Waste-to-Energy Pipeline through Consolidated Fermentation–Microbial Fuel Cell (MFC) System
Abstract
:1. Introduction
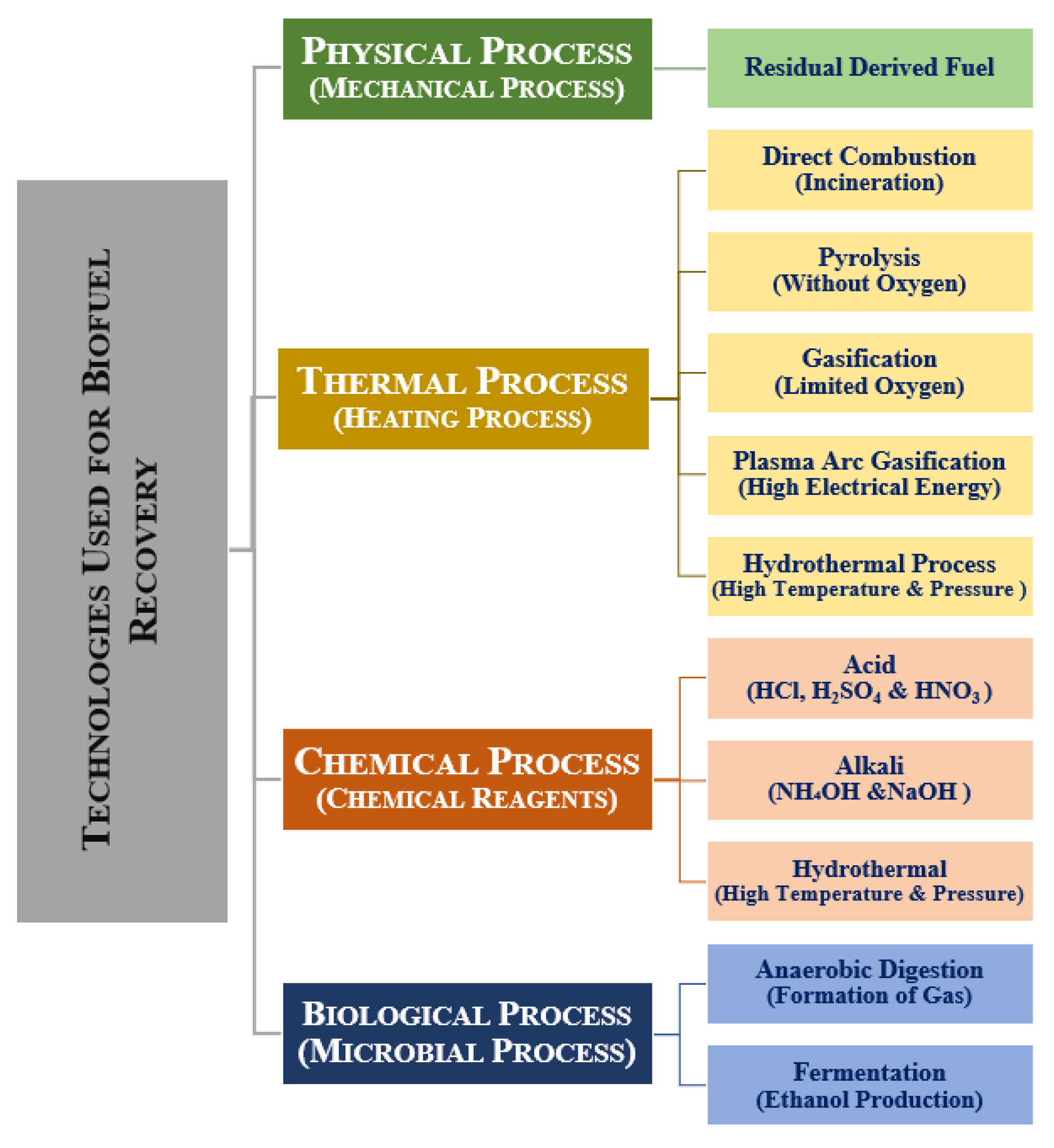
2. Organic Waste
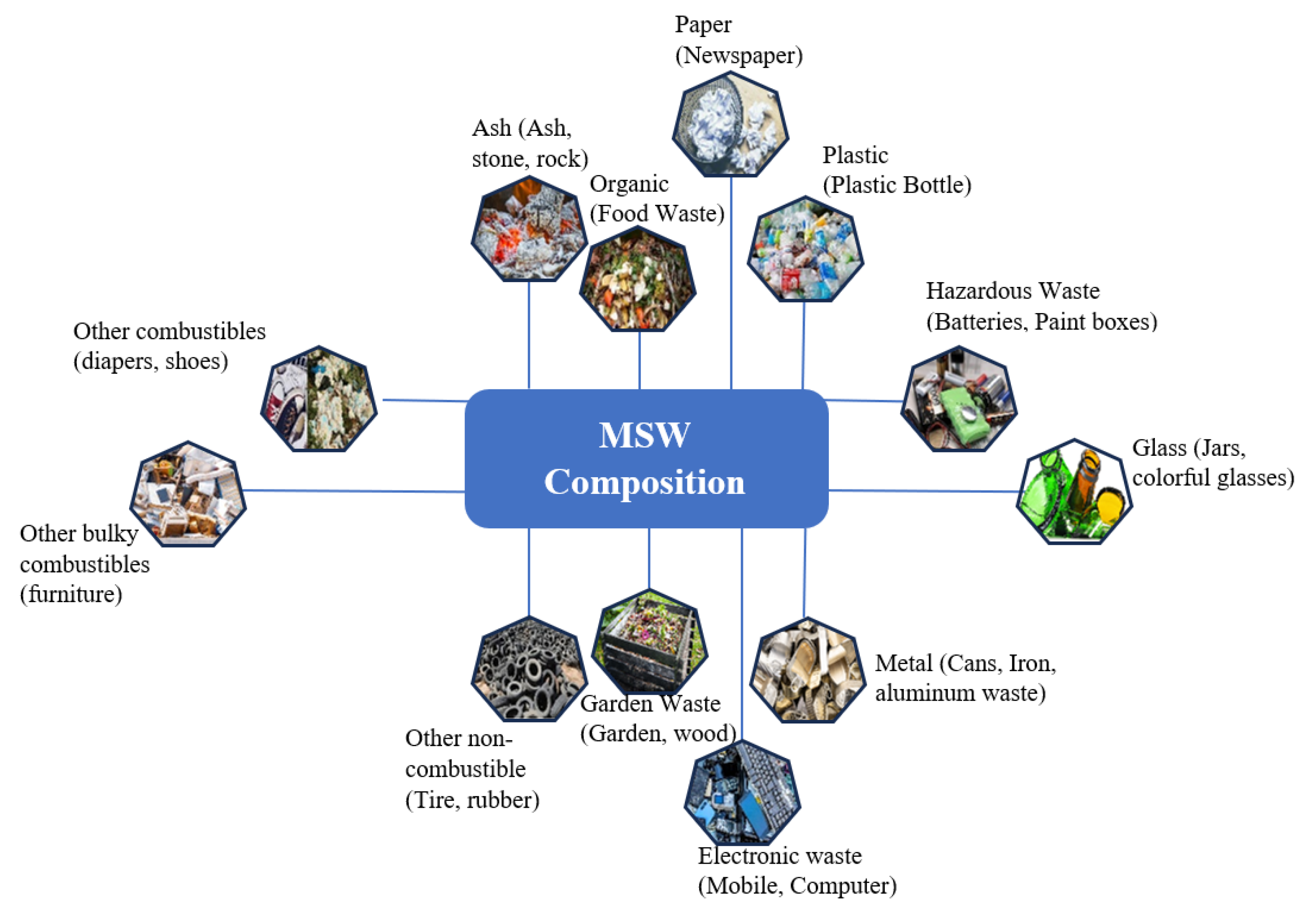
2.1. MSW Sources and Composition
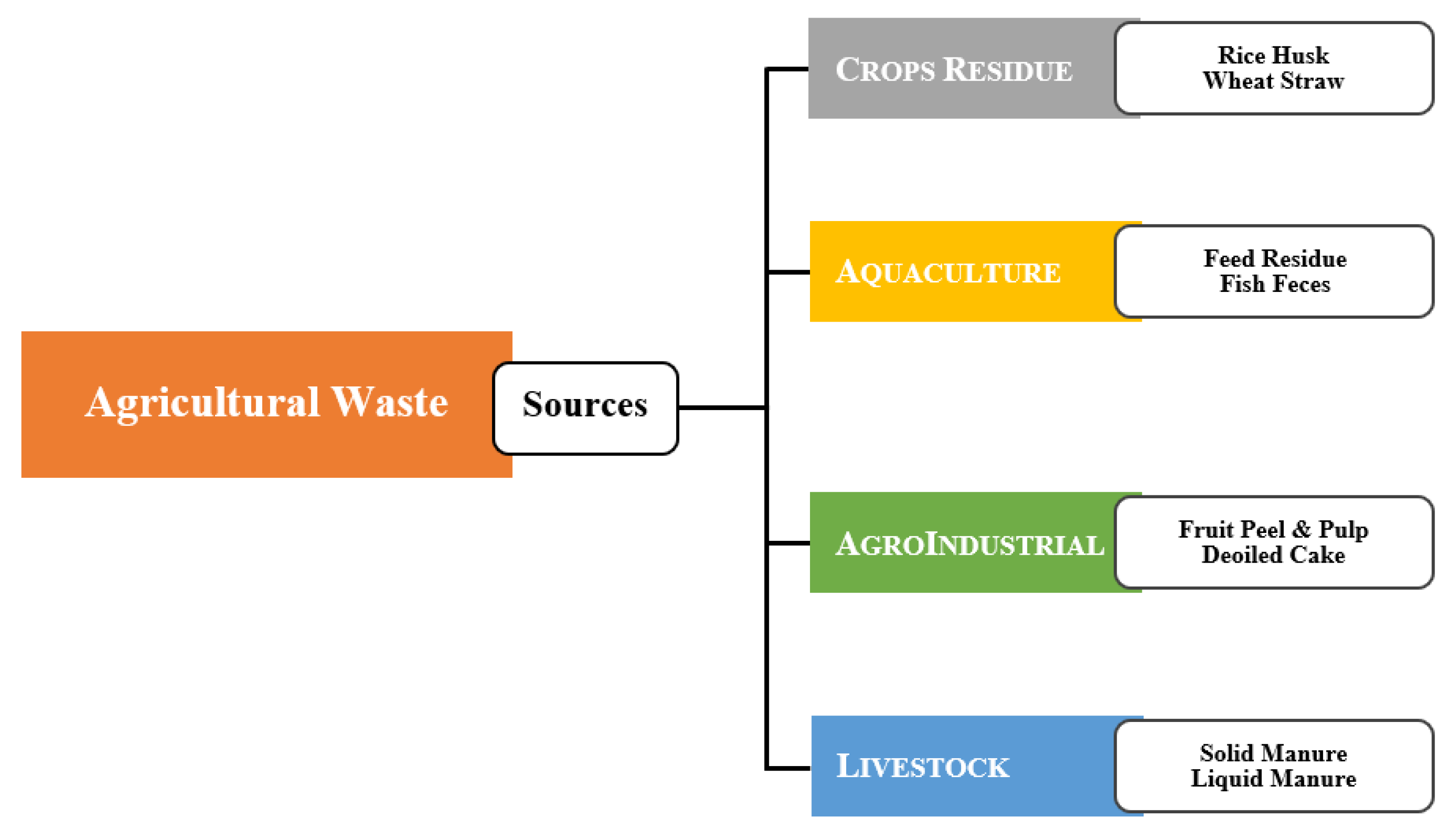
2.2. Agriculture Waste Sources
2.2.1. Crop Residue
2.2.2. Aquaculture Waste
2.2.3. Agro-Industrial Waste
2.2.4. Livestock/Animal Waste
3. Fermentation and MFC for Biofuel, Bioelectricity Generation
3.1. Fermentation
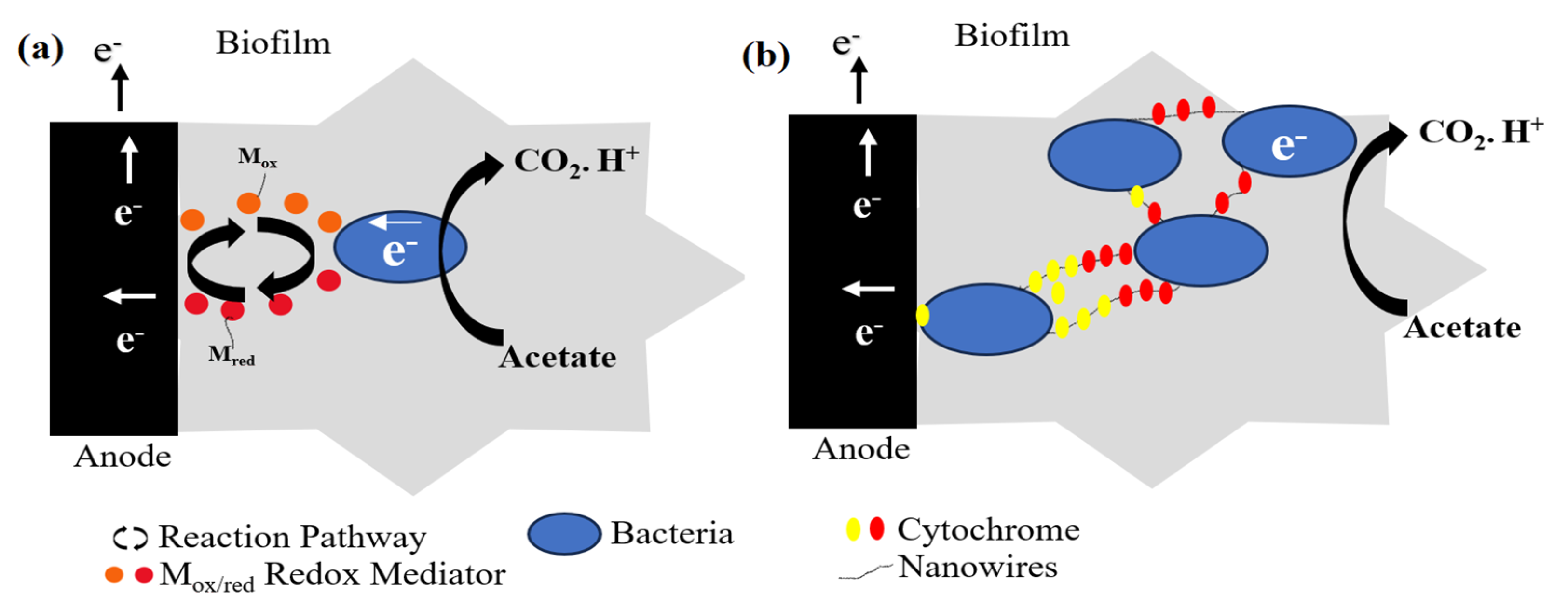
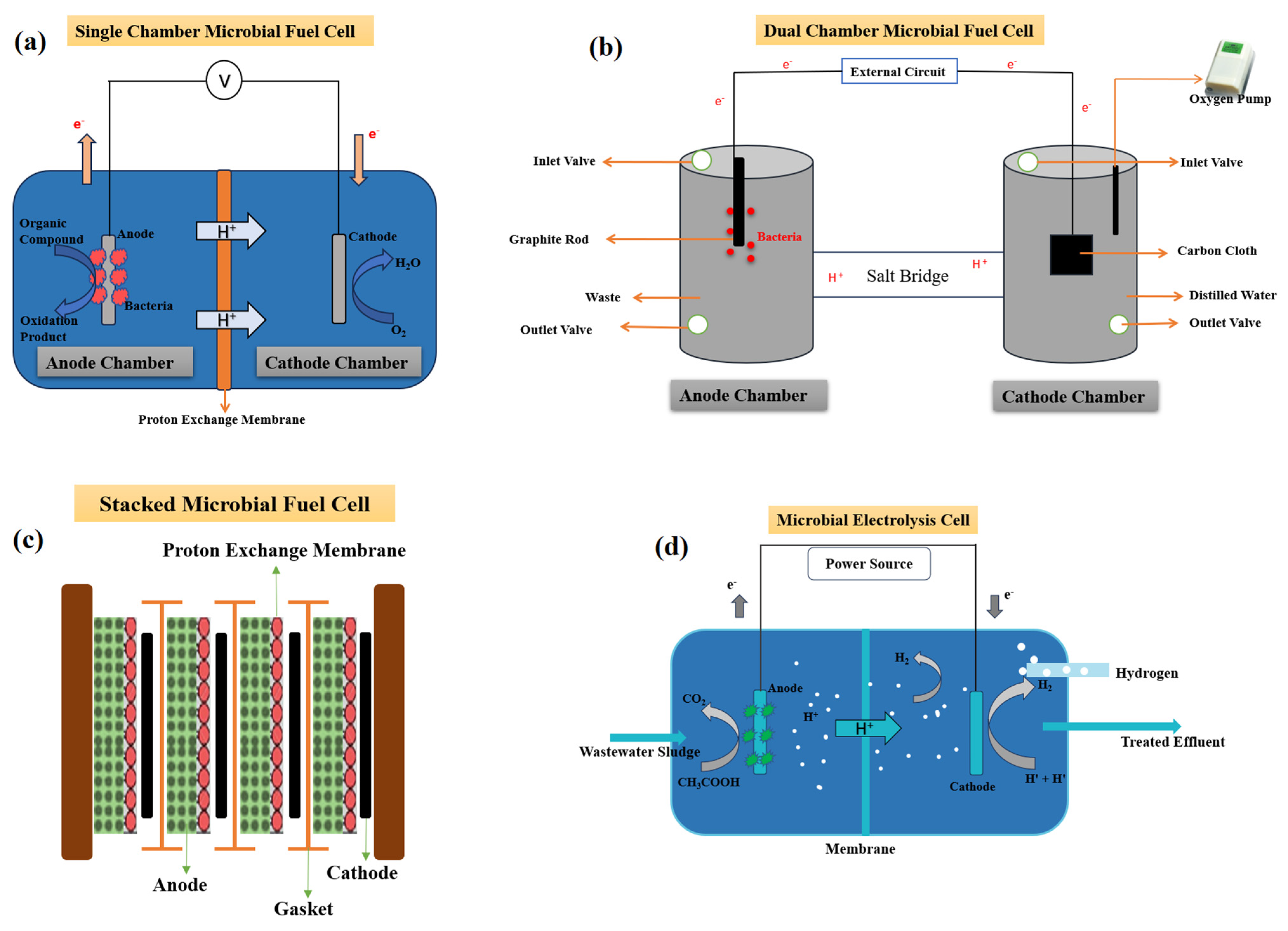
3.2. Microbial Fuel Cells
3.2.1. MFC Configurations
| MFC Configuration | Additional Configuration (Volume, PEM) | Microorganism | Anode/Cathode Material | Organic Waste | Power Output/Voltage Obtained * | Organic Waste Degradation | Reference |
|---|---|---|---|---|---|---|---|
| Single-chamber MFC | 25 mL | Geobacter, Dysgonomonas, and polysaccharide-degrading bacteria | Anode: Graphite brush Cathode: Carbon cloth with Pt catalyst | Potato pulps waste | 32,100 mW/m3 | COD Removal = 68.40% | [109] |
| 120 mL Air cathode | Anaerobic sludge | Anode: Carbon cloth Cathode: Carbon Cloth with 10% Platinum and three diffusion layers | Food waste | 0.51 V | [110] | ||
| 1.5 L and 4 L | Anaerobic sludge seeding | Combination of electrodes:
| Organic fraction of MSW (OFMSW) | 1.5 L (mW/m2)
| [99] | ||
| Dual-chamber MFC | Proton exchange membrane | Coupled with anaerobic digestion | Graphite | Banana waste | 41.3 mW/m2 | COD removal = 85.4 ± 1.0% | [111] |
| 500 mL Connected with salt bridge | Anode: Stainless steel mesh with carbon cloth Cathode: Stainless steel mesh (air cathode) | Raw food waste | 0.0005 V 14,010 mW/m3 | COD removal = 69.78% | [112] | ||
| 1 L Proton exchange membrane | Saccharomyces cerevisiae yeast | Graphite electrodes | Molasses substrate with electrolyte solution | KMnO4 = 0.48 V K3Fe(CN)6 = 0.36 V | [113] | ||
| 4000 mL Connected with salt bridge | Combination of electrodes Cu–Cu, Zn–Cu, Graphite–Cu | Food waste solution | Cu–Cu = 0.936 V Zn–Cu = 0.855 V Graphite–Cu = 0.501 V | [108] | |||
| 150 mL Connected with salt bridge | Cathode: Phlebia floridensis and Phlebia brevispora Anode: Pichia fermentans | Anode: Carbon fibers (100 Cm L, 7 µm) Cathode: Stainless steel (100 cm, 0.05 mm diameter) | Wheat straw | 331.9 mW/m2 | 35% to 38% | [114] | |
| Nafion proton exchange membrane (PEM) | Yeast | Carbon fiber electrode tissue | Inner layer of sugarcane | 5.5 V | [115] | ||
| Outer layer of sugarcane | 6 V | ||||||
| Banana peels | 6 V | ||||||
| H-type Proton exchange membrane | Anaerobic sludge | Anode: Carbon fiber paper Cathode: Carbon cloth coated with a Pt catalyst | Food residue biomass | 29.6 mW/m2 | COD removal efficiency = 71–91% | [116] | |
| 1.5 L and 4 L | Anaerobic sludge seeding | Combination of electrodes
| MSW (organic fraction of MSW) | 1.5 L (mW/m2)
| [99] | ||
| 0.24 L Cation exchange membrane | Anaerobic consortia | Carbon felts | Potato waste | 1.4–6.8 mW/m2 | COD removal = 90% | [117] | |
| U-shaped Cation exchange membrane | Mix microbial culture (composed of anaerobic bacteria) | Graphite rods | Household vegetable waste | 88,990 mW/m2 | [118] | ||
| Proton exchange membrane | Cellulose-degrading bacteria | Non-wet-proof carbon paper | Powdered rice straw | 0.345 V | [101] | ||
| Stacked MFC (Series and Parallel) | 3 MFCs connected | Cellulose-degrading bacteria | Non-wet-proof carbon paper | Powdered rice straw | Series = 2.17 V Parallel = 0.723 V | [101] | |
