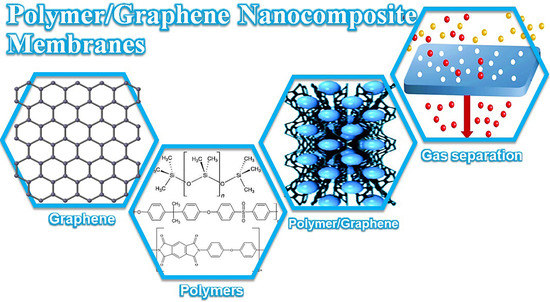
Graphene in Polymeric Nanocomposite Membranes—Current State and Progress
Abstract
:1. Introduction
2. Polymer Nanocomposite-Based Membranes
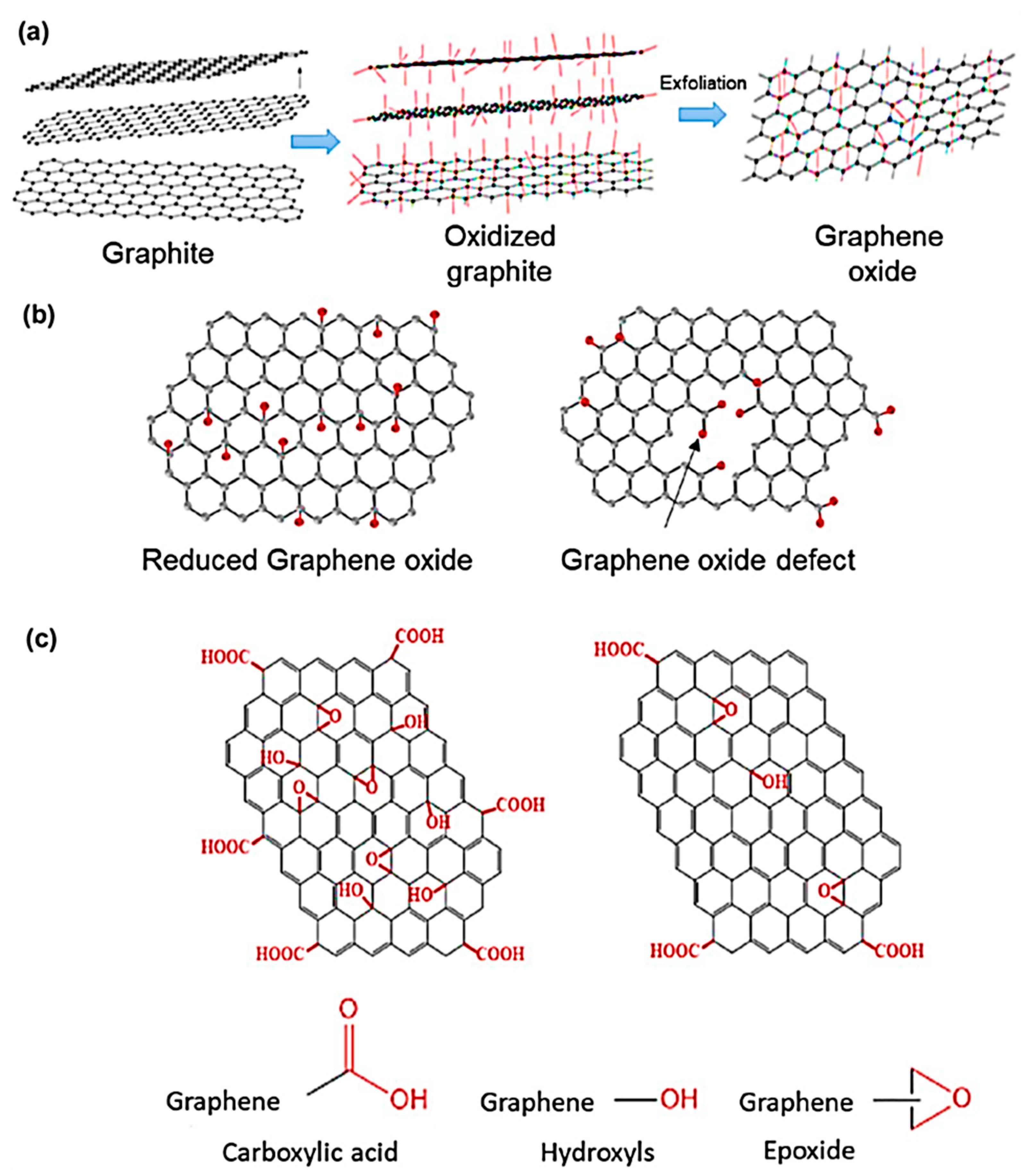
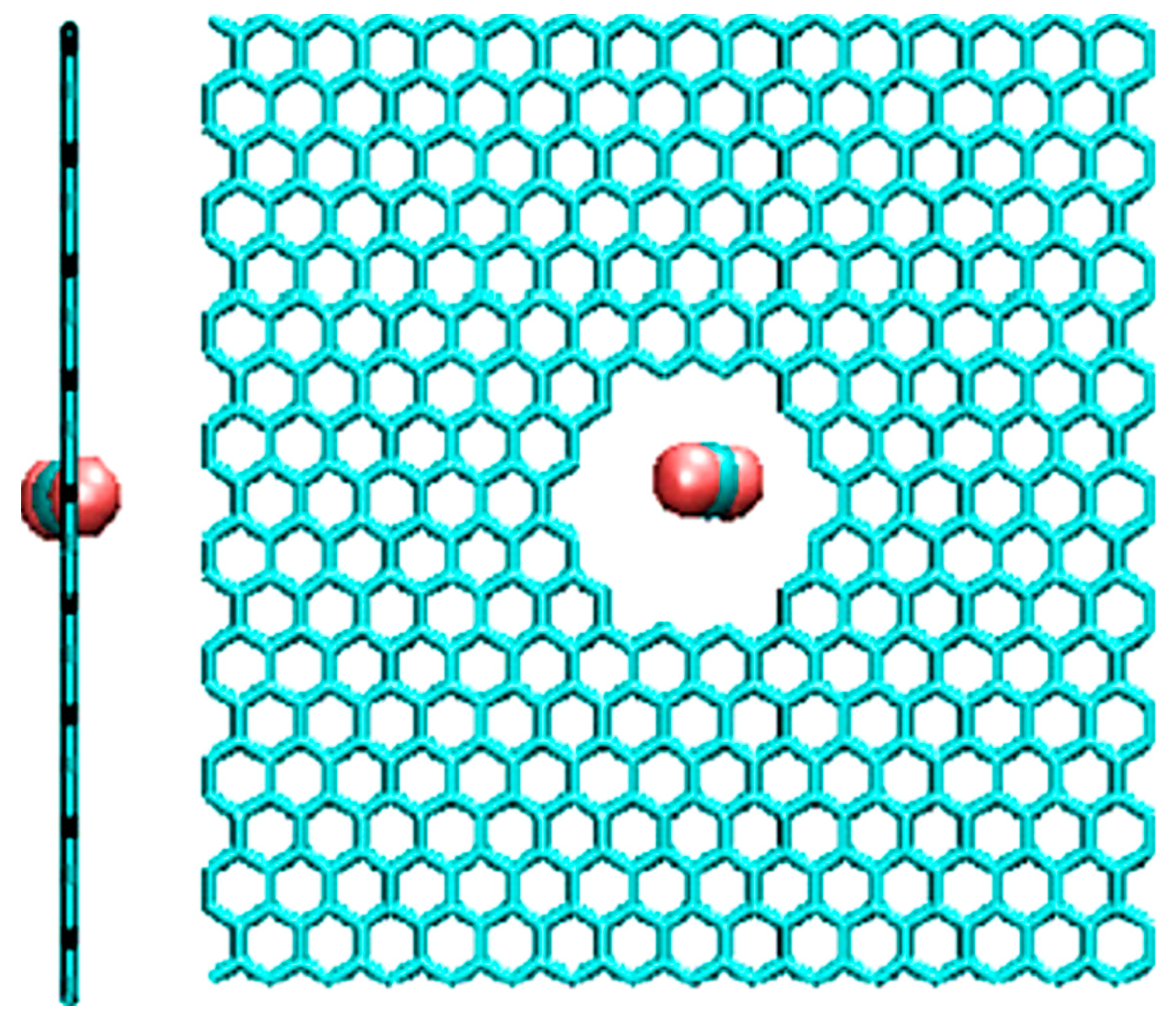
3. Graphene
4. Gas Separation Membranes Derived from Polymer/Graphene Nanocomposites
5. Polymer/Graphene Nanofibers in Membrane Technology
6. Advantages/Shortcomings of Graphene Nanocomposites in Membrane Technology
| Polymer | Nanofiller | Loading (wt.%) | PCO2 (Barrer) | α CO2/CH4 | Refs. |
|---|---|---|---|---|---|
| Polyether ether ketone (PEEK) | Graphene | 0–6 | 292.6–565.3 | 38.1–42.8 | [159] |
| Polyethylene glycol | Graphene oxide | 0.5–3 | 254.2–299.6 | 48.2–59.3 | [160] |
| Polysulfone | Graphene oxide | 0–0.25 | 65.2–74.5 | 17.3–44.4 | [106] |
| Polyethylene glycol-polyether imide | Graphene oxide | 0–10 | 81.9–146 | 18.7–24.4 | [161] |
| Polyethylene glycol | Carbon nanotube | 0–2 | 23.5–35 | 18.7–22.7 | [162] |
| Polyether sulfone | Carbon nanotube | 0–10 | 2.6–4.4 | 11.5–22 | [163] |
| Polysulfone | Carbon nanotube | 0–15 | 3.9–4.52 | 16.1–22.9 | [164] |
| Polyether sulfone | TiO2 | 0–10 | 2–2.9 | 14–15 | [165] |
| Polysulfone | Magnesium oxide | 0–10 | 7.7–9.4 | 25.4–27.7 | [166] |
| Polyimide | SiO2 | 0–0.92 | 9.9–21.3 | 33.2–36.6 | [167] |
7. Prospects and Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vroulias, D.; Staurianou, E.; Ioannides, T.; Deimede, V. Poly (ethylene oxide)-Based Copolymer-IL Composite Membranes for CO2 Separation. Membranes 2022, 13, 26. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Cui, X.; Yan, W.; Su, J.; Jin, L. A Review on the Morphology and Material Properties of the Gas Separation Membrane: Molecular Simulation. Membranes 2022, 12, 1274. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M. State-of-the-Art of Polymer/Fullerene C60 Nanocomposite Membranes for Water Treatment: Conceptions, Structural Diversity and Topographies. Membranes 2023, 13, 27. [Google Scholar] [CrossRef]
- Park, J.M.; Cao, Y.; Xia, L.-Q.; Sun, S.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Robust superconductivity in magic-angle multilayer graphene family. Nat. Mater. 2022, 21, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Goh, K.; Chuah, C.Y.; Wang, R.; Bae, T.-H. Asymmetric mixed-matrix membranes incorporated with nitrogen-doped graphene nanosheets for highly selective gas separation. J. Membr. Sci. 2020, 615, 118293. [Google Scholar] [CrossRef]
- Javed, R.M.N.; Al-Othman, A.; Tawalbeh, M.; Olabi, A.G. Recent developments in graphene and graphene oxide materials for polymer electrolyte membrane fuel cells applications. Renew. Sustain. Energy Rev. 2022, 168, 112836. [Google Scholar] [CrossRef]
- Chumakova, N.A.; Kalai, T.; Rebrikova, A.T.; Sár, C.; Kokorin, A.I. Novel Orientation-Sensitive Spin Probes for Graphene Oxide Membranes Study. Membranes 2022, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, Z.; Liu, G.; Sun, L.; Xu, R.; Zhong, J. Functionalized GO Membranes for Efficient Separation of Acid Gases from Natural Gas: A Computational Mechanistic Understanding. Membranes 2022, 12, 1155. [Google Scholar] [CrossRef]
- Favre, E. Membrane Separation Processes and Post-Combustion Carbon Capture: State of the Art and Prospects. Membranes 2022, 12, 884. [Google Scholar] [CrossRef]
- Lee, J.; Park, C.-Y.; Kong, C.-I.; Lee, J.-H.; Moon, S.-Y. Ultrathin Water-Cast Polymer Membranes for Hydrogen Purification. ACS Appl. Mater. Interfaces 2022, 14, 7292–7300. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Saini, N.; Agarwal, M.; Awasthi, K. Experimental investigation of natural polysaccharide-based mixed matrix membrane modified with graphene oxide and Pd-nanoparticles for enhanced gas separation performance. Int. J. Hydrogen Energy 2022, 47, 41820–41832. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Leaper, S.; Shokri, J.; Alberto, M.; Gorgojo, P. Effect of graphene oxide in the formation of polymeric asymmetric membranes via phase inversion. J. Membr. Sci. 2022, 641, 119924. [Google Scholar] [CrossRef]
- He, X.; Ou, D.; Wu, S.; Luo, Y.; Ma, Y.; Sun, J. A mini review on factors affecting network in thermally enhanced polymer composites: Filler content, shape, size, and tailoring methods. Adv. Compos. Hybrid Mater. 2022, 5, 21–38. [Google Scholar] [CrossRef]
- Fatemi, S.M.; Fatemi, S.J.; Abbasi, Z. Gas separation using graphene nanosheet: Insights from theory and simulation. J. Mol. Model. 2020, 26, 322. [Google Scholar] [CrossRef]
- Liu, M.; Cen, R.; Zhao, J.; Yu, Z.-C.; Chen, L.-X.; Li, Q.; Tao, Z.; Xiao, X. Selective gradient separation of aminophenol isomers by cucurbit [6] uril. Sep. Purif. Technol. 2023, 304, 122342. [Google Scholar] [CrossRef]
- Bahri, M.; Gebre, S.H.; Elaguech, M.A.; Dajan, F.T.; Sendeku, M.G.; Tlili, C.; Wang, D. Recent advances in chemical vapour deposition techniques for graphene-based nanoarchitectures: From synthesis to contemporary applications. Coord. Chem. Rev. 2023, 475, 214910. [Google Scholar] [CrossRef]
- Raza, A.; Hassan, J.Z.; Mahmood, A.; Nabgan, W.; Ikram, M. Recent advances in membrane-enabled water desalination by 2D frameworks: Graphene and beyond. Desalination 2022, 531, 115684. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Xie, F.; Ma, X.; Niu, B.; Chen, M.; Zhang, H.; Zhang, Y.; Long, D. General synthesis of ultrafine metal oxide/reduced graphene oxide nanocomposites for ultrahigh-flux nanofiltration membrane. Nat. Commun. 2022, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Lecaros, R.L.G.; Matira, A.R.; Tayo, L.L.; Hung, W.-S.; Hu, C.-C.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Homostructured graphene oxide-graphene quantum dots nanocomposite-based membranes with tunable interlayer spacing for the purification of butanol. Sep. Purif. Technol. 2022, 283, 120166. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Gao, X.; Zheng, J.; Wang, J.; Volodin, A.; Xie, Y.F.; Huang, X.; Van der Bruggen, B.; Zhu, J. High-performance thin film nanocomposite membranes enabled by nanomaterials with different dimensions for nanofiltration. J. Membr. Sci. 2020, 596, 117717. [Google Scholar] [CrossRef]
