Google Scholar] [CrossRef]
- Vieira Segundo, J.E.D.; Vilar, E.O. Grafeno: Uma Revisão Sobre Propriedades, Mecanismos de Produção e Potenciais Aplicações Em Sistemas Energéticos. Rev. Eletrônica De Mater. E Process. 2016, 11, 54–57. [Google Scholar]
- Yang, Q.; Cao, L.; Li, S.; Zeng, X.; Zhou, W.; Zhang, C. Upgrading Pomelo Peels into Laser-Induced Graphene for Multifunctional Sensors. J. Anal. Appl. Pyrolysis 2023, 173, 106074. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Q.; Cao, L.; Li, S.; Zeng, X.; Zhou, W.; Zhang, C. Synthesis and Application of Porous Carbon Nanomaterials from Pomelo Peels: A Review. Molecules 2023, 28, 4429. [Google Scholar] [CrossRef] [PubMed]
- Staudenmaier, L. Verfahren Zur Darstellung Der Graphitsäure. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hofmann, U.; Rudorff, W. The Formation of Salts from Graphite by Strong Acids. Trans. Faraday Soc. 1938, 45, 1017–1021. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Camargos, J.S.F.; Semmer, A.D.O.; Da Silva, S.N. Características e Aplicações do Grafeno e do Óxido de Grafeno e as Principais Rotas Para Síntese. J. Eng. Exact Sci. 2017, 3, 1118–1130. [Google Scholar] [CrossRef]
- Loudiki, A.; Matrouf, M.; Azriouil, M.; Laghrib, F.; Farahi, A.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Graphene Oxide Synthesized from Zinc-Carbon Battery Waste Using a New Oxidation Process Assisted Sonication: Electrochemical Properties. Mater. Chem. Phys. 2022, 275, 125308. [Google Scholar] [CrossRef]
- Joshi, D.J.; Koduru, J.R.; Malek, N.I.; Hussain, C.M.; Kailasa, S.K. Surface Modifications and Analytical Applications of Graphene Oxide: A Review. TrAC-Trends Anal. Chem. 2021, 144, 116448. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Guo, F.; Du, Y.; Chen, Y. Self-Assembly Sandwich-like Fe, Co, or Ni Nanoparticles/Reduced Graphene Oxide Composites with Excellent Microwave Absorption Performance. Appl. Surf. Sci. 2021, 562, 150212. [Google Scholar] [CrossRef]
- Dong, J.; Sun, T.; Zhang, Y.; Zhang, H.; Lu, S.; Hu, D.; Chen, J.; Xu, L. Mesoporous NiCo Alloy/Reduced Graphene Oxide Nanocomposites as Efficient Hydrogen Evolution Catalysts. J. Colloid Interface Sci. 2021, 599, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Grad, O.; Mihet, M.; Coros, M.; Dan, M.; Lazar, M.D.; Blanita, G. Reduced Graphene Oxide Modified with Noble Metal Nanoparticles for Formic Acid Dehydrogenation. Catal. Today 2021, 366, 41–47. