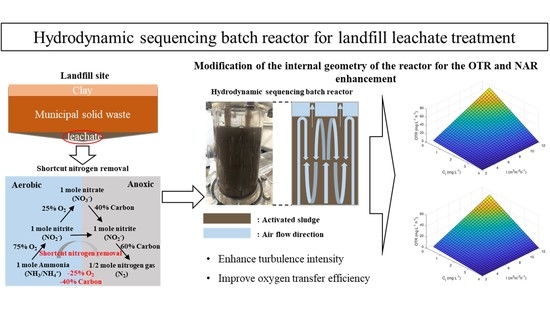
Development of a Novel Hydrodynamic Sequencing Batch Reactor for Landfill Leachate Treatment by Shortcut Biological Nitrogen Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Development and Operation
2.2. Hydrodynamic Mass Transfer Models and Calculating Methods
2.3. Determination of Mass Transfer Coefficient
2.4. Measurement Methods
3. Results and Discussion
3.1. Performance of SBNR Process
3.2. Estimation of Mass Transfer Coefficients
3.3. Modeling Hydrodynamic Oxygen Transfer Rate
3.4. Characteristics of H-SBR Process for Landfill Leachate Treatment
3.5. Performance Comparisons of SBNR Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and Long-Term Composition of MSW Landfill Leachate: A Review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Zhang, S.J.; Peng, Y.Z.; Wang, S.Y.; Zheng, S.W.; Guo, J. Organic matter and concentrated nitrogen removal by shortcut nitrification and denitrification from mature municipal landfill leachate. J. Environ. Sci. 2007, 19, 647–651. [Google Scholar] [CrossRef]
- Huang, X.; Lee, P. Shortcut nitrification/denitrification through limited-oxygen supply with two extreme COD/N-and-ammonia active landfill leachates. Chem. Eng. J. 2001, 404, 126511. [Google Scholar] [CrossRef]
- Surmacz-Gorska, J.; Cichon, A.; Miksch, K. Nitrogen removal from wastewater with high ammonia nitrogen concentration via shorter nitrification and denitrification. Water Sci. Technol. 1997, 36, 73–78. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, G. Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl. Microbiol. Biotechnol. 2006, 73, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Bae, W. Nitrite reduction by a mixed culture under conditions relevant to shortcut biological nitrogen removal. Biodegradation 2002, 13, 163–170. [Google Scholar] [CrossRef]
- Schmidt, I.; Sliekers, O.; Schmid, M.; Bock, E.; Fuerst, J.; Kuenen, J.G.; Jetten, M.S.M.; Strous, M. New concepts of microbial treatment processes for the nitrogen removal in wastewater. FEMS Microbiol. Rev. 2003, 27, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Lee, S.; Kim, M. Removal of organics and nutrients from food wastewater using combined thermophilic two-phase anaerobic digestion and shortcut biological nitrogen removal. Water Res. 2011, 45, 5279–5286. [Google Scholar] [CrossRef]
- Wang, J.L.; Yang, N. Partial nitrification under limited dissolved oxygen conditions. Process Biochem. 2004, 39, 1223–1229. [Google Scholar]
- Cui, B.; Yang, Q.; Liu, X.H.; Huang, S.T.; Yang, Y.B.; Liu, Z.B. The effect of dissolved oxygen concentration on long-term stability of partial nitrification process. J. Environ. Sci. 2020, 90, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.A.; Cheng, T.C.; Demopoulos, G.P. A contribution to the measurement of oxygen mass transfer in a laboratory pressure reactor. J. Chem. Technol. Biotechnol. 2000, 75, 665–672. [Google Scholar]
- Blackburne, R.; Yuan, Z.; Keller, J. Partial nitrification to nitrite using low dissolved oxygen concentration as the main selection factor. Biodegradation 2008, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.J.; Ren, J.Q.; Zhou, F.; Liu, Y.D.; Li, W. Micro-nano aeration is a promising alternative for achieving high-rate partial nitrification. Sci. Total Environ. 2021, 795, 148899. [Google Scholar] [CrossRef]
- Suescun, J.; Irizar, I.; Ostolaza, X.; Ayesa, E. Dissolved oxygen control and simultaneous estimation of oxygen uptake rate in activated-sludge plants. Water Environ. Res. 1998, 70, 316–322. [Google Scholar]
- Shammas, N.K.; Wang, L.K. Pure oxygen activated sludge process. In Handbook of Environmental Engineering; Wang, L.K., Pereira, N.C., Hung, Y.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 8, pp. 283–314. [Google Scholar]
- Duan, Y.; Liu, Y.S.; Zhang, M.M.; Li, Y.Y.; Zhu, W.; Hao, M.Y.; Ma, S.Y. Start-up and operational performance of the partial nitrification process in a sequencing batch reactor (SBR) coupled with a micro-aeration system. Bioresour. Technol. 2019, 296, 122311. [Google Scholar] [CrossRef]
- Khudenko, B.M.; Shpirt, E. Hydrodynamic parameters of diffused air systems. Water Res. 1986, 20, 905–915. [Google Scholar] [CrossRef]