- Nidamanuri, N.; Li, Y.; Li, Q.; Dong, M. Graphene and graphene oxide-based membranes for gas separation. Eng. Sci. 2020, 9, 3–16. [Google Scholar] [CrossRef]
- Rehman, F.; Memon, F.H.; Bhatti, Z.; Iqbal, M.; Soomro, F.; Ali, A.; Thebo, K.H. Graphene-based composite membranes for isotope separation: Challenges and opportunities. Rev. Inorg. Chem. 2022, 42, 327–336. [Google Scholar] [CrossRef]
- Kononova, S.V.; Gubanova, G.N.; Korytkova, E.N.; Sapegin, D.A.; Setnickova, K.; Petrychkovych, R.; Uchytil, P. Polymer nanocomposite membranes. Appl. Sci. 2018, 8, 1181. [Google Scholar] [CrossRef] [Green Version]
- Priya, A.; Gnanasekaran, L.; Kumar, P.S.; Jalil, A.; Hoang, T.K.; Rajendran, S.; Soto-Moscoso, M.; Balakrishnan, D. Recent trends and advancements in nanoporous membranes for water purification. Chemosphere 2022, 303, 135205. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Tariq, S.; Zaman, J.U.; Perales, A.I.M.; Mubashir, M.; Luque, R. Recent trends and challenges with the synthesis of membranes: Industrial opportunities towards environmental remediation. Chemosphere 2022, 306, 135634. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Veloso-Fernández, A.; Vilas-Vilela, J.L.; Ruiz-Rubio, L. Hybrid Organic–Inorganic Membranes for Photocatalytic Water Remediation. Catalysts 2022, 12, 180. [Google Scholar] [CrossRef]
- Roy, S.; Singha, N.R. Polymeric nanocomposite membranes for next generation pervaporation process: Strategies, challenges and future prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Barbaros, I.; Yang, Y.; Safaei, B.; Yang, Z.; Qin, Z.; Asmael, M. State-of-the-art review of fabrication, application, and mechanical properties of functionally graded porous nanocomposite materials. Nanotechnol. Rev. 2022, 11, 321–371. [Google Scholar] [CrossRef]
- Mohamed, A.; Yousef, S.; Tonkonogovas, A.; Makarevicius, V.; Stankevičius, A. High performance of PES-GNs MMMs for gas separation and selectivity. Arab. J. Chem. 2022, 15, 103565. [Google Scholar] [CrossRef]
- Ghazi, F.M.G.; Abbaspour, M.; Rahimpour, M.R. Transport phenomena in gas membrane separations. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 193–208. [Google Scholar]
- Saini, N.; Awasthi, K. Insights into the progress of polymeric nano-composite membranes for hydrogen separation and purification in the direction of sustainable energy resources. Sep. Purif. Technol. 2022, 282, 120029. [Google Scholar] [CrossRef]
- Wang, C.; Park, M.J.; Yu, H.; Matsuyama, H.; Drioli, E.; Shon, H.K. Recent advances of nanocomposite membranes using layer-by-layer assembly. J. Membr. Sci. 2022, 661, 120926. [Google Scholar] [CrossRef]
- Rath, R.; Kumar, P.; Rana, D.; Mishra, V.; Kumar, A.; Mohanty, S.; Nayak, S.K. Sulfonated PVDF nanocomposite membranes tailored with graphene oxide nanoparticles: Improved proton conductivity and membrane selectivity thereof. J. Mater. Sci. 2022, 57, 3565–3585. [Google Scholar] [CrossRef]
- Patel, H.D.; Acharya, N.K. Transport, Spectroscopic, and Electrical Properties of Thermally Rearranged Nanocomposite Membranes. Chem. Eng. Technol. 2022, 45, 2223–2233. [Google Scholar] [CrossRef]
- Ioniţă, M.; Vlăsceanu, G.M.; Watzlawek, A.A.; Voicu, S.I.; Burns, J.S.; Iovu, H. Graphene and functionalized graphene: Extraordinary prospects for nanobiocomposite materials. Compos. Part B Eng. 2017, 121, 34–57. [Google Scholar] [CrossRef]
- Song, P.; Wang, H. High-performance polymeric materials through hydrogen-bond cross-linking. Adv. Mater. 2020, 32, 1901244. [Google Scholar] [CrossRef]
- Vatanpour, V.; Jouyandeh, M.; Akhi, H.; Khadem, S.S.M.; Ganjali, M.R.; Moradi, H.; Mirsadeghi, S.; Badiei, A.; Esmaeili, A.; Rabiee, N. Hyperbranched polyethylenimine functionalized silica/polysulfone nanocomposite membranes for water purification. Chemosphere 2022, 290, 133363. [Google Scholar] [CrossRef]
- Alavi, M.; Thomas, S.; Sreedharan, M. Modification of silica nanoparticles for antibacterial activities: Mechanism of action. Micro Nano Bio Asp. 2022, 1, 49–58. [Google Scholar]
- Barzegar, T.; Hassanajili, S. Fabrication and characterization of dual layer PEBAX-SiO2/polyethersulfone nanocomposite membranes for separation of CO2/CH4 gases. J. Appl. Polym. Sci. 2022, 139, 51624. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Surface-modified nanocomposite membranes. Sep. Purif. Rev. 2018, 47, 288–305. [Google Scholar] [CrossRef]
- Ahmadian-Alam, L.; Mahdavi, H. A novel polysulfone-based ternary nanocomposite membrane consisting of metal-organic framework and silica nanoparticles: As proton exchange membrane for polymer electrolyte fuel cells. Renew. Energy 2018, 126, 630–639. [Google Scholar] [CrossRef] [Green Version]
- Khdary, N.H.; Abdelsalam, M.E. Polymer-silica nanocomposite membranes for CO2 capturing. Arab. J. Chem. 2020, 13, 557–567. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, J.; Zhang, X.; Chen, L.; Zhang, Q.; Ma, L. Recent advances in membrane-based materials for desalination and gas separation. J. Clean. Prod. 2023, 387, 135845. [Google Scholar] [CrossRef]