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic Water Splitting Hydrogen Production via Environmental Benign Carbon Based Nanomaterials. Int. J. Hydrogen Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Jia, X.; Sang, Z.; Sun, L.; Xu, F.; Pan, H.; Zhang, C.; Cheng, R. Graphene-Modified Co-B-P Catalysts for Hydrogen Generation from Sodium Borohydride Hydrolysis. Nanomaterials 2022, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Rabia, M.; Hadia, N.M.A.; Farid, O.M.; Abdelazeez, A.A.A.; Mohamed, S.H.; Shaban, M. Poly(M-toluidine)/Rolled Graphene Oxide Nanocomposite Photocathode for Hydrogen Generation from Wastewater. Int. J. Energy Res. 2022, 46, 11943–11956. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Nickel- and Cobalt-Based Catalysts for Hydrogen Generation by Hydrolysis of Borohydride. J. Alloys Compd. 2006, 415, 288–293. [Google Scholar] [CrossRef]
- Darabdhara, G.; Amin, M.A.; Mersal, G.A.M.; Ahmed, E.M.; Das, M.R.; Zakaria, M.B.; Malgras, V.; Alshehri, S.M.; Yamauchi, Y.; Szunerits, S.; et al. Reduced Graphene Oxide Nanosheets Decorated with Au, Pd and Au-Pd Bimetallic Nanoparticles as Highly Efficient Catalysts for Electrochemical Hydrogen Generation. J. Mater. Chem. A 2015, 3, 20254–20266. [Google Scholar] [CrossRef]
- Sun, L.; Gao, X.; Ning, X.; Qiu, Z.; Xing, L.; Yang, H.; Li, D.; Dou, J.; Meng, Y. Cobalt-Nickel Bimetal Carbon Sphere Catalysts for Efficient Hydrolysis of Sodium Borohydride: The Role of Synergy and Confine Effect. Int. J. Hydrogen Energy 2023, 48, 3413–3428. [Google Scholar] [CrossRef]
- Chou, C.C.; Hsieh, C.H.; Chen, B.H. Hydrogen Generation from Catalytic Hydrolysis of Sodium Borohydride Using Bimetallic NieCo Nanoparticles on Reduced Graphene Oxide as Catalysts. Energy 2015, 90, 1973–1982. [Google Scholar] [CrossRef]
- Karaman, O. Three-Dimensional Graphene Network Supported Nickel-Cobalt Bimetallic Alloy Nanocatalyst for Hydrogen Production by Hydrolysis of Sodium Borohydride and Developing of an Artificial Neural Network Modeling to Forecast Hydrogen Production Rate. Chem. Eng. Res. Des. 2022, 181, 321–330. [Google Scholar] [CrossRef]
- Didehban, A.; Zabihi, M.; Babajani, N. Preparation of the Efficient Nano-Bimetallic Cobalt-Nickel Catalysts Supported on the Various Magnetic Substrates for Hydrogen Generation from Hydrolysis of Sodium Borohydride in Alkaline Solutions. Polyhedron 2020, 180, 114405. [Google Scholar] [CrossRef]
- Yue, C.; Yang, P.; Wang, J.; Zhao, X.; Wang, Y.; Yang, L. Facile Synthesis and Characterization of Nano-Pd Loaded NiCo Microfibers as Stable Catalysts for Hydrogen Generation from Sodium Borohydride. Chem. Phys. Lett. 2020, 743, 137170. [Google Scholar] [CrossRef]