| Thin felt disc | Food waste (mango, banana and orange leftover and peels) | Series = 1.185 V Parallel = 2.05 V | [119] |
3.2.2. Feedstock Used in MFCs
3.3. Consolidated Fermentation–MFC System
4. Waste-to-Energy Role in Circular Economy and Environmental Sustainability
5. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Ghouti, M.A.; Khan, M.; Nasser, M.S.; Al Saad, K.; Heng, O.E. A novel method for metals extraction from municipal solid waste using a microwave-assisted acid extraction. J. Clean. Prod. 2021, 287, 125039. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Woerden, V.F.; Ionkova, K. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Cayumil, R.; Khanna, R.; Konyukhov, Y.; Burmistrov, I.; Kargin, J.B.; Mukherjee, P.S. An Overview on Solid Waste Generation and Management: Current Status in Chile. Sustainability 2021, 13, 11644. [Google Scholar] [CrossRef]
- Bello, A.S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. Sustainable and long-term management of municipal solid waste: A review. Bioresour. Technol. Rep. 2022, 18, 101067. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2021, 206, 112285. [Google Scholar] [CrossRef]
- Khan, S.; Anjum, R.; Raza, S.T.; Ahmed, B.N.; Ihtisham, M. Technologies for municipal solid waste management: Current status, challenges, and future perspectives. Chemosphere 2022, 288, 132403. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Vergara, S.E.; Tchobanoglous, G. Municipal Solid Waste and the Environment: A Global Perspective. Annu. Rev. Environ. Resour. 2012, 37, 277–309. [Google Scholar] [CrossRef]
- Khan, A.H.; Sharholy, M.; Alam, P.; Al-Mansour, A.I.; Ahmad, K.; Kamal, M.A.; Alam, S.; Pervez, M.N.; Naddeo, V. Evaluation of cost benefit analysis of municipal solid waste management systems. J. King Saud Univ. Sci. 2022, 34, 101997. [Google Scholar] [CrossRef]
- Bioenergy—Fuels and Technologies. IEA. Available online: https://www.iea.org/fuels-and-technologies/bioenergy (accessed on 15 July 2023).
- Raturi, A.K. REN21, 2019: Asia and the Pacific Renewable Energy Status Report; REN21 Secretariat: Paris, France, 2019; Available online: https://www.ren21.net/asia-report-2019/ (accessed on 12 July 2023).
- IEA. Global Energy Review 2020; IEA: Paris, France, 2020; Available online: https://www.iea.org/reports/global-energy-review-2020 (accessed on 25 May 2023).
- Omari, A.M.; Kichonge, B.N.; John, G.R.; Njau, N.K.; Mtui, P.L. Potential of Municipal Solid Waste, As Renewable Energy Source-A Case Study of Arusha, Tanzania. Int. J. Renew. Energy Technol. Res. 2014, 3, 1–9. [Google Scholar]
- Zhang, Q.; Dor, L.; Fenigshtein, D.; Yang, W.; Blasiak, W. Gasification of municipal solid waste in the Plasma Gasification Melting process. Appl. Energy 2012, 90, 106–112. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Biomass Conversion Technologies. In Greenhouse Gas Balances of Bioenergy Systems; Academic Press: Cambridge, MA, USA, 2018; pp. 107–139. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 846592. [Google Scholar] [CrossRef]