- Shanmugasundar, S.; Kannan, N.; Sundaravadivel, E.; Zsolt, S.; Mukunthan, K.; Manokaran, J.; Narendranath, J.; Kamalakannan, V.; Kavitha, P.; Prabhu, V. Study on the inflammatory response of PMMA/polystyrene/silica nanocomposite membranes for drug delivery and dental applications. PLoS ONE 2019, 14, e0209948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.-Y.; Yuan, H.-G.; Liu, Y.-Y.; Ren, D.; Su, Y.-C.; Wang, X. Metal–organic framework nanocomposite thin films with interfacial bindings and self-standing robustness for high water flux and enhanced ion selectivity. ACS Nano 2018, 12, 9253–9265. [Google Scholar] [CrossRef] [PubMed]
- Goncharenko, A.A.; Tarasyuk, I.A.; Marfin, Y.S.; Grzhegorzhevskii, K.V.; Muslimov, A.R.; Bondarenko, A.B.; Lebedev, M.D.; Kuz’min, I.A.; Vashurin, A.S.; Lepik, K.V. DDAO Controlled Synthesis of Organo-Modified Silica Nanoparticles with Encapsulated Fluorescent Boron Dipyrrins and Study of Their Uptake by Cancerous Cells. Molecules 2020, 25, 3802. [Google Scholar] [CrossRef]
- Hanif, M.; Shin, H.; Chun, D.; Kim, H.G.; Kwac, L.K.; Kim, Y.S. Photocatalytic VOCs Degradation Efficiency of Polypropylene Membranes by Incorporation of TiO2 Nanoparticles. Membranes 2023, 13, 50. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Z.; Liu, Y.; Li, R.; Xu, Y.; Jakaj, G.; Lin, H. Polymeric membranes incorporated with ZnO nanoparticles for membrane fouling mitigation: A brief review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef]
- Douna, I.; Farrukh, S.; Pervaiz, E.; Hussain, A.; Fan, X.F.; Salahuddin, Z. Blending of ZnO Nanorods in Cellulose Acetate Mixed Matrix Membrane for Enhancement of CO2 Permeability. J. Polym. Environ. 2023, 1–17. [Google Scholar] [CrossRef]
- Yazid, A.F.; Mukhtar, H.; Nasir, R.; Mohshim, D.F. Incorporating Carbon Nanotubes in Nanocomposite Mixed-Matrix Membranes for Gas Separation: A Review. Membranes 2022, 12, 589. [Google Scholar] [CrossRef]
- Lan, Q.; Yan, N.; Yang, H.; Wang, Y. Nanocomposite block copolymer membranes with enhanced permeance and robustness by carbon nanotube doping. Compos. Commun. 2022, 29, 101025. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Borah, A.; Hazarika, S. In situ Assembly of Functionalized Single-Walled Carbon Nanotube with partially reduced Graphene oxide Nanocomposite Membrane for Chiral Separation of β-substituted-α-amino acids. Sep. Purif. Technol. 2022, 283, 120201. [Google Scholar] [CrossRef]
- Kausar, A. Polymeric nanocomposite with polyhedral oligomeric silsesquioxane and nanocarbon (fullerene, graphene, carbon nanotube, nanodiamond)—Futuristic headways. Polym.-Plast. Technol. Mater. 2023, 1–14. [Google Scholar] [CrossRef]
- Kausar, A. Poly (methyl methacrylate)/Fullerene nanocomposite—Factors and applications. Polym.-Plast. Technol. Mater. 2022, 61, 593–608. [Google Scholar] [CrossRef]
- Mohammed, M.; Yahia, I. Towards multifunctional applications of Fullerene-filled (PVA-PEG) polymeric nanocomposite films: Structural, optical, and electrical properties. Phys. Scr. 2022, 97, 065813. [Google Scholar] [CrossRef]
- Norouzi, A.; Lay, E.N.; Hosseinkhani, A.; Chapalaghi, M. Functionalized nanodiamonds in polyurethane mixed matrix membranes for carbon dioxide separation. Results Mater. 2022, 13, 100243. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Bahi, A.; Wuttke, S.; Kamkar, M.; Rojas, O.J.; Ko, F.; Arjmand, M. Electrospun Nanofibers of Chitosan/Polyvinyl Alcohol/UiO-66/Nanodiamond: Versatile Adsorbents for Wastewater Remediation and Organic Dye Removal. Chem. Eng. J. 2022, 457, 141176. [Google Scholar] [CrossRef]
- Pandey, K.; Dwivedi, M.M.; Sanjay, S.S. A brief review on synthesis and application of polymer–nanodiamond composite. Mater. Today Proc. 2022, 68, 2772–2780. [Google Scholar] [CrossRef]
- Johnson, D.J.; Hilal, N. Nanocomposite nanofiltration membranes: State of play and recent advances. Desalination 2022, 524, 115480. [Google Scholar] [CrossRef]
- Wu, J.; Chung, T.S. Supramolecular Polymer Network Membranes with Molecular-Sieving Nanocavities for Efficient Pre-Combustion CO2 Capture. Small Methods 2022, 6, 2101288. [Google Scholar] [CrossRef]
- de Sá, M.; Pinto, A.; Oliveira, V. Passive direct methanol fuel cells as a sustainable alternative to batteries in hearing aid devices–An overview. Int. J. Hydrogen Energy 2022, 47, 16552–16567. [Google Scholar] [CrossRef]
- You, X.; Zhang, Q.; Yang, J.; Dong, S. Review on 3D-printed graphene-reinforced composites for structural applications. Compos. Part A Appl. Sci. Manuf. 2023, 167, 107420. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Hong, N.; Kireev, D.; Zhao, Q.; Chen, D.; Akinwande, D.; Li, W. Roll-to-Roll Dry Transfer of Large-Scale Graphene. Adv. Mater. 2022, 34, 2106615. [Google Scholar] [CrossRef]
- Chen, X.; Fan, K.; Liu, Y.; Li, Y.; Liu, X.; Feng, W.; Wang, X. Recent advances in fluorinated graphene from synthesis to applications: Critical review on functional chemistry and structure engineering. Adv. Mater. 2022, 34, 2101665. [Google Scholar] [CrossRef]
- Fadil, Y.; Thickett, S.C.; Agarwal, V.; Zetterlund, P.B. Synthesis of graphene-based polymeric nanocomposites using emulsion techniques. Prog. Polym. Sci. 2022, 125, 101476. [Google Scholar] [CrossRef]
- Narayanam, P.K.; Botcha, V.D.; Ghosh, M.; Major, S.S. Growth and Photocatalytic Behaviour of Transparent Reduced GO-ZnO Nanocomposite Sheets. Nanotechnology 2019, 30, 485601. [Google Scholar] [CrossRef]
- Zandiatashbar, A.; Lee, G.-H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.J.; Zeng, X.L.; Dang, C.Y. Graphene Composites. Handb. Graphene 2019, 1, 1–25. [Google Scholar]
- Zhou, Q.; Xia, G.; Du, M.; Lu, Y.; Xu, H. Scotch-tape-like exfoliation effect of graphene quantum dots for efficient preparation of graphene nanosheets in water. Appl. Surf. Sci. 2019, 483, 52–59. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.S. Interlayer Distance Controlled Graphene, Supercapacitor and Method of Producing the Same. U.S. Patent 10,214,422, 26 February 2019. [Google Scholar]