- Su, S.; Chen, K.; Yang, X.; Dang, D. Coronavirus-like Core—Shell-Structured Co@C for Hydrogen Evolution via Hydrolysis of Sodium Borohydride. Molecules 2023, 28, 2–9. [Google Scholar] [CrossRef]
- Zhao, W.; Kido, G.; Hara, K.; Noguchi, H. Characterization of Neutralized Graphite Oxide and Its Use in Electric Double Layer Capacitors. J. Electroanal. Chem. 2014, 712, 185–193. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, X.; Jin, G.; Jiao, Y.; Li, D. Preparation, Deposited Behavior and Hydrophobic Property of Modified Graphene Oxide Reinforced Ni Composite Coatings by Magnetic Field Assisted Electro-Brush Plating. Surf. Coat. Technol. 2020, 403, 126363. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene Oxide and Reduced Graphene Oxide Studied by the XRD, TEM and Electron Spectroscopy Methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Saxena, S.; Tyson, T.A.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of Structural and Electronic Properties of Graphene Oxide. Appl. Phys. Lett. 2011, 99, 5–8. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Mohan, M.; Dhanyaprabha, K.C.; Thomas, H. NiCo2O4 Nanoparticles Anchored on Reduced Graphene Oxide with Enhanced Catalytic Activity towards the Reduction of P-Nitrophenol in Water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128717. [Google Scholar] [CrossRef]
- Xu, H.; Shi, Z.X.; Tong, Y.X.; Li, G.R. Porous Microrod Arrays Constructed by Carbon-Confined NiCo@NiCoO2 Core@Shell Nanoparticles as Efficient Electrocatalysts for Oxygen Evolution. Adv. Mater. 2018, 30, e1705442. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Cao, H.; Li, S.; Guo, L.; Wang, Y.; Yang, J. Flexible Nickel Cobalt Metal-Organic Frameworks/Reduced Graphene Oxide Hybrid Film for High-Performance Supercapacitors. J. Energy Storage 2022, 54, 105270. [Google Scholar] [CrossRef]
- Rattana, T.; Chaiyakun, S.; Witit-Anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and Characterization of Graphene Oxide Nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef]
- Naveen, A.N.; Selladurai, S. Novel Low Temperature Synthesis and Electrochemical Characterization of Mesoporous Nickel Cobaltite-Reduced Graphene Oxide (RGO) Composite for Supercapacitor Application. Electrochim. Acta 2015, 173, 290–301. [Google Scholar] [CrossRef]