- Glover, G.C.; Printemps, C.; Essemiani, K.; Meinhold, J. Modelling of wastewater treatment plants—How far shall we go with sophisticated modelling tools? Water Sci. Technol. 2006, 53, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liotta, F.; Chatellier, P.; Esposito, G.; Fabbricino, M.; Van Hullebusch, E.D.; Lens, P.N.L. Hydrodynamic Mathematical Modelling of Aerobic Plug Flow and Nonideal Flow Reactors: A Critical and Historical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2642–2673. [Google Scholar] [CrossRef]
- Ertekin, E.; Kavanagh, J.M.; Fletcher, D.F.; McClure, D.D. Validation studies to assist in the development of scale and system independent CFD models for industrial bubble columns. Chem. Eng. Res. Des. 2021, 171, 1–12. [Google Scholar] [CrossRef]
- Nadal-Rey, G.; McClure, D.D.; Kavanagh, J.M.; Cassells, B.; Cornelissen, S.; Fletcher, D.F.; Gernaey, K.V. Computational fluid dynamics modelling of hydrodynamics, mixing and oxygen transfer in industrial bioreactors with Newtonian broths. Biochem. Eng. J. 2022, 177, 108265. [Google Scholar] [CrossRef]
- Jun, K.S.; Jain, S.C. Oxygen-transfer in bubbly turbulent shear-flow. J. Hydraul. Eng. 1993, 119, 21–36. [Google Scholar] [CrossRef]
- Herrmann-Heber, R.; Reinecke, S.F.; Hampel, U. Dynamic aeration for improved oxygen mass transfer in the wastewater treatment process. Chem. Eng. J. 2020, 386, 122068. [Google Scholar] [CrossRef]
- Schwarz, M.; Behnisch, J.; Trippel, J.; Engelhart, M.; Wagner, M. Oxygen transfer in two-stage activated sludge wastewater treatment plants. Water 2023, 13, 1964. [Google Scholar] [CrossRef]
- Wang, J.W.; Yang, L.X.R.; Zhang, Y.; Zhang, H.P.; Liu, J.J. Partial nitrification characteristics of an immobilized carrier in municipal wastewater under low-temperature shock: The role of the nitrifying bacterial community structure. Water 2023, 15, 1714. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Zhang, Y.; Chen, Y.G. Recent advances in partial denitrification in biological nitrogen removal: From enrichment to application. Bioresour. Technol. 2020, 298, 122444. [Google Scholar] [CrossRef]
- Guo, H.L.; He, T.Y.; Chang, J.S.; Liu, P.; Lee, D.J. Nitrogen removal from low C/N wastewater in a novel Sharon&DSR (denitrifying sulfide removal) reactor. Bioresour. Technol. 2022, 362, 127789. [Google Scholar]
- Shalini, S.S.; Joseph, K. Combined SHARON and ANAMMOX processes for ammoniacal nitrogen stabilisation in landfill bioreactors. Bioresour. Technol. 2018, 250, 723–732. [Google Scholar] [CrossRef]
- Kosgey, K.; Zungu, P.V.; Bux, F.; Kumari, S. Biological nitrogen removal from low carbon wastewater. Front. Microbiol. 2022, 13, 968812. [Google Scholar] [CrossRef]
- Magri, A.; Ruscalleda, M.; Vila, A.; Akaboci, T.R.V.; Balaguer, M.D.; Llenas, J.M.; Colprim, J. Scaling-up and long-term operation of a full-scale two-stage partial nitritation-anammox system treating landfill leachate. Processes 2021, 9, 800. [Google Scholar] [CrossRef]
- Bae, W.; Kim, S.; Park, S.; Ryu, H.; Chung, J. Evaluation of predominant factor for shortcut biological nitrogen removal in sequencing batch reactor at ambient temperature. Bioprocess. Biosyst. Eng. 2019, 42, 1195–1204. [Google Scholar] [CrossRef]
- Scitovski, R. A special nonlinear least-squares problem. J. Comput. Appl. Math. 1994, 53, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.; Park, C.; Kim, M. Application of curve-fitting techniques to develop numerical calibration procedures for a river water quality model. J. Environ. Manag. 2019, 249, 109375. [Google Scholar] [CrossRef]
- Klockner, W.; Gacem, R.; Anderlei, T.; Raven, N.; Schillberg, S.; Lattermann, C.; Buchs, J. Correlation between mass transfer coefficient k(L)a and relevant operating parameters in cylindrical disposable shaken bioreactors on a bench-to-pilot scale. J. Biol. Eng. 2014, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, K.G. An Introductory Engineering and Life Science Approach. Chapter 8—The Oxygen Transfer Rate and Overall Volumetric Oxygen Transfer Coefficient. Bioprocess Engineering; Elsevier Science: Amsterdam, The Netherlands, 2013; pp. 147–170. [Google Scholar]