- Omran, B.; Baek, K.-H. Graphene-derived antibacterial nanocomposites for water disinfection: Current and future perspectives. Environ. Pollut. 2022, 298, 118836. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhao, H.; Yu, H. Bio-inspired Multifunctional Graphene–Epoxy Anticorrosion Coatings by Low-Defect Engineered Graphene. ACS Nano 2022, 16, 710–720. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, Y.; Kong, Q.; Pang, H. Applications of Metal–Organic Framework–Graphene Composite Materials in Electrochemical Energy Storage. FlatChem 2022, 32, 100332. [Google Scholar] [CrossRef]
- Aiswaria, P.; Mohamed, S.N.; Singaravelu, D.L.; Brindhadevi, K.; Pugazhendhi, A. A review on graphene/graphene oxide supported electrodes for microbial fuel cell applications: Challenges and prospects. Chemosphere 2022, 296, 133983. [Google Scholar]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Obraztsov, I.; Bakandritsos, A.; Šedajová, V.; Langer, R.; Jakubec, P.; Zoppellaro, G.; Pykal, M.; Presser, V.; Otyepka, M.; Zbořil, R. Graphene Acid for Lithium-Ion Batteries—Carboxylation Boosts Storage Capacity in Graphene. Adv. Energy Mater. 2022, 12, 2103010. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.; Yang, M.; Qian, G.; Peng, S.; Qi, F.; Shuai, C. Semicoherent strengthens graphene/zinc scaffolds. Mater. Today Nano 2022, 17, 100163. [Google Scholar] [CrossRef]
- Grasseschi, D.; Silva, W.C.; de Souza Paiva, R.; Starke, L.D.; do Nascimento, A.S. Surface coordination chemistry of graphene: Understanding the coordination of single transition metal atoms. Coord. Chem. Rev. 2020, 422, 213469. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Abdelaziz, A.; Bessa, W.; Hussin, M.H.; Brosse, N.; Thakur, V.K. Cellulose nanofibrils–graphene hybrids: Recent advances in fabrication, properties, and applications. Nanoscale 2022, 14, 12515–12546. [Google Scholar] [CrossRef]
- Nimbalkar, A.S.; Tiwari, S.K.; Ha, S.K.; Hong, C.K. An efficient water saving step during the production of graphene oxide via chemical exfoliation of graphite. Mater. Today Proc. 2020, 21, 1749–1754. [Google Scholar] [CrossRef]
- Balaji, A.; Yang, S.; Wang, J.; Zhang, J. Graphene oxide-based nanostructured DNA sensor. Biosensors 2019, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Halim, A.; Luo, Q.; Ju, Y.; Song, G. A mini review focused on the recent applications of graphene oxide in stem cell growth and differentiation. Nanomaterials 2018, 8, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Arvizu-Rodriguez, L.; Paramo-García, U.; Caballero-Briones, F. Low resistance, high mobility reduced graphene oxide films prepared with commercial antioxidant supplements. Mater. Lett. 2020, 276, 128176. [Google Scholar] [CrossRef]
- Lin, T.-N.; Santiago, S.R.M.; Yuan, C.-T.; Chiu, K.-P.; Shen, J.-L.; Wang, T.-C.; Kuo, H.-C.; Chiu, C.-H.; Yao, Y.-C.; Lee, Y.-J. Enhanced performance of GaN-based ultraviolet light emitting diodes by photon recycling using graphene quantum dots. Sci. Rep. 2017, 7, 7108. [Google Scholar] [CrossRef] [Green Version]
- Magne, T.M.; de Oliveira Vieira, T.; Alencar, L.M.R.; Junior, F.F.M.; Gemini-Piperni, S.; Carneiro, S.V.; Fechine, L.M.; Freire, R.M.; Golokhvast, K.; Metrangolo, P. Graphene and its derivatives: Understanding the main chemical and medicinal chemistry roles for biomedical applications. J. Nanostructure Chem. 2021, 12, 693–727. [Google Scholar] [CrossRef]
- Deng, X.; Zou, C.; Han, Y.; Lin, L.-C.; Ho, W.W. Computational Evaluation of Carriers in Facilitated Transport Membranes for Postcombustion Carbon Capture. J. Phys. Chem. C 2020, 124, 46. [Google Scholar] [CrossRef]
- Szomek, M.; Moesgaard, L.; Reinholdt, P.; Hald, S.B.H.; Petersen, D.; Krishnan, K.; Covey, D.F.; Kongsted, J.; Wüstner, D. Membrane organization and intracellular transport of a fluorescent analogue of 27-hydroxycholesterol. Chem. Phys. Lipids 2020, 233, 105004. [Google Scholar] [CrossRef]
- Lee, J.; Aluru, N.R. Water-solubility-driven separation of gases using graphene membrane. J. Membr. Sci. 2013, 428, 546–553. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, J.; Hou, W.; Yang, X.; Zhou, J. Unsaturated Zn–N2–O active sites derived from hydroxyl in graphene oxide and zinc atoms in core shell ZIF-8@ ZIF-67 nanocomposites enhanced CO2 adsorption capacity. Microporous Mesoporous Mater. 2020, 312, 110786. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.-C. High selective mixed membranes based on mesoporous MCM-41 and MCM-41-NH2 particles in a polysulfone matrix. Front. Chem. 2019, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally stable polymers for advanced high-performance gas separation membranes. Prog. Energy Combust. Sci. 2018, 66, 1–41. [Google Scholar] [CrossRef]
- Koenig, S.P.; Wang, L.; Pellegrino, J.; Bunch, J.S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 2012, 7, 728–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.C.; Huang, L.J.; Wang, Y.X.; Yang, K.; Tang, J.G.; Wang, Y.; Cheng, M.M.; Zhang, Y.; Kipper, M.J.; Belfiore, L.A. Recent developments in graphene-based polymer composite membranes: Preparation, mass transfer mechanism, and applications. J. Appl. Polym. Sci. 2019, 136, 47761. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, molecular-sieving graphene oxide membranes for selective hydrogen separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef]
- Dong, G.; Hou, J.; Wang, J.; Zhang, Y.; Chen, V.; Liu, J. Enhanced CO2/N2 separation by porous reduced graphene oxide/Pebax mixed matrix membranes. J. Membr. Sci. 2016, 520, 860–868. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Banihashemi, F.; Lin, Y. Graphene oxide membranes with narrow inter-sheet galleries for enhanced hydrogen separation. Chem. Commun. 2019, 55, 3077–3080. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Super gas barrier and selectivity of graphene oxide-polymer multilayer thin films. Adv. Mater. 2013, 25, 503–508. [Google Scholar] [CrossRef]