- Caridad, J.M.; Rossella, F.; Bellani, V.; Grandi, M.S.; Diez, E. Automated Detection and Characterization of Graphene and Few-Layer Graphite via Raman Spectroscopy. J. Raman Spectrosc. 2011, 42, 286–293. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-Friendly Method to Produce Graphene That Employs Vitamin C and Amino Acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Gao, M.; Pan, H. Tailoring Thermodynamics and Kinetics for Hydrogen Storage in Complex Hydrides towards Applications. Chem. Rec. 2016, 16, 189–204. [Google Scholar] [CrossRef]
- Zou, Y.; Yin, Y.; Gao, Y.; Xiang, C.; Chu, H.; Qiu, S.; Yan, E.; Xu, F.; Sun, L. Chitosan-Mediated Co–Ce–B Nanoparticles for Catalyzing the Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2018, 43, 4912–4921. [Google Scholar] [CrossRef]
- Kiren, B.; Ayas, N. Nickel Modified Dolomite in the Hydrogen Generation from Sodium Borohydride Hydrolysis. Int. J. Hydrogen Energy 2021, 47, 19702–19717. [Google Scholar] [CrossRef]
- Paksoy, A.; Kurtoğlu, S.F.; Dizaji, A.K.; Altıntaş, Z.; Khoshsima, S.; Uzun, A.; Balcı, Ö. Nanocrystalline Cobalt–Nickel–Boron (Metal Boride) Catalysts for Efficient Hydrogen Production from the Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2021, 46, 7974–7988. [Google Scholar] [CrossRef]
- Guo, J.; Hou, Y.; Li, B.; Liu, Y. Novel Ni–Co–B Hollow Nanospheres Promote Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2018, 43, 15245–15254. [Google Scholar] [CrossRef]
- Fu, F.; Wang, C.; Wang, Q.; Martinez-Villacorta, A.M.; Escobar, A.; Chong, H.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E.; et al. Highly Selective and Sharp Volcano-Type Synergistic Ni2Pt@ZIF-8-Catalyzed Hydrogen Evolution from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and Its Use as a Reducing Agent and in the Generation of Hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Amendola, S.C.; Sharp-Goldman, S.L.; Saleem Janjua, M.; Kelly, M.T.; Petillo, P.J.; Binder, M. An Ultrasafe Hydrogen Generator: Aqueous, Alkaline Borohydride Solutions and Ru Catalyst. J. Power Sources 2000, 85, 186–189. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Cobalt Ferrite Supported Platinum Nanoparticles: Superb Catalytic Activity and Outstanding Reusability in Hydrogen Generation from the Hydrolysis of Ammonia Borane. J. Colloid Interface Sci. 2021, 596, 100–107. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Guo, Q. Research Progress on Catalysts for Hydrogen Generation through Sodium Borohydride Alcoholysis. Int. J. Hydrogen Energy 2022, 47, 5929–5946. [Google Scholar] [CrossRef]