- Wang, Y.; Zhu, T.; Chang, M.; Jin, D. Performance of a hybrid membrane aerated biofilm reactor (H-MBfR) for shortcut nitrification. Biochem. Eng. J. 2021, 173, 108089. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, Y.; Wang, S.; Wang, Z.; Jiang, H. Efficient step-feed partial nitrification, simultaneous Anammox and denitrification (SPNAD) equipped with real-time control parameters treating raw mature landfill leachate. J. Hazard. Mater. 2019, 364, 163–172. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Zhao, C.; Liang, D.; Peng, Y. A novel partial-denitrification strategy for post-anammox to effectively remove nitrogen from landfill leachate. Sci. Total Environ. 2018, 633, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kulikowska, D.; Bernat, K. Nitritation–denitritation in landfill leachate with glycerine as a carbon source. Bioresour. Technol. 2013, 142, 297–303. [Google Scholar] [CrossRef] [PubMed]





| Parameter | Unit | Value (Average ± Standard Deviation) |
|---|---|---|
| Total chemical oxygen demand (TCOD) | mg L−1 | 1050 ± 230 |
| Soluble chemical oxygen demand (SCOD) | mg L−1 | 667 ± 120 |
| Total phosphorous (T-P) | mg P L−1 | 25 ± 4 |
| Total nitrogen (T-N) | mg N L−1 | 268 ± 23 |
| Ammonia nitrogen (NH4+-N) | mg N L−1 | 93 ± 15 |
| Nitrite nitrogen (NO2-N) | mg N L−1 | 140 ± 10 |
| Nitrate nitrogen (NO3-N) | mg N L−1 | 41 ± 5.9 |
| Alkalinity | mg CaCO3 L−1 | 459 ± 24 |
| Model Type | Equation | Description | Reference |
|---|---|---|---|
| Oxygen transfer model | Oxygenation capacity qc refers to the amount of oxygen transferred from the gas phase (bubble) to the unit liquid phase (water) per unit time in an aeration system under standard conditions. | [13] | |
| Dimensional model | Influencing variables for kLa in cylindrical orbital shaken bioreactors and their corresponding units are the volumetric mass transfer coefficient for oxygen, the reactor diameter (d), the shaking diameter (d0), the shaking frequency (n), the liquid volume (VL), the diffusion coefficient for oxygen (DO2), the kinematic viscosity (ν), and the gravitational acceleration (g). | [34] | |
| Hydrodynamic model | The rate of oxygen transfer in an air-water mixture is assumed to be proportional to the product of the DO deficit, defined as the difference between the saturation concentration and the ambient concentration of dissolved oxygen in water, and the total air-water interfacial area. | [22] |
| Symbol | Description | Unit | H-SBR | N-SBR |
|---|---|---|---|---|
| H | The liquid depth in the reactor | m | 1.1 | 1.2 |
| h | The submergence of the aerator | m | 1.0 | 1.1 |
| B | The width of the reactor | m | 0.12 | 0.12 |
| f | The width of the aeration band | m | 0.08 | 0.08 |
| I | The intensity of aeration | m3 m−2 h−1 | 2–12 | 2–12 |
| d | The average diameter of bubbles | m | 0.003 | 0.003 |
| SO2 | The solubility of oxygen | mg L−1 | 8.0 | 8.0 |
| k | The correction factor for oxygen solubility | |||
| j | The amount of oxygen in g m−3 of air | - | 299 | 299 |
| P | The atmospheric pressure | kPa | 101.325 | 101.325 |
| α | The calibrating constant of the KLa model | - | 0.7361 | 0.6841 |
| β | The calibrating constant of the KLa model | - | 1.2639 | 1.3170 |
| SBNR Process | Wastewater | C/N Ratio (COD to TN) | OTR (mg L−1 h−1) | NAR (%) | T-N Removal (%) | Reference |
|---|---|---|---|---|---|---|
| H-MBfR | Synthetic wastewater | Partial nitrification | 25.6 | 84 | 87 | [36] |
| SPNAD | Landfill leachate | 1.0 | No data | 88 | 98 | [37] |
| A/O/A | Landfill leachate | 3–4 | No data | 68 | 94 | [38] |
| Nano bubble SBR | Synthetic wastewater | Partial nitrification | 36 | 60 | 96 | [13] |
| SBR | Landfill leachate | 1.3 | No data | 98 | 78 | [39] |
| H-SBR | Landfill leachate | 2–3 | 63 | 60 | 96 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Mo, K.; Kim, M.; Cui, F. Development of a Novel Hydrodynamic Sequencing Batch Reactor for Landfill Leachate Treatment by Shortcut Biological Nitrogen Removal. Processes 2023, 11, 1868. https://doi.org/10.3390/pr11071868
Kim M, Mo K, Kim M, Cui F. Development of a Novel Hydrodynamic Sequencing Batch Reactor for Landfill Leachate Treatment by Shortcut Biological Nitrogen Removal. Processes. 2023; 11(7):1868. https://doi.org/10.3390/pr11071868
Chicago/Turabian StyleKim, Minkyung, Kyung Mo, Moonil Kim, and Fenghao Cui. 2023. "Development of a Novel Hydrodynamic Sequencing Batch Reactor for Landfill Leachate Treatment by Shortcut Biological Nitrogen Removal" Processes 11, no. 7: 1868. https://doi.org/10.3390/pr11071868