- Vinodh, R.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent advancements in polysulfone based membranes for fuel cell (PEMFCs, DMFCs and AMFCs) applications: A critical review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; Shahat, A.; Ayoub, T.I.; Kamel, R.M. Fabrication of high flux polysulfone/mesoporous silica nanocomposite ultrafiltration membranes for industrial wastewater treatment. Biointerface Res. Appl. Chem. 2022, 12, 7556–7572. [Google Scholar]
- Sherugar, P.; Déon, S.; Nagaraja, K.; Padaki, M. Tailoring the structure of polysulfone nanocomposite membranes by incorporating iron oxide doped aluminium oxide for excellent separation performance and antifouling property. Environ. Sci. Water Res. Technol. 2022, 8, 1059–1077. [Google Scholar] [CrossRef]
- Costa Flores, M.; Figueiredo, K.C.d.S. Asymmetric oxygen-functionalized carbon nanotubes dispersed in polysulfone for CO2 separation. J. Appl. Polym. Sci. 2023, 140, e53303. [Google Scholar] [CrossRef]
- Said, N.; Mansur, S.; Zainol Abidin, M.N.; Ismail, A.F. Fabrication and characterization of polysulfone/iron oxide nanoparticle mixed matrix hollow fiber membranes for hemodialysis: Effect of dope extrusion rate and air gap. J. Membr. Sci. Res. 2023, 9, 1972553. [Google Scholar]
- Zahri, K.; Goh, P.; Ismail, A. The incorporation of graphene oxide into polysulfone mixed matrix membrane for CO2/CH4 separation. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2016; p. 012007. [Google Scholar]
- Zahri, K.; Wong, K.; Goh, P.; Ismail, A. Graphene oxide/polysulfone hollow fiber mixed matrix membranes for gas separation. RSC Adv. 2016, 6, 89130–89139. [Google Scholar] [CrossRef]
- Sainath, K.; Modi, A.; Bellare, J. In-situ growth of zeolitic imidazolate framework-67 nanoparticles on polysulfone/graphene oxide hollow fiber membranes enhance CO2/CH4 separation. J. Membr. Sci. 2020, 614, 118506. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Hao, J.; Bao, B.; Bian, X.; Jiang, X.; Pang, J.; Zhang, H.; Jiang, Z.; Jiang, L. A charge-density-tunable three/two-dimensional polymer/graphene oxide heterogeneous nanoporous membrane for ion transport. ACS Nano 2017, 11, 10816–10824. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pan, Y.; Xu, J.; Wang, Z.; Zhu, H.; Liu, G.; Zhong, J.; Jin, W. Introducing amphipathic copolymer into intermediate layer to fabricate ultra-thin Pebax composite membrane for efficient CO2 capture. J. Membr. Sci. 2023, 667, 121183. [Google Scholar] [CrossRef]
- Hieu, N.H.; Phat, N.T.; Giang, N.T.H.; Tinh, N.T.; Dat, N.M.; Phong, M.T. Recovery of furfural by pervaporation technology using the ceramic tubular supported graphene-polydimethylsiloxane nanocomposite membranes. FlatChem 2022, 34, 100402. [Google Scholar] [CrossRef]
- Yang, J.; Chen, A.; Liu, F.; Gu, L.; Xie, X.; Ding, Z. Hybrid coating of polydimethylsiloxane with nano-ZrO2 on magnesium alloy for superior corrosion resistance. Ceram. Int. 2022, 48, 35280–35289. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Wang, B.; Han, Y.; Zhang, R. High-strength electrospun polydimethylsiloxane/polytetrafluoroethylene hybrid membranes with stable and controllable coral-like structures. Compos. Part A Appl. Sci. Manuf. 2023, 164, 107316. [Google Scholar] [CrossRef]
- Berean, K.J.; Ou, J.Z.; Nour, M.; Field, M.R.; Alsaif, M.M.; Wang, Y.; Ramanathan, R.; Bansal, V.; Kentish, S.; Doherty, C.M. Enhanced gas permeation through graphene nanocomposites. J. Phys. Chem. C 2015, 119, 13700–13712. [Google Scholar] [CrossRef]
- Koolivand, H.; Sharif, A.; Chehrazi, E.; Kashani, M.R.; Paran, S.M.R. Mixed-matrix membranes comprising graphene-oxide nanosheets for CO2/CH4 separation: A comparison between glassy and rubbery polymer matrices. Polym. Sci. Ser. A 2016, 58, 801–809. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Fan, H.; Feng, L.; Wen, G.; Qin, L.-C. Synthesis of graphene oxide using boric acid in hummers method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129802. [Google Scholar] [CrossRef]
- Ha, H.; Park, J.; Ando, S.; Kim, C.B.; Nagai, K.; Freeman, B.D.; Ellison, C.J. Gas permeation and selectivity of poly (dimethylsiloxane)/graphene oxide composite elastomer membranes. J. Membr. Sci. 2016, 518, 131–140. [Google Scholar] [CrossRef]
- Tumnantong, D.; Srisamrid, K.; Poompradub, S.; Prasassarakich, P. Preparation of poly (methyl methacrylate)-silica nanocomposites via DMP-assisted RAFT polymerization and NR/PMMA-RAFT-SiO2 hybrid membrane for pervaporation. Eur. Polym. J. 2022, 168, 111088. [Google Scholar] [CrossRef]
- Zakaria, N.; Zaliman, S.; Leo, C.; Ahmad, A.; Ooi, B.; Poh, P.E. Electrochemical cleaning of superhydrophobic polyvinylidene fluoride/polymethyl methacrylate/carbon black membrane after membrane distillation. J. Taiwan Inst. Chem. Eng. 2022, 138, 104448. [Google Scholar] [CrossRef]
- Mohammed, M.; Khafagy, R.; Hussien, M.S.; Sakr, G.; Ibrahim, M.A.; Yahia, I.; Zahran, H. Enhancing the structural, optical, electrical, properties and photocatalytic applications of ZnO/PMMA nanocomposite membranes: Towards multifunctional membranes. J. Mater. Sci. Mater. Electron. 2022, 33, 1977–2002. [Google Scholar] [CrossRef]
- Baldanza, A.; Pastore Carbone, M.G.; Brondi, C.; Manikas, A.C.; Mensitieri, G.; Pavlou, C.; Scherillo, G.; Galiotis, C. Chemical Vapour Deposition Graphene–PMMA Nanolaminates for Flexible Gas Barrier. Membranes 2022, 12, 611. [Google Scholar] [CrossRef]