- Magalhães, A.I.; Pereira, M.; Karp, S.G.; Câmara, M.C.; Coral Medina, J.D.; Soccol, C.R. Lignocellulosic biomass from agro-industrial residues in South America: Current developments and perspectives. Biofuels Bioprod. Biorefining 2019, 13, 1505–1519. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Boas, J.V.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Review on microbial fuel cells applications, developments and costs. J. Environ. Manag. 2022, 307, 114525. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiao, S.; Ma, M.; Peng, S. Microbial fuel cell system: A promising technology for pollutant removal and environmental remediation. Environ. Sci. Pollut. Res. 2020, 27, 6749–6764. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Pai, T.-Y.; Lin, K.-L.; Ganesan, S.; Ponnusamy, V.K.; Lo, F.-C.; Chiu, H.-Y.; Banks, C.J.; Lo, H.-M. Electricity production from municipal solid waste using microbial fuel cells with municipal solid waste incinerator bottom ash as electrode plate. Bioresour. Technol. Rep. 2022, 19, 619–629. [Google Scholar] [CrossRef]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Leropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Schroeder, U.; Harnisch, F. Biofilms, Electroactive. Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 120–126. [Google Scholar] [CrossRef]
- Christwardana, M.; Joelianingsih, J.; Yoshi, L.A. Performance of Yeast Microbial Fuel Cell Integrated with Sugarcane Bagasse Fermentation for COD Reduction and Electricity Generation. Bull. Chem. React. Eng. Catal. 2021, 16, 446–458. [Google Scholar] [CrossRef]
- Borole, A.P.; Mielenz, J.R.; Vishnivetskaya, T.A.; Hamilton, C.Y. Controlling accumulation of fermentation inhibitors in biorefinery recycle water using microbial fuel cells. Biotechnol. Biofuels 2009, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Kaur, G.J.; Routray, W.; Rahimi, J.; Nair, G.R.; Singh, A. Recent advances in utilization of municipal solid waste for production of bioproducts: A bibliometric analysis. Case Stud. Chem. Environ. Eng. 2021, 4, 100164. [Google Scholar] [CrossRef]
- Sharma, K.D.; Jain, S. Municipal solid waste generation, composition, and management: The global scenario. Soc. Responsib. J. 2020, 16, 917–948. [Google Scholar] [CrossRef]
- Hoang, A.T.; Varbanov, P.S.; Nižetić, S.; Sirohi, R.; Pandey, A.; Luque, R.; Ng, K.H.; Pham, V.V. Perspective review on Municipal Solid Waste-to-energy route: Characteristics, management strategy, and role in circular economy. J. Clean. Prod. 2022, 359, 131897. [Google Scholar] [CrossRef]
- Ozcan, H.K.; Guvenc, S.Y.; Guvenc, L.; Demir, G. Municipal Solid Waste Characterization According to Different Income Levels: A Case Study. Sustainability 2016, 8, 1044. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafy, H.I.; Mansour, M.S. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Dehkordi, S.M.M.N.; Jahromi, A.R.T.; Ferdowsi, A.; Shumal, M.; Dehnavi, A. Investigation of biogas production potential from mechanical separated municipal solid waste as an approach for developing countries (case study: Isfahan-Iran). Renew. Sustain. Energy Rev. 2020, 119, 109586. [Google Scholar] [CrossRef]
- Oluseun Adejumo, I.; Adebukola Adebiyi, O. Agricultural Solid Wastes: Causes, effects, and effective management. In Strategies of Sustainable Solid Waste Management; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Obi, F.; Ugwuishiwu, B.; Nwakaire, J. Agricultural waste concept, generation, utilization, and Management. Niger. J. Technol. 2016, 35, 957. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Chuang, W.; Lin, L.; Shih, H.; Shy, Y.; Chang, S.; Lee, T. The Potential Utilization of High-Fiber Agricultural By-Products as Monogastric Animal Feed and Feed Additives: A Review. Animals 2021, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, R.K. Selection of sustainable solutions for crop residue burning: An environmental issue in northwestern states of India. Environ. Dev. Sustain. 2020, 23, 3696–3730. [Google Scholar] [CrossRef]