- Khan, S.B.; Ali, F.; Asiri, A.M. Metal Nanoparticles Supported on Polyacrylamide Water Beads as Catalyst for Efficient Generation of H2 from NaBH4 Methanolysis. Int. J. Hydrogen Energy 2020, 45, 1532–1540. [Google Scholar] [CrossRef]
- Khan, S.B. Metal Nanoparticles Containing Chitosan Wrapped Cellulose Nanocomposites for Catalytic Hydrogen Production and Reduction of Environmental Pollutants. Carbohydr. Polym. 2020, 242, 116286. [Google Scholar] [CrossRef] [PubMed]



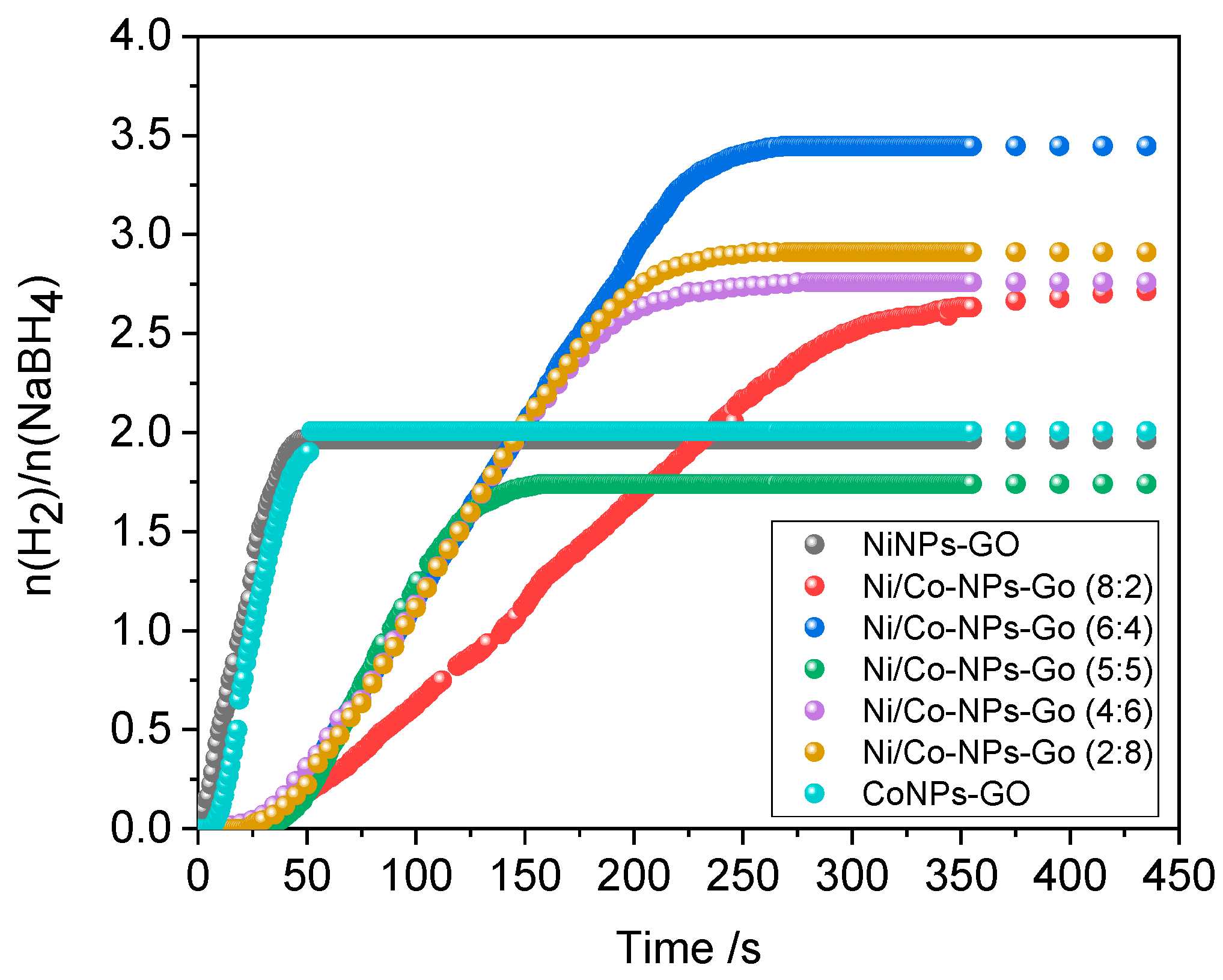




| System | Composition |
|---|---|
| NiNPs-GO | NiNPs |
| Co/Ni-NPs-GO (20:80 w/w) | Co/Ni ratio 20:80 w/w |
| Co/Ni-NPs-GO (40:60 w/w) | Co/Ni ratio 40:60 w/w |
| Co/Ni-NPs-GO (60:40 w/w) | Co/Ni ratio 60:40 w/w |
| Co/Ni-NPs-GO (80:20 w/w) | Co/Ni ratio 80:20 w/w |
| CoNPs-GO | CoNPs |
| Catalyst | Hydrolysis Conditions | Ea * | HGR ** | Reference |
|---|---|---|---|---|
| Raney Ni–Co | 0.5 g catalyst; 1 g NaBH4; 10% wt NaOH; 293 K | 52.3 | 228.5 | [20] |
| Co-NiƟC | 50 mg catalyst; 0.1 g NaBH4; 0.1 g NaOH; 298 K | 30.3 | 6364 | [22] |
| Ni-Co/r-GO | 5 g of 10 wt% NaBH4; 5 wt% NaOH; 0.05 g catalysts; 25 °C | 55.12 | 1280 | [23] |
| Ni-Co@3DG | 0.02 g catalyst; 25 °C; 1 mol L−1 NaBH4; 20.0 mL NaOH; (pH = 10.0) | Not informed. | 82.65 | [24] |
| Co@C-650 | 10 mg of catalyst/5 mL H2O 2% m/m NaOH; 2% m/m NaBH4, 30 °C | 41.5 | 330 | [27] |
| Ni/Dolomita | 5 mL de 0.25 mol L−1 of NaOH; 60 °C; 100 mg NaBH4; catalyst: 100 mg. | 38.33 | 88.16 | [44] |
| CoB-Ni4B3 | 25 °C; 20 mg of catalyst; 10 mmol NaOH; 5 mL NaBH4 0.2 mol L−1 | 32.7 | 404.6 | [45] |
| Ni/Co-GO NPs | 0.500 mol L−1 NaBH4; catalyst: 100 mg; 296.15 K; Without of NaOH | 51.6 | 212.1 | This work |
| Temperature (Kelvin) | Reaction Kinetic Constant (s−1) |
|---|---|
| 296.15 | 0.0230 |
| 304.15 | 0.0606 |
| 313.15 | 0.0893 |
| 321.15 | 0.1047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperandio, G.; Junior, I.M.; Bernardo, E.; Moreira, R. Graphene Oxide from Graphite of Spent Batteries as Support of Nanocatalysts for Fuel Hydrogen Production. Processes 2023, 11, 3250. https://doi.org/10.3390/pr11113250
Sperandio G, Junior IM, Bernardo E, Moreira R. Graphene Oxide from Graphite of Spent Batteries as Support of Nanocatalysts for Fuel Hydrogen Production. Processes. 2023; 11(11):3250. https://doi.org/10.3390/pr11113250
Chicago/Turabian StyleSperandio, Gabriel, Iterlandes Machado Junior, Esteefany Bernardo, and Renata Moreira. 2023. "Graphene Oxide from Graphite of Spent Batteries as Support of Nanocatalysts for Fuel Hydrogen Production" Processes 11, no. 11: 3250. https://doi.org/10.3390/pr11113250