- Pavlou, C.; Pastore Carbone, M.G.; Manikas, A.C.; Trakakis, G.; Koral, C.; Papari, G.; Andreone, A.; Galiotis, C. Effective EMI shielding behaviour of thin graphene/PMMA nanolaminates in the THz range. Nat. Commun. 2021, 12, 4655. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.V.; Benck, J.D.; Yuan, Z.; Misra, R.P.; Govind Rajan, A.; Eatmon, Y.; Kale, S.; Chu, X.S.; Li, D.O.; Gong, C. Fabrication, pressure testing, and nanopore formation of single-layer graphene membranes. J. Phys. Chem. C 2017, 121, 14312–14321. [Google Scholar] [CrossRef]
- Jin, J.; Zhu, S.; Wang, Z.; Bi, X.; Shi, Y.; Shi, Y.; Zhang, Y. Thin Film of Polyimide/Mof Nanocomposite Membrane with Substantially Improved Stability for Co2/Ch4 Separation; Social Science Research Network; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–30. [Google Scholar]
- Muhammad, S.; Niazi, J.H.; Shawuti, S.; Qureshi, A. Functional POSS based polyimide nanocomposite for enhanced structural, thermal, antifouling and antibacterial properties. Mater. Today Commun. 2022, 31, 103287. [Google Scholar] [CrossRef]
- Zhu, S.; Bi, X.; Shi, Y.; Shi, Y.; Zhang, Y.; Jin, J.; Wang, Z. Thin Films Based on Polyimide/Metal–Organic Framework Nanoparticle Composite Membranes with Substantially Improved Stability for CO2/CH4 Separation. ACS Appl. Nano Mater. 2022, 5, 8997–9007. [Google Scholar] [CrossRef]
- Melicchio, A.; Favvas, E.P. Preparation and characterization of graphene oxide as a candidate filler material for the preparation of mixed matrix polyimide membranes. Surf. Coat. Technol. 2018, 349, 1058–1068. [Google Scholar] [CrossRef]
- Shishatskiy, S.; Makrushin, V.; Levin, I.; Merten, P.; Matson, S.; Khotimskiy, V. Effect of Immobilization of Phenolic Antioxidant on Thermo-Oxidative Stability and Aging of Poly (1-trimethylsilyl-1-propyne) in View of Membrane Application. Polymers 2022, 14, 462. [Google Scholar] [CrossRef]
- Golubev, G.; Sokolov, S.; Rokhmanka, T.; Makaev, S.; Borisov, I.; Khashirova, S.; Volkov, A. High Efficiency Membranes Based on PTMSP and Hyper-Crosslinked Polystyrene for Toxic Volatile Compounds Removal from Wastewater. Polymers 2022, 14, 2944. [Google Scholar] [CrossRef] [PubMed]
- Kalmykov, D.; Balynin, A.; Yushkin, A.; Grushevenko, E.; Sokolov, S.; Malakhov, A.; Volkov, A.; Bazhenov, S. Membranes Based on PTMSP/PVTMS Blends for Membrane Contactor Applications. Membranes 2022, 12, 1160. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, L.; Ligi, S.; De Angelis, M.G.; Cucca, G.; Pettinau, A. Effect of graphene and graphene oxide nanoplatelets on the gas permselectivity and aging behavior of poly (trimethylsilyl propyne)(PTMSP). Ind. Eng. Chem. Res. 2015, 54, 11199–11211. [Google Scholar] [CrossRef]
- Alberto, M.; Bhavsar, R.; Luque-Alled, J.M.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Impeded physical aging in PIM-1 membranes containing graphene-like fillers. J. Membr. Sci. 2018, 563, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Xu, S.; Wan, R.; Yang, Y.; He, R. Functionalized graphene oxide cross-linked poly (2, 6-dimethyl-1, 4-phenylene oxide)-based anion exchange membranes with superior ionic conductivity. J. Power Sources 2022, 517, 230720. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Shen, C.; Gao, S. Preparation and Characterization of Non-N-Bonded Side-Chain Anion Exchange Membranes Based on Poly (2, 6-dimethyl-1, 4-phenylene oxide). Ind. Eng. Chem. Res. 2022, 61, 1715–1724. [Google Scholar] [CrossRef]
- Chu, X.; Miao, S.; Zhou, A.; Liu, S.; Liu, L.; Li, N. A strategy to design quaternized poly (2, 6-dimethyl-1, 4-phenylene oxide) anion exchange membranes by atom transfer radical coupling. J. Membr. Sci. 2022, 649, 120397. [Google Scholar] [CrossRef]
- Rea, R.; Ligi, S.; Christian, M.; Morandi, V.; Giacinti Baschetti, M.; De Angelis, M.G. Permeability and selectivity of PPO/graphene composites as mixed matrix membranes for CO2 capture and gas separation. Polymers 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theravalappil, R.; Rahaman, M. Patents on graphene-based polymer composites and their applications. In Polymer Nanocomposites Containing Graphene; Elsevier: Amsterdam, The Netherlands, 2022; pp. 615–638. [Google Scholar]
- Ren, F.; Tan, W.; Duan, Q.; Jin, Y.; Pei, L.; Ren, P.; Yan, D. Ultra-low gas permeable cellulose nanofiber nanocomposite films filled with highly oriented graphene oxide nanosheets induced by shear field. Carbohydr. Polym. 2019, 209, 310–319. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on immobilization of enzymes on synthetic polymeric nanofibers fabricated by electrospinning. Biotechnol. Bioeng. 2022, 119, 9–33. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Y.; Zhang, S.; Li, T.; Ramakrishna, S.; Liu, Y. Progress of improving mechanical strength of electrospun nanofibrous membranes. Macromol. Mater. Eng. 2020, 305, 2000230. [Google Scholar] [CrossRef]
- Peng, K.; Huang, H. Investigating the origin of the core-shell structure of polymeric nanofibers during fabrication process at the atomistic scale. Appl. Surf. Sci. 2023, 608, 155105. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Bräse, S. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Du, Y.; Ji, Y.; Zhou, Y.; Zhao, P.; Yu, D.G. Modernization of traditional Chinese condiments via electrospun polymeric nanocomposites. ES Food Agrofor. 2022, 8, 47–56. [Google Scholar] [CrossRef]
- Mora-Boza, A.; López-Ruiz, E.; López-Donaire, M.L.; Jiménez, G.; Aguilar, M.R.; Marchal, J.A.; Pedraz, J.L.; Vázquez-Lasa, B.; Román, J.S.; Gálvez-Martín, P. Evaluation of Glycerylphytate Crosslinked Semi-and Interpenetrated Polymer Membranes of Hyaluronic Acid and Chitosan for Tissue Engineering. Polymers 2020, 12, 2661. [Google Scholar] [CrossRef]