- Gomes-Araújo, R.; Martínez-Vázquez, D.G.; Charles-Rodríguez, A.V.; Rangel-Ortega, S.; Robledo-Olivo, A. Bioactive Compounds from Agricultural Residues, Their Obtaining Techniques, and the Antimicrobial Effect as Postharvest Additives. Int. J. Food Sci. 2020, 2021, 9936722. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Dien, B.S.; Jin, Y.-S.; Thompson, S.; Shin, J.; Watson Slininger, P.J.; Qureshi, N.; Singh, V. Conversion of High-Solids Hydrothermally Pretreated Bioenergy Sorghum to Lipids and Ethanol Using Yeast Cultures. ACS Sustain. Chem. Eng. 2021, 9, 8515–8525. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Sukhesh, M.J.; Rao, P.V. Anaerobic digestion of crop residues: Technological developments and environmental impact in the Indian context. Biocatal. Agric. Biotechnol. 2018, 16, 513–528. [Google Scholar] [CrossRef]
- Aquaculture Industry Summary. International Trade Administration|Trade.gov. Available online: https://www.trade.gov/aquaculture-industry-summary (accessed on 15 July 2023).
- Dauda, A.B.; Ajadi, A.; Tola-Fabunmi, A.S. Laboratory of Aquaculture, 109, & 101. Waste in Aquaculture: Part 1—Responsible Seafood Advocate. Global Seafood Alliance. Available online: https://www.globalseafood.org/advocate/waste-in-aquaculture-part-1/ (accessed on 2 May 2023).
- Akinwole, A.O.; Dauda, A.B.; Ololade, O.A. Growth Performance of African Catfish (Clarias gariepinus) Juveniles Reared in Wastewater Treated with Alum and Moringa oleifera Seed. J. Aquac. Res. Dev. 2016, 7, 460. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Waiho, K.; Azwar, E.; Fazhan, H.; Peng, W.; Ishak, S.D.; Tabatabaei, M.; Yek, P.N.Y.; Almomani, F.; Aghbashlo, M.; et al. A state-of-the-art review on producing engineered biochar from shellfish waste and its application in aquaculture wastewater treatment. Chemosphere 2022, 288, 132559. [Google Scholar] [CrossRef]
- Esteves, A.F.; Soares, S.M.; Salgado, E.M.; Boaventura, R.A.R.; Pires, J.C.M. Microalgal Growth in Aquaculture Effluent: Coupling Biomass Valorisation with Nutrients Removal. Appl. Sci. 2022, 12, 12608. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Wan Mahari, W.A.; Kong, S.H.; Foong, S.Y.; Peng, W.; Ting, H.; Liew, R.K.; Xia, C.; Sonne, C.; Tabatabaei, M.; et al. Pilot-scale co-processing of lignocellulosic biomass, algae, shellfish waste via thermochemical approach: Recent progress and future directions. Bioresour. Technol. 2022, 347, 126687. [Google Scholar] [CrossRef] [PubMed]
- Amulya, K.; Morris, S.; Lens, N.L. Aquatic biomass as sustainable feedstock for biorefineries. Biofuels Bioprod. Biorefining 2023, 17, 1012–1029. [Google Scholar] [CrossRef]
- Godvin, S.V.; Kumar, M.D.; Pugazhendi, A.; Bajhaiya, A.K.; Gugulothu, P.; Rajesh, B.J. Biofuel production from Macroalgae: Present scenario and future scope. Bioengineered 2021, 12, 9216–9238. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K. Source, Treatment, and Disposal of Aquaculture Solid Waste: A Review. J. Environ. Eng. 2021, 147, 03120012. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Pedersen, P.B. Solid and suspended/dissolved waste (N, P, O) from rainbow trout (Oncorynchus mykiss). Aquaculture 2011, 313, 92–99. [Google Scholar] [CrossRef]
- Henriksson, P.J.G.; Belton, B.; Murshed-e-Jahan, K.; Rico, A. Measuring the potential for sustainable intensification of aquaculture in Bangladesh using life cycle assessment. Proc. Natl. Acad. Sci. USA 2018, 115, 2958–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masabni, J.; Niu, G. Aquaponics. Plant Factory Basics, Applications and Advances; Academic Press: Cambridge, MA, USA, 2022; pp. 167–180. [Google Scholar] [CrossRef]