- Morales-Zamudio, L.; Lozano, T.; Caballero-Briones, F.; Zamudio, M.A.; Angeles-San Martin, M.E.; de Lira-Gomez, P.; Martinez-Colunga, G.; Rodriguez-Gonzalez, F.; Neira, G.; Sanchez-Valdes, S. Structure and Mechanical Properties of Graphene Oxide-Reinforced Polycarbonate. Mater. Chem. Phys. 2020, 261, 124180. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, S.J.; Jeon, I.-Y. Graphene/Polymer Nanocomposites: Preparation, Mechanical Properties, and Application. Polymers 2022, 14, 4733. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Ghaferi, A.A. Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review. Sensors 2022, 22, 8661. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, D.H.; Chung, Y.S.; Hwang, S.K.; Hwang, H.Y.; Kim, S.S. Effect of processing parameters of the continuous wet spinning system on the crystal phase of PVDF fibers. J. Appl. Polym. Sci. 2018, 135, 45712. [Google Scholar] [CrossRef]
- Ganguly, S. Preparation/processing of polymer-graphene composites by different techniques. In Polymer Nanocomposites Containing Graphene; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–74. [Google Scholar]
- Orasugh, J.T.; Ray, S.S. Graphene-Based Electrospun Fibrous Materials with Enhanced EMI Shielding: Recent Developments and Future Perspectives. ACS Omega 2022, 7, 33699–33718. [Google Scholar] [CrossRef]
- Medeiros, G.B.; Lima, F.d.A.; de Almeida, D.S.; Guerra, V.G.; Aguiar, M.L. Modification and Functionalization of Fibers Formed by Electrospinning: A Review. Membranes 2022, 12, 861. [Google Scholar] [CrossRef]
- Kausar, A.; Bocchetta, P. Polymer/graphene nanocomposite membranes: Status and emerging prospects. J. Compos. Sci. 2022, 6, 76. [Google Scholar] [CrossRef]
- Kausar, A. Reinforced polyaniline nanocomposite nanofibers: Cutting-edge potential. Polym.-Plast. Technol. Mater. 2022, 61, 1–14. [Google Scholar] [CrossRef]
- Widakdo, J.; Huang, T.-J.; Subrahmanya, T.; Austria, H.F.M.; Chou, H.-L.; Hung, W.-S.; Wang, C.-F.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Bioinspired ionic liquid-graphene based smart membranes with electrical tunable channels for gas separation. Appl. Mater. Today 2022, 27, 101441. [Google Scholar] [CrossRef]
- Cooley, J.F. Improved methods of and apparatus for electrically separating the relatively volatile liquid component from the component of relatively fixed substances of composite fluids. U. K. Pat. 1900, 6385, 19. [Google Scholar]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2014, 131, 39628. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906–7915. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.; Wang, W.; Zhu, S.; Guo, L.; Zhang, Z.; Li, P. Effects of different functional groups in graphene nanofiber on the mechanical property of polyvinyl alcohol composites by the molecular dynamic simulations. J. Mol. Liq. 2019, 277, 261–268. [Google Scholar] [CrossRef]
- Xin, Q.; Li, Z.; Li, C.; Wang, S.; Jiang, Z.; Wu, H.; Zhang, Y.; Yang, J.; Cao, X. Enhancing the CO2 separation performance of composite membranes by the incorporation of amino acid-functionalized graphene oxide. J. Mater. Chem. A 2015, 3, 6629–6641. [Google Scholar] [CrossRef]
- Quan, S.; Li, S.W.; Xiao, Y.C.; Shao, L. CO2-selective mixed matrix membranes (MMMs) containing graphene oxide (GO) for enhancing sustainable CO2 capture. Int. J. Greenh. Gas Control 2017, 56, 22–29. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 capture by functionalized graphene oxide nanosheets as fillers to fabricate multi-permselective mixed matrix membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Huang, S.; Wu, H.; Li, Y.; Tian, Z.; Jiang, Z. Pebax–PEG–MWCNT hybrid membranes with enhanced CO2 capture properties. J. Membr. Sci. 2014, 460, 62–70. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, Z.; Li, F.; Liu, S.; Wang, L.; Tang, X.; Rudolph, V. Investigation of Gas permeability in carbon nanotube (CNT)− polymer matrix membranes via modifying CNTs with functional groups/metals and controlling modification location. J. Phys. Chem. C 2011, 115, 6661–6670. [Google Scholar] [CrossRef]
- Kim, S.; Chen, L.; Johnson, J.K.; Marand, E. Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: Theory and experiment. J. Membr. Sci. 2007, 294, 147–158. [Google Scholar] [CrossRef]
- Liang, C.-Y.; Uchytil, P.; Petrychkovych, R.; Lai, Y.-C.; Friess, K.; Sipek, M.; Reddy, M.M.; Suen, S.-Y. A comparison on gas separation between PES (polyethersulfone)/MMT (Na-montmorillonite) and PES/TiO2 mixed matrix membranes. Sep. Purif. Technol. 2012, 92, 57–63. [Google Scholar] [CrossRef]
- Momeni, S.; Pakizeh, M. Preparation, characterization and gas permeation study of PSf/MgO nanocomposite membrane. Braz. J. Chem. Eng. 2013, 30, 589–597. [Google Scholar]
- Chen, X.Y.; Razzaz, Z.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on silica nanoparticles and microcellular polymers for CO2/CH4 separation. J. Cell. Plast. 2018, 54, 309–331. [Google Scholar] [CrossRef]
- Karki, S.; Gohain, M.B.; Yadav, D.; Ingole, P.G. Nanocomposite and bio-nanocomposite polymeric materials/membranes development in energy and medical sector: A review. Int. J. Biol. Macromol. 2021, 193, 2121–2139. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Paula, C.D.d.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the barrier and mechanical behavior of different nanofillers in chitosan films for food packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Nanomaterials and nanotechnology for water treatment: Recent advances. Inorg. Nano-Met. Chem. 2021, 51, 1615–1645. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Junaidi, N.F.D.; Othman, N.H.; Shahruddin, M.Z.; Alias, N.H.; Lau, W.J.; Ismail, A.F. Effect of graphene oxide (GO) and polyvinylpyrollidone (PVP) additives on the hydrophilicity of composite polyethersulfone (PES) membrane. Malays. J. Fundam. Appl. Sci. 2019, 15, 361–366. [Google Scholar] [CrossRef]
- Mahdavi Far, R.; Van der Bruggen, B.; Verliefde, A.; Cornelissen, E. A review of zeolite materials used in membranes for water purification: History, applications, challenges and future trends. J. Chem. Technol. Biotechnol. 2021, 97, 575–596. [Google Scholar] [CrossRef]
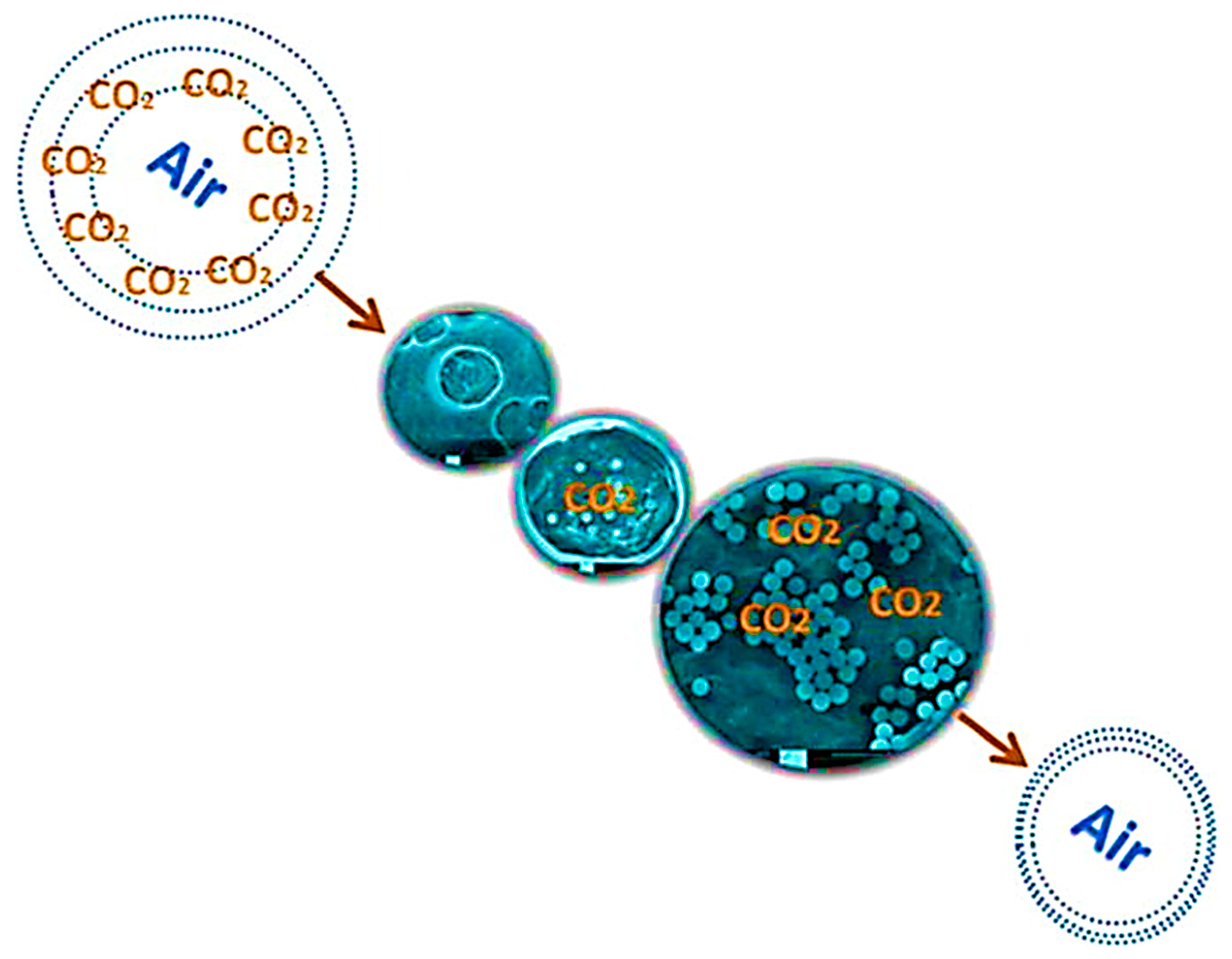
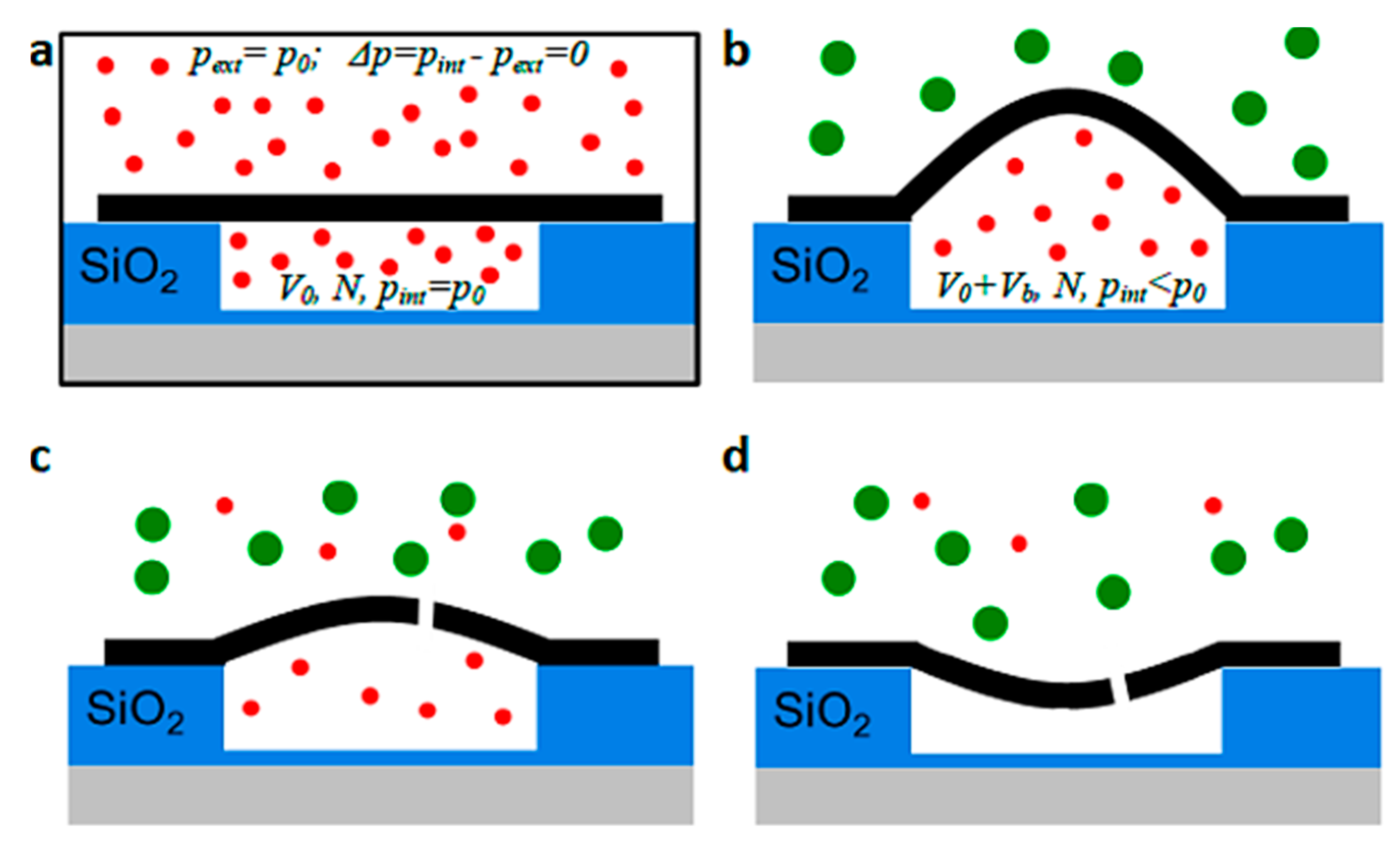
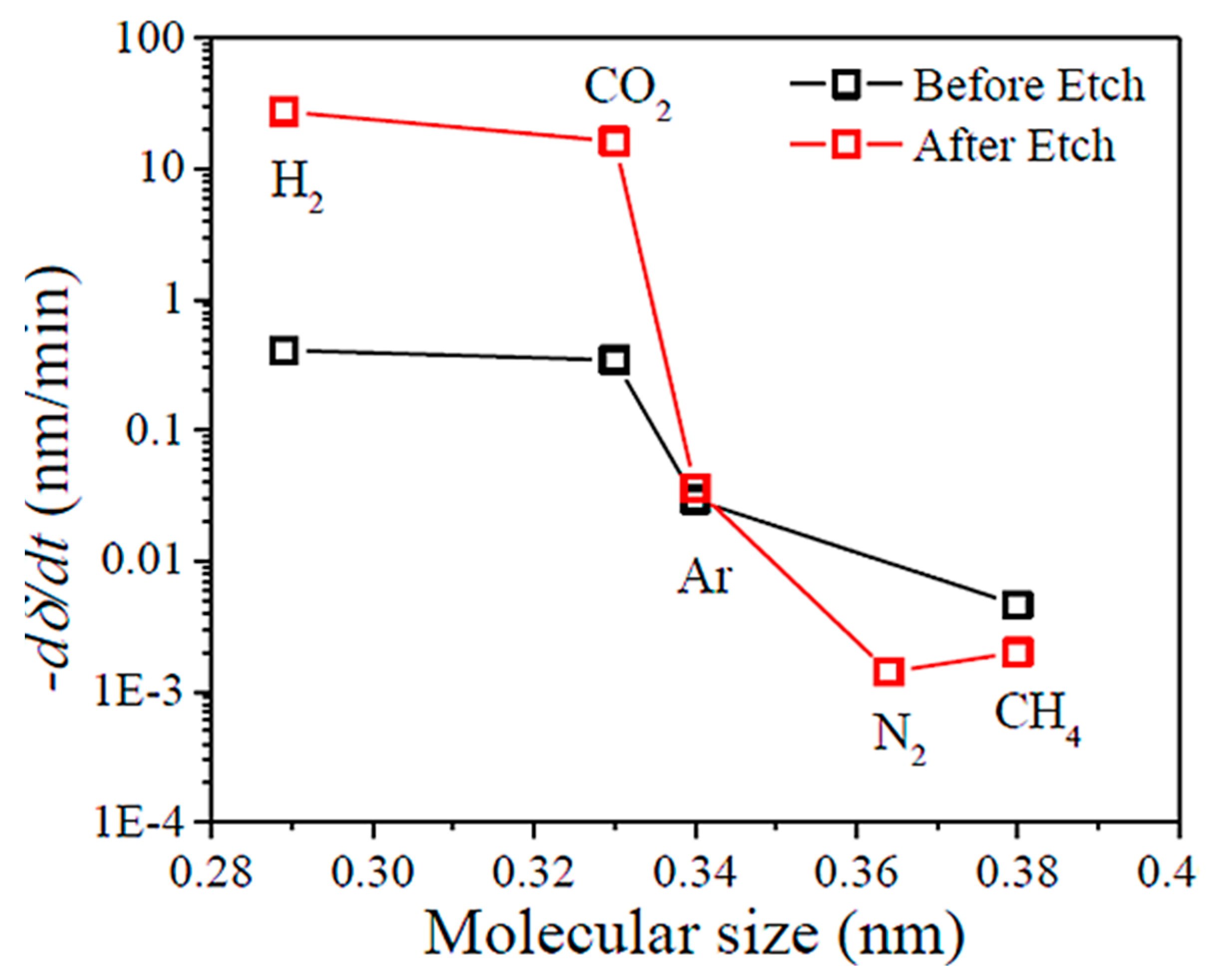


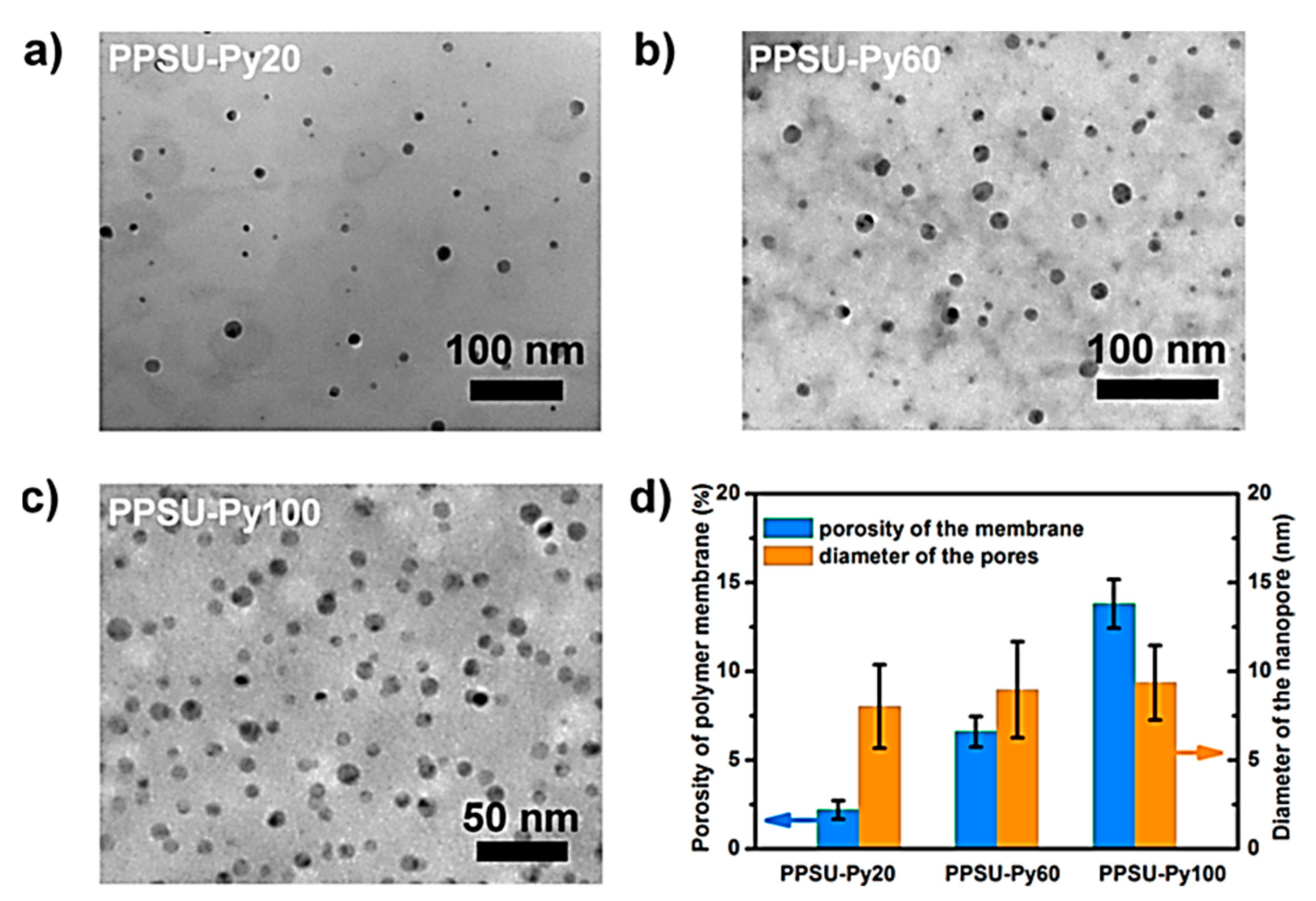


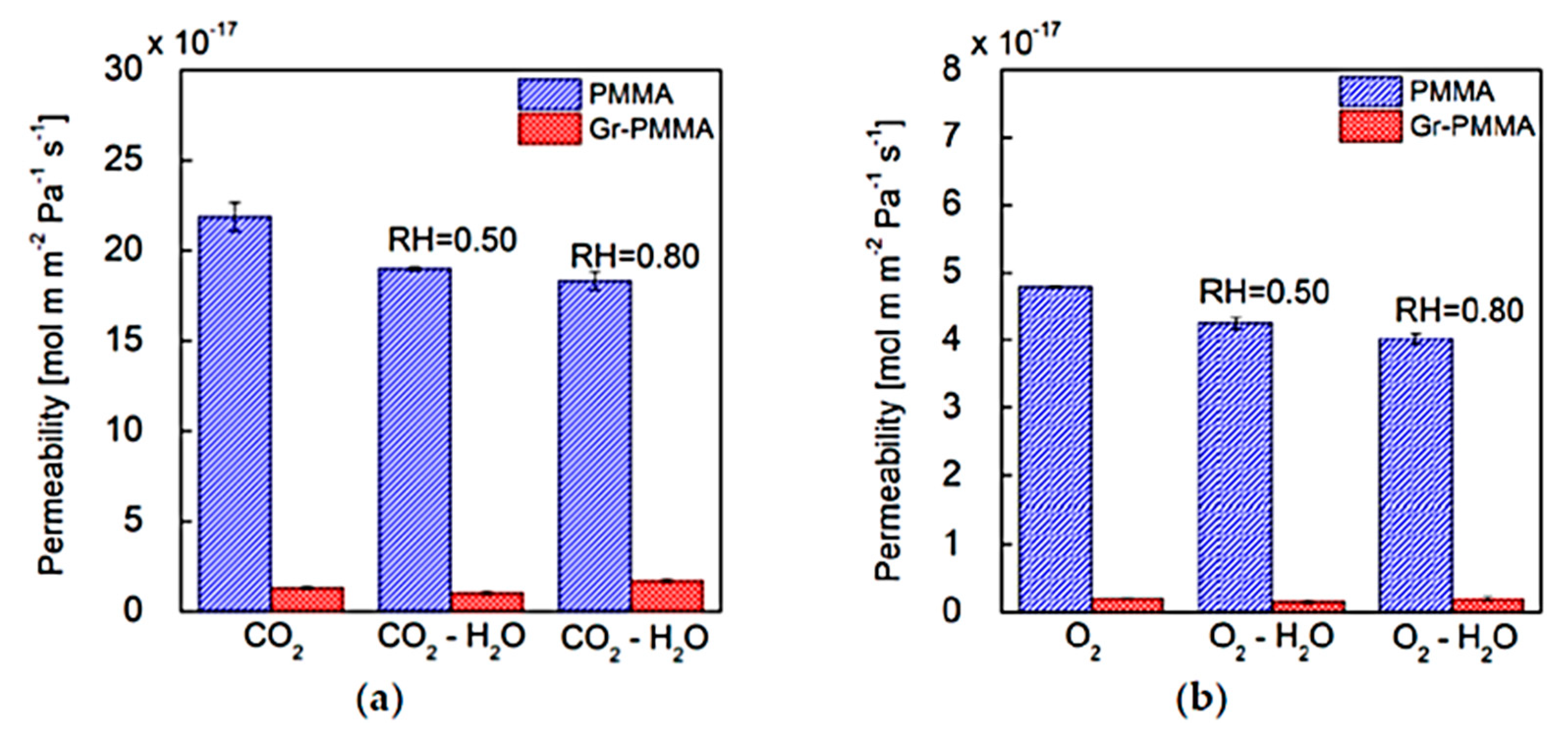

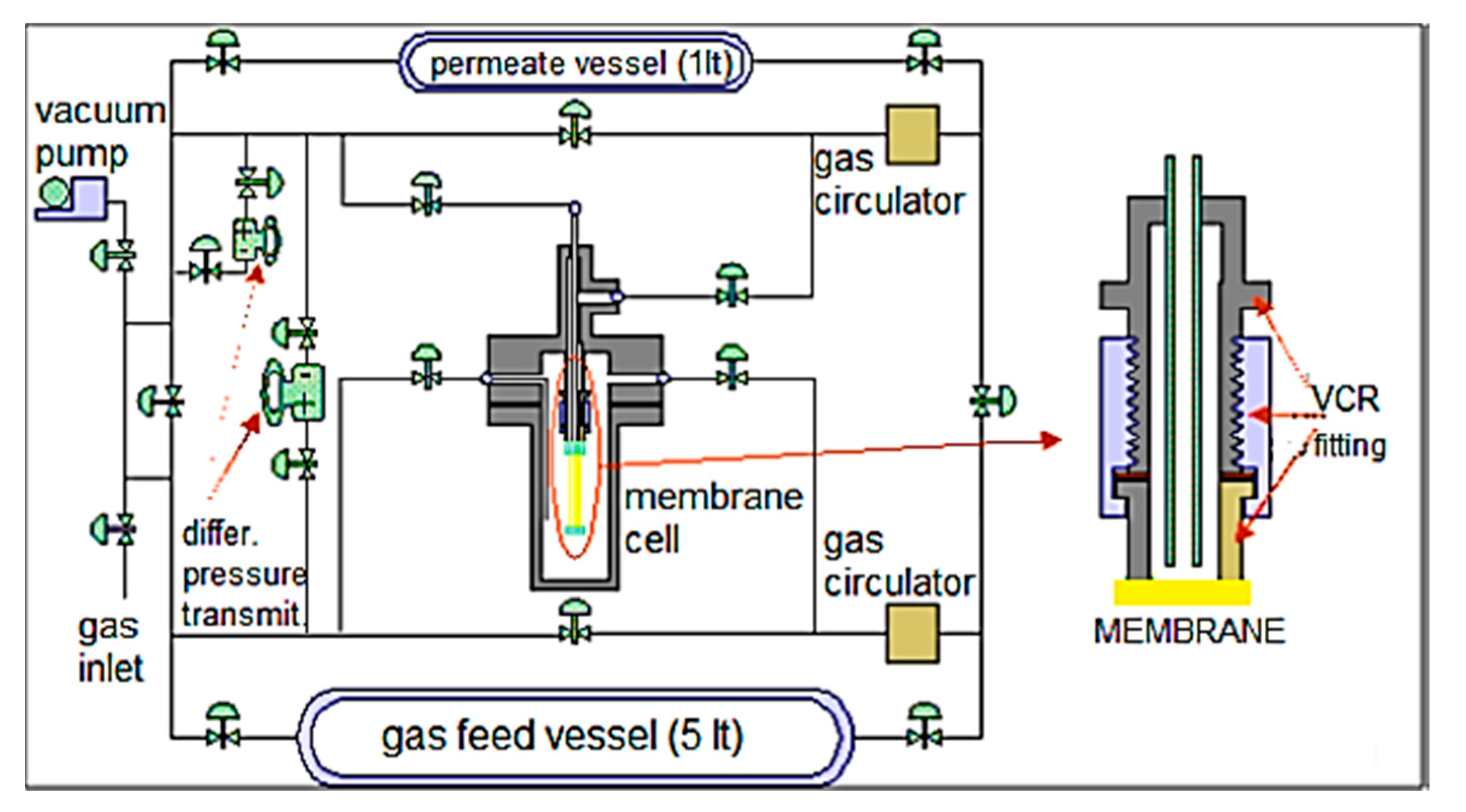
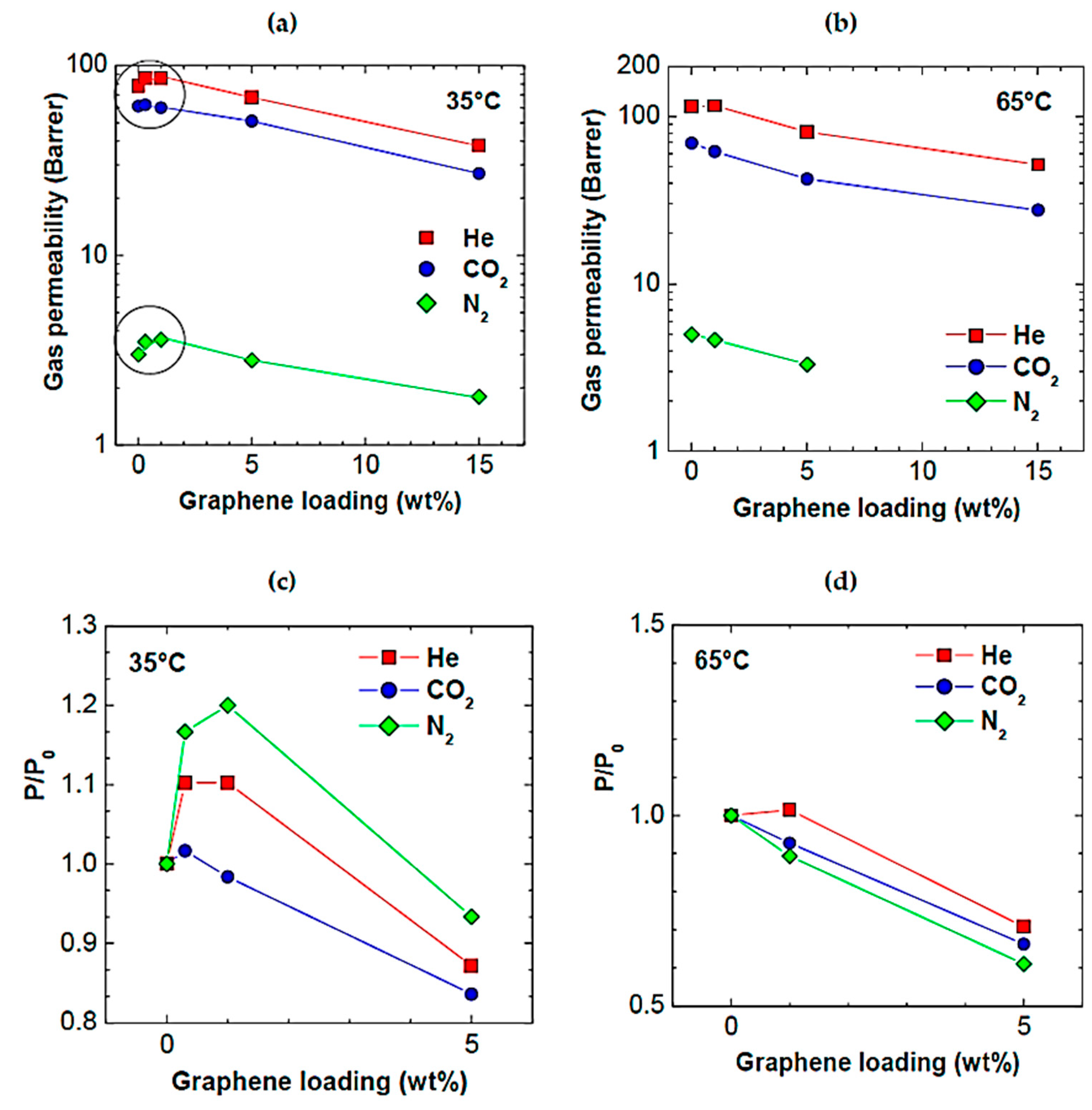
| Gas Mixture or Gas | CO2 Flux (No. of Molecules) | Selectivity | Size of Gas Molecules Kinetic Diameter (pm) |
|---|---|---|---|
| CO2/O2 | 0.43 ± 0.04 | 9.5 ± 0.7 | - |
| CO2/N2 | 0.23 ± 0.06 | 14.4 ± 1.4 | - |
| CO2/CH4 | 0.35 ± 0.12 | 9.9 ± 0.7 | - |
| H2 | - | - | 289 |
| CO2 | - | - | 330 |
| O2 | - | - | 346 |
| N2 | - | - | 364 |
| CH4 | - | - | 384 |
| Nanolaminate/Permeating Gas | P [mol·m·m−2·Pa−1·s−1] | P [Barrer] |
|---|---|---|
| PMMA/CO2 | 21.9 (±0.8) × 10−17 | 6.5 (±0.2) × 10−1 |
| Gr-PMMA/CO2 | 1.30 (±0.1) × 10−17 | 0.39 (±0.03) × 10−1 |
| PMMA/O2 | 4.79 (±0.01) × 10−17 | 1.434 (±0.003) × 10−1 |
| Gr-PMMA/O2 | 0.21 (±0.01) × 10−17 | 0.063 (±0.003) × 10−1 |
| Polymer | Nanofiller | Fabrication Route | Physicochemical Properties | Membrane Properties | Ref. |
|---|---|---|---|---|---|
| Polymer | Graphene or graphene oxide | Solution casting | Ion-molecule interaction; 1.8–20 nm thickness | H2/N2 selectivity 900; H2/CO2 selectivity 3400; pore size ~0.34 nm | [96] |
| Polysulfone | Graphene oxide | Dry-wet phase inversion techniques; dimethyl acetamide solvent; hollow fiber mixed matrix membrane | Graphene oxide own π-π stacking form interaction with CO2 gas and polar gas molecules; multi-layered porous structure with large macro-voids | 0.25 wt.% nanofiller; CO2/CH4 separation 25; CO2 permeance 86.80 GPU | [105] |
| Polysulfone | Graphene | Phase inversion; hollow fiber mixed matrix membrane | Nanosize synthesized graphene; interfacial interaction between graphene and polymer matrix | CO2/N2 selectivity 158%; CO2/CH4 selectivity 74% | [106] |
| Polysulfone | Graphene oxide | Solution route; N-Methyl-2-pyrrolidone solvent | Physical interaction between oxygenated functional groups of graphene oxide and polymer; interactions between functional groups of nanocomposites and gas molecules | CO2/CH4 selectivity ~45 | [107] |
| Polyphenylsulfone-pyridine | Graphene oxide | Vacuum infiltration technique | Wettability and surface charge response to pH; acidic pH = 3 form hydrophilic state contact angle 63.3°; alkaline pH = 11 form hydrophobic state contact angle 106.5°; charge-density-tunable nanoporous; power of ≈0.76 W m–2 | Dispersion; morphology | [108] |
| Poly(dimethyl siloxane) | Graphene | Solution casting; p-xylene solvent | π-π interactions in matrix-nanofiller | 0.2 wt.% nanofiller; N2, CO2, Ar, and CH4 permeation 60%; CO2/CH4 selectivity 4.2 | [113] |
| Poly(dimethyl siloxane) | Graphene oxide | Solution/ ultrasonication methods; tetrahydrofuran solvent | Interfacial interactions between functional groups of graphene oxide and polymer; density 1.09–1.12; thickness 1.9–2.8 nm | 5 wt.% nanofiller; CO2/CH4 selectivity 112%; CO2 permeability 29% | [114] |
| Poly(dimethyl siloxane) | Graphene oxide | Solution casting | Matrix-nanofiller interactions; interaction between graphene oxide and polymer | 8 wt.% nanofiller; H2, O2, N2, CH4 and CO2 permeability 99.9% | [116] |
| Poly(methyl methacrylate) | Graphene | Wet deposition method | Water adsorption; membrane wrinkles; degree of dispersion/orientation of the graphene nanosheet; structure organization of polymeric chains at the interface with graphene nanosheet | CO2 permeability coefficient 1.30 × 10−17 mol·m·m−2·Pa−1·s−1; O2 permeability coefficient 0.21 × 10−17 mol·m·m−2·Pa−1·s−1 | [120] |
| Polyimide | Graphene oxide | Knife casting technique | Graphene oxide nanoflake anchoring; polymer-graphene interphasedensity; water and testing gas sorption | 0.57 vol.% nanofiller; H2 permeability 28 Barrer; CO2 permeability 8 Barrer; H2/CO2 selectivity 3.5 | [126] |
| Poly(1-trimethylsilyl-1-propyne) | Graphene oxide | Solution casting; chloroform solvent | Anchoring of graphene oxide nanosheets lowers membrane flexibility; less free volume; covalent cross-linking of polymer | 1 wt.% graphene; diffusion coefficient of CO2 (25%); N2 (14); CH4 (9%) | [130] |
| Poly(1-trimethylsilyl-1-propyne) | Graphene | Solution route | Interaction between filler and polymer matrix; 0.93–1.36 MPa; 38–44 MPa | 0.05 wt.% nanofiller; CO2 permeability 3.5 × 103 Barrer | [131] |
| Poly(2,6-dimethyl-1,4-phenylene oxide) | Graphene | Solution route | Void formation at interface; glassy polymer filled with graphene;graphene inclusion for physical constraint to relaxation of polymer chains | 0.3–15 wt.% nanofiller reduced permeability | [135] |
| Cellulose | Graphene oxide | Solution route | Graphene oxide exfoliation in polymer; physical interactions; tensile strength and Young’s modulus increase by 2 and 57 times, respectively | 3.66 vol.% nanofiller; oxygen permeability coefficient 1.4 × 10−17 cm3cm·cm−2s−1Pa−1 | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Eisa, M.H. Graphene in Polymeric Nanocomposite Membranes—Current State and Progress. Processes 2023, 11, 927. https://doi.org/10.3390/pr11030927
Kausar A, Ahmad I, Zhao T, Aldaghri O, Eisa MH. Graphene in Polymeric Nanocomposite Membranes—Current State and Progress. Processes. 2023; 11(3):927. https://doi.org/10.3390/pr11030927
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, Tingkai Zhao, O. Aldaghri, and M. H. Eisa. 2023. "Graphene in Polymeric Nanocomposite Membranes—Current State and Progress" Processes 11, no. 3: 927. https://doi.org/10.3390/pr11030927