- El-Sayed, A.-M. Technological Innovations. Tilapia Culture, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 297–328. [Google Scholar] [CrossRef]
- Pattanaik, L.; Pattnaik, F.; Saxena, D.K.; Naik, S.N. Biofuels from agricultural waste. In Second and Third Generation of Feedstocks; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–142. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging? Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, Z.; Rasit, N.; Umor, N.A.; Ismail, S. The effect of A. Fumigatus SK1 and trichoderma sp. on the biogas production from cow manure. Malays. J. Fundam. Appl. Sci. 2018, 14, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Orlando, M.; Borja, V. Pretreatment of Animal Manure Biomass to Improve Biogas Production: A Review. Energies 2020, 13, 3573. [Google Scholar] [CrossRef]
- Tarafdar, A.; Gaur, V.K.; Rawat, N.; Wankhade, P.R.; Gaur, G.K.; Awasthi, M.K.; Sagar, N.A.; Sirohi, R. Advances in biomaterial production from animal derived waste. Bioengineered 2021, 12, 8247–8258. [Google Scholar] [CrossRef] [PubMed]
- Sharholy, M.; Ahmad, K.; Mahmood, G.; Trivedi, R. Municipal solid waste management in Indian cities—A review. Waste Manag. 2008, 28, 459–467. [Google Scholar] [CrossRef]
- Ramamurthy, P.C.; Singh, S.; Kapoor, D.; Parihar, P.; Samuel, J.; Prasad, R.; Kumar, A.; Singh, J. Microbial biotechnological approaches: Renewable bioprocessing for the future energy systems. Microb. Cell Factories 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S. Solid State Fermentation and Its Applications. Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2007; pp. 465–489. [Google Scholar] [CrossRef]
- Morales-Martínez, T.K.; Díaz-Blanco, D.I.; Rodríguez-de la Garza, J.A.; Morlett-Chávez, J.; Castro-Montoya, A.J.; Quintero, J.; Aroca, G.; Rios-González, L.J. Assessment of different saccharification and fermentation configurations for eth-anol production from Agave lechuguilla. BioRes 2017, 12, 8093–8105. [Google Scholar] [CrossRef]
- Kassim, M.A.; Meng, T.K.; Kamaludin, R.; Hussain, A.H.; Bukhari, N.A. Bioprocessing of sustainable renewable biomass for bioethanol production. In Value-Chain of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–234. [Google Scholar] [CrossRef]
- Chacón, M.G.; Ibenegbu, C.; Leak, D.J. Simultaneous saccharification and lactic acid fermentation of the cellulosic fraction of municipal solid waste using Bacillus smithii. Biotechnol. Lett. 2020, 43, 667–675. [Google Scholar] [CrossRef]
- Aulitto, M.; Fusco, S.; Nickel, D.B.; Bartolucci, S.; Contursi, P.; Franzén, C.J. Seed culture pre-adaptation of Bacillus coagulans MA-13 improves lactic acid production in simultaneous saccharification and fermentation. Biotechnol. Biofuels 2019, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Pandiyan, K.; Singh, A.; Singh, S.; Saxena, A.K.; Nain, L. Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew. Energy 2019, 132, 723–741. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Wang, L.; York, S.W.; Ingram, L.O.; Shanmugam, K. Simultaneous fermentation of biomass-derived sugars to ethanol by a co-culture of an engineered Escherichia coli and Saccharomyces cerevisiae. Bioresour. Technol. 2019, 273, 269–276. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; Da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef]
- Akinosho, H.; Yee, K.; Close, D.; Ragauskas, A. The emergence of Clostridium thermocellum as a high utility candidate for consolidated bioprocessing applications. Front. Chem. 2014, 2, 100795. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.; Kanthaliya, B.; Meena, S.; Khan, F.; Arora, J. Process consolidation approaches for cellulosic ethanol production. In Sustainable Biofuels; Academic Press: Cambridge, MA, USA, 2021; pp. 43–72. [Google Scholar] [CrossRef]
- Environmental Protection Agency. EPA. Available online: https://www.epa.gov/agstar/how-does-anaerobic-digestion-work (accessed on 22 June 2023).
- Yoshizu, D.; Kouzuma, A.; Watanabe, K. Use of Microbial Fuel Cells for the Treatment of Residue Effluents Discharged from an Anaerobic Digester Treating Food Wastes. Microorganisms 2023, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K. Recent Developments in Microbial Fuel Cell Technologies for Sustainable Bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef]
- Asai, Y.; Miyahara, M.; Kouzuma, A.; Watanabe, K. Comparative evaluation of wastewater-treatment microbial fuel cells in terms of organics removal, waste-sludge production, and electricity generation. Bioresour. Bioprocess. 2017, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Moqsud, M.A. Bioelectricity from Organic Solid Waste. In Strategies of Sustainable Solid Waste Management; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Yaakop, A.S.; Ahmad, A.; Hussain, F.; Oh, S.-E.; Alshammari, M.B.; Chauhan, R. Domestic Organic Waste: A Potential Source to Produce the Energy via a Single-Chamber Microbial Fuel Cell. Int. J. Chem. Eng. 2023, 2023, 1–10. [Google Scholar] [CrossRef]
- Hamed, M.S.; Majdi, H.S.; Hasan, B.O. Effect of Electrode Material and Hydrodynamics on the Produced Current in Double Chamber Microbial Fuel Cells. ACS Omega 2020, 5, 10339–10348. [Google Scholar] [CrossRef]
- Sreelekshmy, B.R. Exploration of Electrochemcially Active Bacterial Strains for Microbial Fuel Cells: An Innovation in Bioelectricity Generation. J. Pure Appl. Microbiol. 2020, 14, 103–122. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Xing, D.; Zhang, L.; Sun, R.; Zhang, J.; Zhong, Y.; Feng, Y.; Ren, N. Interaction of bacteria and archaea in a microbial fuel cell with ITO anode. RSC Adv. 2018, 8, 28487–28495. [Google Scholar] [CrossRef]
- Sarma, H.; Bhattacharyya, P.; Jadhav, D.A.; Pawar, P.; Thakare, M.; Pandit, S.; Mathuriya, A.S.; Prasad, R. Fungal-mediated electrochemical system: Prospects, applications and challenges. Curr. Res. Microb. Sci. 2021, 2, 100041. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Uria, N.; Ferrera, I.; Mas, J. Electrochemical performance and microbial community profiles in microbial fuel cells in relation to electron transfer mechanisms. BMC Microbiol. 2017, 17, 208. [Google Scholar] [CrossRef] [Green Version]
- Schröder, U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef]
- Malvankar, N.S.; Lovley, D.R. Microbial Nanowires: A New Paradigm for Biological Electron Transfer and Bioelectronics. Chemsuschem 2012, 5, 1039–1046. [Google Scholar] [CrossRef]
- Vishwanathan, A.S. Microbial fuel cells: A comprehensive review for beginners. 3 Biotech 2021, 11, 248. [Google Scholar] [CrossRef]
- Babanova, S.; Carpenter, K.; Phadke, S.; Suzuki, S.; Ishii, S.; Phan, T.; Grossi-Soyster, E.; Flynn, M.; Hogan, J.; Bretschger, O. The Effect of Membrane Type on the Performance of Microbial Electrosynthesis Cells for Methane Production. J. Electrochem. Soc. 2016, 164, H3015–H3023. [Google Scholar] [CrossRef]
- Bose, P. Recent Microbial Fuel Cell Applications and Developments. AZoM.com. Available online: https://www.azom.com/article.aspx?ArticleID=19737 (accessed on 17 June 2023).
- Silva, M.; Yang, Y.; Mwaniki, K. Energy-Nutrient-Water-Nexus by Microbial Fuel Cell: A Potential Smart Water Solution for Wastewater Treatment Plants. J. Sustain. Bioenergy Syst. 2022, 12, 12–19. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity Generation Using an Air-Cathode Single Chamber Microbial Fuel Cell in the Presence and Absence of a Proton Exchange Membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Wang, Z.; Mahadevan, G.D.; Wu, Y.; Zhao, F. Progress of air-breathing cathode in microbial fuel cells. J. Power Sources 2017, 356, 245–255. [Google Scholar] [CrossRef]
- Ho, N.; Babel, S.; Sombatmankhong, K. Bio-electrochemical system for recovery of silver coupled with power generation and wastewater treatment from silver(I) diammine complex. J. Water Process. Eng. 2018, 23, 186–194. [Google Scholar] [CrossRef]
- Hidayat, A.R.P.; Widyanto, A.R.; Asranudin, A.; Ediati, R.; Sulistiono, D.O.; Putro, H.S.; Sugiarso, D.; Prasetyoko, D.; Purnomo, A.S.; Bahruji, H.; et al. Recent development of double chamber microbial fuel cell for hexavalent chromium waste removal. J. Environ. Chem. Eng. 2022, 10, 107505. [Google Scholar] [CrossRef]
- Miskan, M.; Ismail, M.; Ghasemi, M.; Md Jahim, J.; Nordin, D.; Abu Bakar, M.H. Characterization of membrane biofouling and its effect on the performance of microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 543–552. [Google Scholar] [CrossRef]
- Chiu, H.; Pai, T.; Liu, M.; Chang, C.; Lo, F.; Chang, T.; Lo, H.; Chiang, C.; Chao, K.; Lo, W.; et al. Electricity production from municipal solid waste using microbial fuel cells. Waste Manag. Res. J. Sustain. Circ. Econ. 2016, 34, 619–629. [Google Scholar] [CrossRef]
- Touqeer, T.; Miran, W.; Mumtaz, M.W.; Mukhtar, H. Design and Configuration of Microbial Fuel Cells. In Microbial Fuel Cells for Environmental Remediation. Sustainable Materials and Technology; Ahmad, A., Mohamad Ibrahim, M.N., Yaqoob, A.A., Mohd Setapar, S.H., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Hassan, S.H.; Gad El-Rab, S.M.; Rahimnejad, M.; Ghasemi, M.; Joo, J.; Sik-Ok, Y.; Kim, I.S.; Oh, S. Electricity generation from rice straw using a microbial fuel cell. Int. J. Hydrogen Energy 2014, 39, 9490–9496. [Google Scholar] [CrossRef]
- Fudge, T.; Bulmer, I.; Bowman, K.; Pathmakanthan, S.; Gambier, W.; Dehouche, Z.; Majed, S.; Constantinou, A. Microbial Electrolysis Cells for Decentralised Wastewater Treatment: The Next Steps. Water 2021, 13, 445. [Google Scholar] [CrossRef]
- Kalathil, S.; Patil, S.; Pant, D. Microbial Fuel Cells: Electrode Materials. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–318. [Google Scholar] [CrossRef]
- Mustakeem, N. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Sharma, T.; Mohana reddy, A.L.; Chandra, T.; Ramaprabhu, S. Development of carbon nanotubes and nanofluids based microbial fuel cell. Int. J. Hydrogen Energy 2008, 33, 6749–6754. [Google Scholar] [CrossRef]
- Zhu, X.; Logan, B.E. Copper anode corrosion affects power generation in microbial fuel cells. J. Chem. Technol. Biotechnol. 2014, 89, 471–474. [Google Scholar] [CrossRef]
- Masud, N.; Al-Mustasin, A.H.; Md Jawarul, M.; Ali, M. Performance evaluation of microbial fuel cell with food waste solution as a potential energy storage medium. Proc. Int. Exch. Innov. Conf. Eng. Sci. (IEICES) 2021, 7, 96–102. [Google Scholar] [CrossRef]
- Tian, Y.; Mei, X.; Liang, Q.; Wu, D.; Ren, N.; Xing, D. Biological degradation of potato pulp waste and microbial community structure in microbial fuel cells. RSC Adv. 2017, 7, 8376–8380. [Google Scholar] [CrossRef] [Green Version]
- Flimban, S.G.; Ismail, I.M.I.; Kim, T.; Oh, S. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tian, Y.; Zuo, W.; Zhang, J.; Pan, X.; Li, L.; Su, X. Electricity generation from food wastes and characteristics of organic matters in microbial fuel cell. Bioresour. Technol. 2016, 205, 104–110. [Google Scholar] [CrossRef]
- Rincón-Catalán, N.I.; Cruz-Salomón, A.; Sebastian, P.J.; Pérez-Fabiel, S.; Hernández-Cruz, M.D.C.; Sánchez-Albores, R.M.; Hernández-Méndez, J.M.; Domínguez-Espinosa, M.E.; Esquinca-Avilés, H.A.; Ríos-Valdovinos, E.I.; et al. Banana Waste-to-Energy Valorization by Microbial Fuel Cell Coupled with Anaerobic Digestion. Processes 2022, 10, 1552. [Google Scholar] [CrossRef]
- Sathyamoorthy, G.L.; Sushmitha, A.S. Sustainable energy production from food waste using microbial fuel cell (MFC). In Proceedings of the 8th Annual International Seminar on Trends in Science and Science Education (AISTSSE), Coimbatore, India, 23–25 July 2021. [Google Scholar] [CrossRef]
- Rusdin, A.; Ahmad, A.; Karim, A.; Wahab, A.W.; Dali, S.; Taba, P.; Natsir, H.; Baharuddin, M. Production of electricity and bioethanol with microbial fuel cell (MFC) technology on molasses substrate. In Proceedings of the 9th International Conference of the Indonesian Chemical Society ICICS 2021: Toward a Meaningful Society, Mataram, India, 11–13 August 2021. [Google Scholar] [CrossRef]
- William, P.; Wen, E.T.X.; Jenevieve, H.J.Y. Microbial Fuel Cells: Food Waste as a Sugar Source. In IRC-SET 2018; Guo, H., Ren, H., Bandla, A., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Pal, M.; Sharma, R.K. Development of wheat straw based catholyte for power generation in microbial fuel cell. Biomass-Bioenergy 2020, 138, 105591. [Google Scholar] [CrossRef]
- Chatzikonstantinou, D.; Tremouli, A.; Papadopoulou, K.; Kanellos, G.; Lampropoulos, I.; Lyberatos, G. Bioelectricity production from fermentable household waste in a dual-chamber microbial fuel cell. Waste Manag. Res. 2018, 36, 1037–1042. [Google Scholar] [CrossRef]
- Du, H.; Li, F. Size effects of potato waste on its treatment by microbial fuel cell. Environ. Technol. 2015, 37, 1305–1313. [Google Scholar] [CrossRef]
- Javed, M.M.; Nisar, M.A.; Ahmad, M.U.; Muneer, B. Production of Bioelectricity from Vegetable Waste Extract by Designing a U-shaped Microbial Fuel Cell. Pak. J. Zool. 2017, 49, 711–716. [Google Scholar] [CrossRef]
- Rahman, W.; Yusup, S.; Mohammad, S.N. Screening of fruit waste as substrate for microbial fuel cell (MFC). In Proceedings of the 4th International Sciences, Technology and Engineering Conference (ISTEC) 2020: Exploring Materials for the Future, Arau, Malaysia, 8 October 2020. [Google Scholar] [CrossRef]
- Chatterjee, P.; Ghangrekar, M.M.; Leech, M. A brief review on recent advances in Air-Cathode Microbial Fuel Cells. Environ. Eng. Manag. J. 2018, 17, 1531–1544. [Google Scholar] [CrossRef]
- Cheng, K.; Kaksonen, A. Integrating Microbial Electrochemical Technologies With Anaerobic Digestion for Waste Treatment: Possibilities and Perspectives. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–221. [Google Scholar] [CrossRef]
- Fradler, K.R.; Kim, J.R.; Shipley, G.; Massanet-Nicolau, J.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Operation of a bioelectrochemical system as a polishing stage for the effluent from a two-stage biohydrogen and biomethane production process. Biochem. Eng. J. 2014, 85, 125–131. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, X.; Pei, Y.; Chen, W.; Lin, Q.; Huang, J.; Hou, P.; Tang, J.; Han, W. Process design and techno-economic analysis of fuel ethanol production from food waste by enzymatic hydrolysis and fermentation. Bioresour. Technol. 2022, 363, 127882. [Google Scholar] [CrossRef]
- Gubicza, K.; Nieves, I.U.; Sagues, W.J.; Barta, Z.; Shanmugam, K.; Ingram, L.O. Techno-economic analysis of ethanol production from sugarcane bagasse using a Liquefaction plus Simultaneous Saccharification and co-Fermentation process. Bioresour. Technol. 2016, 208, 42–48. [Google Scholar] [CrossRef]
- Sun, C.; Xia, A.; Liao, Q.; Fu, Q.; Huang, Y.; Zhu, X. Life-cycle assessment of biohythane production via two-stage anaerobic fermentation from microalgae and food waste. Renew. Sustain. Energy Rev. 2019, 112, 395–410. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wu, X.E.J.; Lu, N.; Wang, T.; Zuo, H. System development and environmental performance analysis of a pilot scale microbial electrolysis cell for hydrogen production using urban wastewater. Energy Convers. Manag. 2019, 193, 52–63. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Mathur, S.; Balan, V.; Liao, W.; Pareek, N.; Vivekanand, V. Organic Fraction of Municipal Solid Waste: Overview of Treatment Methodologies to Enhance Anaerobic Biodegradability. Front. Energy Res. 2018, 6, 384716. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Bhagia, S.; Smith, M.D.; Petridis, L.; Ong, R.G.; Cai, C.M.; Mittal, A.; Himmel, M.H.; Balan, V.; Dale, B.E.; et al. Cellulose–hemicellulose interactions at elevated temperatures increase cellulose recalcitrance to biological conversion. Green Chem. 2018, 20, 921–934. [Google Scholar] [CrossRef]
- Pereira, T.D. Sorghum: Cultivation, Varieties and Uses; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Krishnan, M.S.; Ho, N.W.Y.; Tsao, G.T. Fermentation Kinetics of Ethanol Production from Glucose and Xylose by Recombinant Saccharomyces 1400(pLNH33). Appl. Biochem. Biotechnol. 1999, 78, 373–388. [Google Scholar] [CrossRef]
- ETHANOL2023 Data—2005–2022 Historical-2024 Forecast-Price-Quote-Chart. Ethanol—2023 Data—2005–2022 Historical—2024 Forecast-Price-Quote-Chart. Available online: https://tradingeconomics.com/commodity/ethanol (accessed on 10 July 2023).
- Gerbrandt, K.; Chu, P.L.; Simmonds, A.; Mullins, K.A.; MacLean, H.L.; Griffin, M.W.; Saville, B.A. Life cycle assessment of lignocellulosic ethanol: A review of key factors and methods affecting calculated GHG emissions and energy use. Curr. Opin. Biotechnol. 2016, 38, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, K.S. How does electron transfer occur in microbial fuel cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef]


| Feedstock | Technology | MESP | Final Fuel Products | GHG Emission | Reference |
|---|---|---|---|---|---|
| Food Waste | Enzymatic Hydrolysis + Fermentation | 19.36 cents/L (USD 548.48 t−1) | Ethanol | N/A | [124] |
| Sugarcane Bagasse | Liquification + Simultaneous Saccharification and Co-fermentation | 52.61–64.3 cents/L (USD 627.2 t−1) | Ethanol * | N/A | [125] |
| Food Waste, Microalgae | Fermentation | N/A | H2, CH4 | 15.1 kg CO2-eq/kg H2 | [126] |
| Urban Wastewater | MEC | N/A | H2, | 18.8 kg CO2-eq/kg H2 | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.; Ding, L.; Zhao, H.; Cheng, M.-H. Waste-to-Energy Pipeline through Consolidated Fermentation–Microbial Fuel Cell (MFC) System. Processes 2023, 11, 2451. https://doi.org/10.3390/pr11082451
Kumar K, Ding L, Zhao H, Cheng M-H. Waste-to-Energy Pipeline through Consolidated Fermentation–Microbial Fuel Cell (MFC) System. Processes. 2023; 11(8):2451. https://doi.org/10.3390/pr11082451
Chicago/Turabian StyleKumar, Kundan, Ling Ding, Haiyan Zhao, and Ming-Hsun Cheng. 2023. "Waste-to-Energy Pipeline through Consolidated Fermentation–Microbial Fuel Cell (MFC) System" Processes 11, no. 8: 2451. https://doi.org/10.3390/pr11082451






